Abstract
CTLA-4 (CD152) negatively regulates T cell activation signaling, and the cytoplasmic domain of CTLA-4 (ctCTLA-4) itself has the capacity to inhibit T cell activation in vitro and in vivo. In this study, the inhibitory mechanisms of the cell-permeable recombinant protein Hph-1-ctCTLA-4 on T cell activation and its ability to prevent collagen-induced arthritis were analyzed. Hph-1-ctCTLA-4 prevented human and mouse T cell activation and proliferation by inhibition of T cell receptor-proximal signaling and the arrest of the cell cycle. Furthermore, Hph-1-ctCTLA-4 protected human umbilical vein endothelial cells (HUVEC) from the human CTL allo-response. The incidence and severity of collagen-induced arthritis were significantly reduced and the erosion of cartilage and bone was effectively prevented by i.v. injection and transdermal administration of Hph-1-ctCTLA-4. Inflammatory cytokine production (IL-1β, IL-6, TNF-α, IL-17A) and collagen-specific antibody levels were significantly reduced, and the numbers of activated T cells and infiltrating granulocytes were substantially decreased. These results demonstrate that systemic or transdermal application of a cell-permeable form of the cytoplasmic domain of CTLA-4 offers an effective therapeutic approach for autoimmune diseases such as rheumatoid arthritis.
Keywords: autoimmune disease, costimulatory molecule
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disorder that leads to progressive joint destruction (1). Hallmarks of RA are joint inflammation, proliferation of synovial cells, and attachment and invasion of synovial fibroblasts into adjacent cartilage and bone (2, 3). Cumulative evidence has indicated that CD4+ T cell-mediated autoimmune responses play a critical role in the pathogenesis of RA, in conjunction with activation of B cells and macrophages that infiltrate the synovium (4, 5). Recent biological therapies against IL-1β, IL-6, or TNF-α have demonstrated promising effects against progressive joint destruction (6–8). However, the clinical use of these therapies has been limited because of several issues, including side effects and the increase of therapy-insensitive patients.
Recently, several reports demonstrated that regulation of costimulatory signaling in the T cell is an important target for treatment of autoimmune diseases such as arthritis (9, 10). CTLA-4 is an activation-induced surface molecule on T cells and is essential for the negative regulation of T cell activation; its inhibitory effects can be accomplished either by competition with CD28 for their ligands B7–1 and -2 or by transmission of negative signals through its intracellular domain (11, 12). The cytoplasmic domain of CTLA-4 (ctCTLA-4), which contains a tyrosine phosphorylation motif, has been found to be 100% conserved among different species, suggesting that this domain is critical for CTLA-4 functions (13, 14). In addition, there are several splicing variants of CTLA-4, including full-length CTLA-4, soluble CTLA-4 that lacks the transmembrane domain, ligand-independent CTLA-4 (liCTLA-4) that lacks the extracellular domain, and only the cytoplasmic domain of CTLA-4 (15). However, the function of these spicing variants in various T cell subsets is not fully understood.
Intracellular delivery of many therapeutic and diagnostic agents can be challenging because the plasma membrane forms a formidable barrier against entry of biomolecules. The discovery of the protein transduction domain (PTD) has made it possible to transduce therapeutically active agents into living cells (16–18). PTDs such as Tat, Antp, Vp22, MTS, Pep-1, and R7 have protein transduction efficiency good enough to be used in protein drug development; however, these PTDs are either of virus or of Drosophila origin, or are generated by assembling different peptide sequences (19–24). In our previous report, we identified a novel human PTD, Hph-1, and Hph-1-PTD has efficient protein transduction efficiency and can deliver therapeutic protein to prevent airway inflammation via an intranasal route (25).
In this study, we describe possible mechanisms for the inhibitory function of the cell-permeable cytoplasmic domain of CTLA-4 (Hph-1-ctCTLA-4) in T cell activation and the therapeutic effects via systemic or transdermal application in the collagen type II (CII)-induced arthritis (CIA) model in vivo. These results demonstrate that blockade of the T cell costimulation pathway by Hph-1-ctCTLA-4 prevents arthritic symptoms effectively and provides preclinical support for the potential therapeutic efficacy of Hph-1-ctCTLA-4 against autoimmune diseases like human RA via systemic or transdermal administration.
Results
Regulation of T Cell Function by Hph-ctCTLA-4.
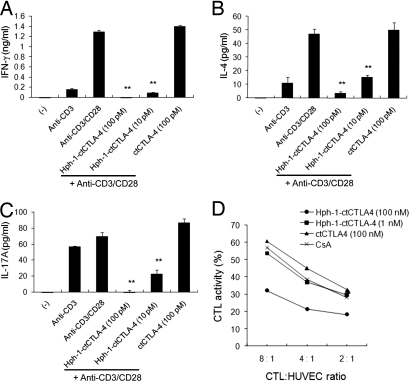
To evaluate how ctCTLA-4 regulates T cell activation, a cell-permeable form of ctCTLA-4 (Hph-1-ctCTLA-4), the tyrosine mutant form of ctCTLA-4 (Hph-1-ctCTLA-4YF), and ctCTLA-4 without the Hph-1-PTD were designed as previously described (25). We purified these proteins under denaturing conditions and the purified proteins were characterized by Western blot analysis with anti-HA antibody [supporting information (SI) Fig. S1]. In mouse splenocytes, Hph-1-ctCTLA-4 strongly inhibited secretion of inflammatory cytokines, including IFN-γ, IL-4, and IL-17 (Fig. 1 A–C). ctCTLA-4 that lacks Hph-1-PTD did not have an inhibitory effect on cytokine secretion. Moreover, to examine whether Hph-1-ctCTLA-4 also can inhibit the CD8 T cell response, a human allo-CTL line against primary human umbilical vein endothelial cells (HUVEC) was generated, and the inhibitory effect of Hph-1-ctCTLA-4 on its CTL activity was tested (Fig. 1D). Hph-1-ctCTLA-4 suppressed CTL activity against target cells (HUVEC) in a concentration-dependent manner. However, ctCTLA-4 and cyclosporine A (CsA), a calcineurin inhibitor, had no effect. These results suggest the intracellular cytoplasmic domain of CTLA-4 strongly inhibits cytotoxic CD8 T cell activation as well as helper T cell activation.
Fig. 1.
Inhibition of T cell activation by Hph-1-ctCTLA-4. After Hph-1-ctCTLA-4 or ctCTLA-4 was incubated with mouse splenocytes for 1 h, cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 24 h. (A) IFN-γ, (B) IL-4, and (C) IL-17A levels in the culture media were analyzed by ELISA. (D) HUVEC-specific human primary CTL was generated, and Hph-1-ctCTLA-4-transduced CTL was cocultured with calcein-treated HUVEC cells for 4 h. CTL killing activity was calculated as killing percentage. Bars represent mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the activated T cell group.
Inhibition of T Cell Activation by Hph-1-ctCTLA-4 via TcR-Proximal Signaling Events.
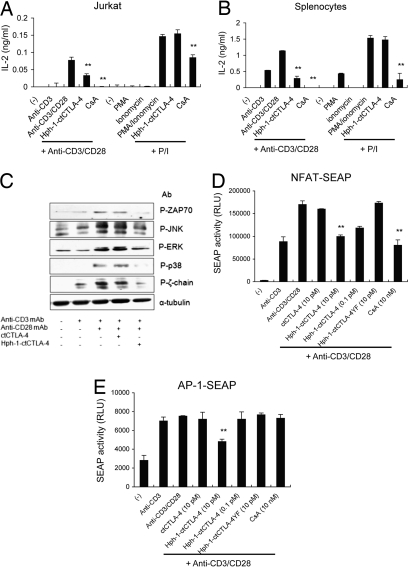
The inhibitory mechanism of Hph-1-ctCTLA-4 in T cell signaling pathway was examined with human Jurkat T cells and mouse splenocytes activated by T cell Receptor (TcR)-mediated or TcR-bypassed stimulation. We found that 10 pM of Hph-1-ctCTLA-4 or 10 nM of CsA could effectively inhibit IL-2 secretion from Jurkat T cells and mouse splenocytes activated by anti-CD3 and anti-CD28 antibodies. However, in contrast to CsA, Hph-1-ctCTLA-4 could not suppress Phorbol 12-Myristate 13-Acetate (PMA)/ionomycin-induced T cell activation, confirming that Hph-1-ctCTLA-4 specifically acts on TcR-proximal signaling events (Fig. 2 A and B). Because the inhibitory function of Hph-1-ctCTLA-4 seems to be dependent on TcR stimulation, the effect of Hph-1-ctCTLA-4 on phosphorylation of intracellular signaling proteins including the ζ-chain of the TcR complex, ZAP70, p38, ERK, and JNK was analyzed by Western blot. (Fig. 2C). Phosphorylation of all these signaling molecules was significantly inhibited by 10 pM of Hph-1-ctCTLA-4 although there was no meaningful difference in total lysate control and also in total amount of nonphosphoproteins (data not shown).
Fig. 2.
Hph-1-ctCTLA-4 suppresses T cell activation by inhibition of TcR-proximal signals. (A) Jurkat T cells or (B) mouse splenocytes were incubated with Hph-1-ctCTLA-4, and its inhibitory function on IL-2 secretion was stimulated by plate-bound anti-CD3 and soluble anti-CD28 antibodies or PMA/ionomycin was analyzed by ELISA. (C) After ctCTLA-4 or Hph-1-ctCTLA-4 was incubated with Jurkat T cells for 30 min, cells were activated with anti-CD3 and anti-CD28 antibodies for 5 min at 37 °C, cell lysates were analyzed by Western blot analysis with anti-Phospho-ERK, -JNK, -p38, -ZAP70, -ζ-chain antibodies, and anti-α-tubulin antibody was used as lysate control. (D and E) After each protein was incubated with (D) NFAT- or (E) AP-1-SEAP Jurkat T cells for 30 min, cells were stimulated with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody for 24 h and chemiluminescent SEAP activity in the culture media was analyzed. Bars represent mean ± SD of three independent experiments. *, P < 0.05; **, P < 0.01 compared with the activated T cell group.
Next, we examined whether Hph-1-ctCTLA-4 can inhibit the transcriptional promoter activities of Nuclear Factor of Activated T-cells (NFAT) and AP-1 (Fig. 2 D and E). We used stably transfected Jurkat cell lines with an NFAT- or an AP-1-Secreted form of Embryonic Alkaline Phosphatase (SEAP) reporter gene, in which SEAP expression is driven by the NFAT or the AP-1 binding site, respectively. CsA, a calcineurin inhibitor, showed an inhibitory effect on NFAT promoter activity but not on the AP-1 promoter. However, Hph-1-ctCTLA-4 could suppress both NFAT and AP-1 promoter activity as well, suggesting that Hph-1-ctCTLA-4 seems to target the signaling events of T cell activation upstream of NFAT and AP-1 activation.
Inhibition of Proliferation and Cell Cycle of Activated T Cells by Hph-1-ctCTLA-4.
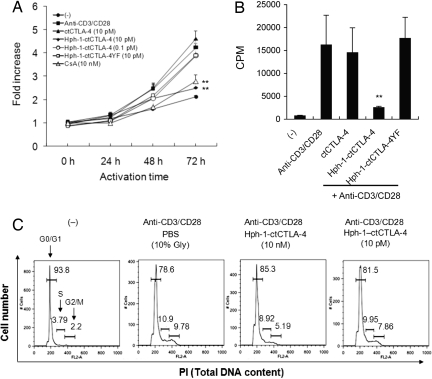
Next, we examined the effect of Hph-1-ctCTLA-4 on T cell proliferation and cell cycle progression. After Jurkat T cells were activated with soluble anti-CD28 antibody and plate-bound anti-CD3 antibody for 24–72 h, total live cells were stained with the WST-8 dye and data were presented as fold increase compared to the value of initial cells (Fig. 3A). Hph-1-ctCTLA-4 or CsA significantly inhibited T cell proliferation; however, ctCTLA-4 without Hph-1-PTD had no effect. Additionally, an inhibitory effect of Hph-1-ctCTLA-4 on T cell proliferation was confirmed by thymidine incorporation assay (Fig. 3B). To test whether inhibition of T cell proliferation by Hph-1-ctCTLA-4 was because of cell cycle arrest, we stained the total DNA content of activated T cells by Propidium Iodide (PI) (Fig. 3C). Following treatment of Hph-1-ctCTLA-4 there was a reduction in the G2/M and an increase in the G0/G1 population. In agreement with the earlier findings using the CTLA-4/B7–1/B7–2 TKO and the B7–1/B7–2 DKO system (26), the cell cycle progression of T cells incubated with Hph-1-ctCTLA-4 was effectively arrested at the G0/G1 phase. Taken together, Hph-1-ctCTLA-4 specifically inhibits tyrosine phosphorylation of TcR-proximal key signal mediators, leading to suppression of T cell proliferation via cell cycle arrest.
Fig. 3.
Hph-1-ctCTLA-4 suppresses proliferation and cell cycle of activated T cells. (A) After each protein was incubated with Jurkat T cells for 30 min, cells were stimulated with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody for 24–72 h. WST-8 dye was added to each well to stain total live cells. (B) After splenocytes were incubated with each protein and stimulated as described above, proliferation of cells was indicated by the number of thymidine-incorporating cells. (C) After splenocytes were stimulated as described above for 3 days, cells were fixed and intracellular DNA was stained by PI, and the cell cycle was analyzed by FACS. Bars represent mean ± SD of three independent experiments. *, P < 0.05; **, P < 0.01 compared with the activated T cell group.
Inhibitory Effect of Hph-1-ctCTLA-4 on Joint Inflammation in a Collagen-Induced Arthritis Model.
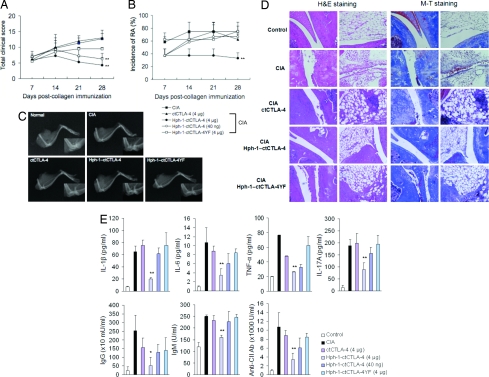
To show the therapeutic effect of Hph-1-ctCTLA-4 in the CIA model, 7 days after the boost injection of CII antigen, 4 μg or 40 ng of ctCTLA-4, Hph-1-ctCLTA-4, or Hph-1-ctCTLA-4YF recombinant proteins were injected i.v. into mice three times a week for 3 weeks. Mice treated with Hph-1-ctCTLA-4 displayed a significantly lower arthritis severity index and incidence of CIA, and improvement of the arthritis severity index and incidence of RA by Hph-1-ctCTLA-4 was clearly dose dependent (Fig. 4 A and B). Importantly, radiographic analysis that was performed at the conclusion of the experiment showed markedly less bone narrowing or fusion and cartilage damage in Hph-1-ctCTLA-4-treated mice than in mice treated with ctCTLA-4 or Hph-1-ctCTLA-4YF (Fig. 4C). In histological analysis, severe cartilage erosion, synovial membrane destruction, synovial cell infiltration, collagen deposition, and angiogenesis were detected in CIA mice (Fig. 4D). In contrast, treatment of CIA mice with 4 μg of Hph-1-ctCTLA-4 clearly suppressed articular destruction and inflammatory cell infiltration. The number of infiltrating granulocytes was also significantly decreased in the knee joint of CIA mice treated with Hph-1-ctCTLA-4, and the Hph-1-CT4YF mutant partially inhibited the infiltration of granulocytes into the knee joint (Table S1).
Fig. 4.
Inhibition of inflammation and bone destruction by Hph-1-ctCTLA-4 in the collagen-induced arthritis model. Seven days after boost injection of CII antigen, each protein was i.v. injected three times per week for 3 weeks. (A) Severity and (B) incidence of the arthritis symptom were assessed by clinical scoring. (C) X-ray radiographic analysis was performed with the legs of the mice treated with the corresponding protein. (D) Each joint sample was analyzed for histological analysis by H&E staining (Left) and M-T staining (Right) to examine inflammatory tissue damage and collagen deposition, respectively. (E) The levels of IL-1β, IL-6, TNF-α, IL-17A, IgM, IgG, and anti-type II collagen Ab in the serum from CIA mice treated with the corresponding protein were analyzed by ELISA. Bars represent mean ± SEM of three independent experiments from four mice per group. *, P < 0.05; **, P < 0.01 compared with the control CIA group.
To investigate immunological factors for the therapeutic effect of Hph-1-ctCTLA-4 on CIA more in detail, we measured various parameters and markers important for induction and maintenance of disease progression in CIA mice. The level of inflammatory cytokines such as IL-1β, IL-6, TNF-α, and IL-17A was increased significantly in CIA-induced mice; however, Hph-1-ctCLTA-4 treatment significantly reduced the level of these cytokines (Fig. 4E). We also examined the level of total IgG and IgM antibodies, and antibody to type II collagen, which is a marker for arthritis secreted by activated B cells in CIA mice (Fig. 4E). Hph-1-ctCTLA-4 effectively lowered serum antibodies specific to type II collagen as well as total IgG and IgM levels in a concentration-dependent manner; however, ctCTLA-4 or Hph-1-ctCTLA-4YF showed little effect. These results indicate that the inhibitory effect of the Hph-1-ctCTLA-4 on production of antibodies and the secretion of inflammatory cytokines contributes to the prevention of inflammation in joint tissue and bone destruction in CIA mice.
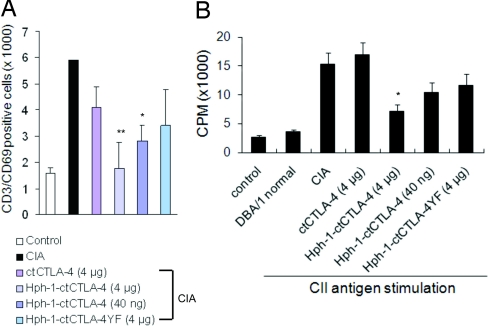
To verify whether the decrease of inflammatory cytokine secretion and antibody production in Hph-1-ctCTLA-4-treated mice was due to inhibition of T cell activation by Hph-1-ctCTLA-4 in vivo, we analyzed the number of activated T cells (CD3+/CD69+) in spleen (Fig. 5A) from CIA mice. The number of CD3+/CD69+ T cells was significantly reduced in Hph-1-ctCTLA-4-treated mice. To analyze inhibition of antigen-specific T cell proliferation by Hph-1-ctCTLA-4 treatment, splenocytes from CIA mice were stimulated with plate-bound CII antigen, and proliferation was measured by thymidine incorporation assay (Fig. 5B). Proliferation of CII antigen-specific cells was strongly inhibited in the cells from the Hph-1-ctCTLA-4-treated mice although ctCTLA-4 or the tyrosine mutant form of ctCTLA-4 had no effect on proliferation. These results demonstrate that Hph-1-ctCTLA-4 inhibits collagen-induced arthritis by inhibition of CII antigen-specific T cell activation and proliferation.
Fig. 5.
Therapeutic activity of Hph-1-ctCTLA-4 in the collagen-induced arthritis model is mediated by inhibition of T cell activation. (A) The absolute number of CD3- and CD69-positive cells from spleen was analyzed by flow cytometry. (B) CII antigen-specific T cell proliferation was analyzed by thymidine incorporation assay in CIA-induced mice treated with the different protein. Bars represent mean ± SEM of three independent experiments from four mice per group. *, P < 0.05; **, P < 0.01 compared with the control CIA group.
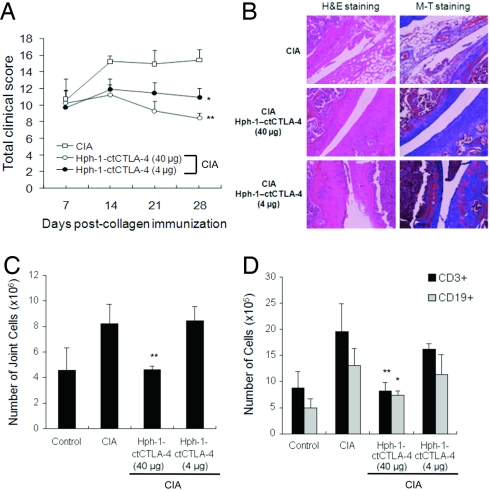
Transdermal Administration of Hph-1-ctCTLA-4 on the Joints of CIA Mice Suppresses Arthritis Symptoms.
Taking advantage of the fact that Hph-1-PTD can deliver a protein through local administration routes such as skin (25), we tested whether transdermal administration of Hph-1-ctCTLA-4 also could prevent arthritis symptoms in CIA mice. Beginning at 7 days after boost injection of CII antigen, when arthritis symptoms appeared in mice, Hph-1-ctCTLA-4 in an ointment mixture was applied to joint skin of CIA mice five times a week for 4 weeks. The total arthritis clinical score was significantly reduced by topical application of Hph-1-ctCTLA-4 (Fig. 6A). In addition, histological analysis of the joint area after transdermal administration of 40 μg of Hph-1-ctCTLA-4 revealed significant reductions in joint tissue damage from inflammation and inflammatory cell infiltration, cartilage erosion, and synovial membrane destruction (Fig. 6B). We also found the total number of joint cells and the number of T cells and B cells were dramatically decreased by Hph-1-ctCTLA-4 treatment on the joint area (Fig. 6 C and D). Taken together, these results showed that both systemic and transdermal administration of Hph-1-ctCTLA-4 had remarkable therapeutic effects in the CIA mouse model.
Fig. 6.
Transdermal application of Hph-1-ctCTLA-4 in the collagen-induced arthritis model. (A) Seven days after boost injection of CII antigen, Hph-1-ctCTLA-4 and ointment mixture were administered on the joint skin of the mice five times a week for 4 weeks. Severity of the arthritis symptom was measured by clinical scoring. (B) Each joint sample was analyzed for histological analysis by H&E and M-T staining to examine inflammatory tissue damage and collagen deposition, respectively. (C) The number of total joint cells and (D) the number of CD3+ or CD19+ cells were calculated with the percentage of each staining and the total number of cells by FACS. Bars represent mean ± SEM of two independent experiments from four mice per group. *, P < 0.05; **, P < 0.01 compared with the control CIA group.
Discussion
CTLA-4 is a costimulatory molecule for negative regulation of T cell activation. The fact that the amino acid sequences of the intracellular tail of CTLA-4 are 100% conserved among mammalian species suggests that signal transduction via this cytoplasmic tail has an important inhibitory role in T cell activation (12, 27). The cytoplasmic portion of CTLA-4 is 36 aa in length, lacks any intrinsic enzymatic activity, and does not contain a bona fide ITIM motif; however, it contains many other potential protein–protein interacting motifs (15, 28, 29).
In recent studies, mice expressing CTLA-4 lacking the cytoplasmic tail still showed a lymphoproliferative phenotype, albeit a much less aggressive one, suggesting that antagonism of CD28 costimulation requires the cytoplasmic domain to inhibit T cell activation (30, 31). In another report, overexpression of the ligand-independent form of CTLA-4 (liCTLA-4) inhibited T cell activation in TKO (B7.1, B7.2, CTLA-4−/−) mice (32). In a separate study, expression of a non-ligand-binding CTLA-4 molecule inhibited the in vitro response of CTLA-4 KO T cells to B7 and protected mice from the massive hyperproliferation and tissue infiltration observed in CTLA-4-deficient animals (33). Therefore, the cytoplasmic domain of CTLA-4 provides a promising therapeutic target for the development of immunotherapeutic drugs for allergic diseases, autoimmune diseases, and graft rejection.
In this study, transduction of Hph-1-CTLA-4, a fusion protein between the cytoplasmic domain of CTLA-4 and a novel human origin Hph-1-PTD, into T cells was examined, and the molecular mechanism of action involved in negative regulation of Hph-1-ctCTLA-4 in T cell activation was examined. Hph-1-ctCTLA-4 specifically inhibited TcR-proximal signaling events such as phosphorylation of ZAP70, JNK, P38, ERK, and the ζ-chain of the TcR complex, leading to prevention of secretion of various cytokines characteristic of Th-1, Th2, and Th-17 cells. Previously CTLA-4 signaling resulted in inhibition of ZAP70 and ERK or secretion of IFN-γ and IL-2 (34, 35). Interestingly Hph-1-ctCTLA-4 could inhibit TcR-induced activation, but not PMA/ionomycin-induced activation, which bypasses signaling events in the proximity of the TcR complex. In contrast, CsA, which inhibits NFAT function via calcineurin, can suppress TcR- and PMA/ion-induced T cell activation. This inhibitory function of Hph-1-ctCTLA-4 specific to TcR-proximal signaling events is in agreement with that of liCTLA-4 (32) and non-ligand-binding CTLA-4 (33). In the previous report, B7–1/B7–2/CTLA-4 TKO T cells readily progress from the G0/G1 to S and G2/M phase compared to B7–1/B7–2 DKO T cells, suggesting that CTLA-4 prevented the degradation of p27kip1 and inhibited cell cycle progression (26). Consistent with these results, Hph-1-ctCTLA-4 effectively arrested the cell cycle progression of T cells at the G0/G1 stage. The cell-permeable cytoplasmic domain of CTLA-4 also could effectively suppress human allo-CTL activity against human primary HUVEC cells in vitro, suggesting it has an inhibitory function on TcR-mediated activation of CD8 T cells and CD4 T cells. However, Hph-1-ctCTLA-4 could not block the signaling through TLR2 or TLR4 on dendritic cells and macrophages stimulated by CpG or LPS (data not shown). These results clearly demonstrate that the inhibitory action of Hph-1-ctCTLA-4 is specific to TcR-mediated activation signaling.
Next, we examined the therapeutic potential of Hph-1-ctCTLA-4 in a collagen II-induced arthritis model in mice. It could effectively subdue the arthritic symptoms and this therapeutic effect was attributed to inhibition of the production of inflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-17A). It has been suggested that these inflammatory cytokines are produced through continuous activation of T cells in RA (36). We found that the numbers of total infiltrating cells and CD11b+/Gr1+ granulocytes in joint tissue were significantly reduced in Hph-1-ctCTLA-4-treated mice and also it can decrease the number of CD4+ and CD8+ T cells in lymph node and spleen (Fig. S2). When Hph-1-ctCTLA-4 was administered through the joint skin area of the CIA mice, arthritis clinical score and incidence, and histology of the joint area, were greatly improved. All of these in vivo therapeutic effects of Hph-1-ctCTLA-4 were dose dependent, but Hph-1-ctCTLA-4-YF, a nonfunctional mutant form of Hph-1-ctCTLA-4, had significantly fewer or negligible inhibitory effects in vivo, suggesting the importance of the two tyrosine residues for in vitro and in vivo function of Hph-1-ctCTLA-4.
From our study, the possible mechanism underlying the inhibitory effect of Hph-1-ctCTLA-4 on synovial inflammation in vivo is that the presence of the cytoplasmic domain of CTLA-4 containing an ITIM-like motif (YVKM motif) or other protein–protein interacting motif might block T cell activation, thereby preventing joint inflammation as previously suggested by other groups (37, 38). We administered Hph-1-ctCTLA-4 after the arthritis clinical score “2” was observed in mice, indicating that Hph-1-ctCTLA-4 has therapeutic and preventive effects on RA. In vivo immunogenicity and toxicity of Hph-1-ctCTLA-4 were not detected even when 1–5 mg/kg of recombinant protein (fivefold higher dose than the therapeutic dose) were injected i.v. into mice (25).
Importantly, transdermal administration of Hph-1-ctCTLA-4 to the joint skin area of the disease-induced animal significantly improved joint inflammation and arthritis symptoms. This report demonstrates that an intracellular signaling protein with therapeutic potential in T cells can be used for the treatment of arthritis via transdermal application.
Materials and Methods
Purification of Hph-1-PTD-Conjugated Proteins.
Generation of ctCTLA-4, Hph-1-ctCTLA-4, and Hph-1-ctCTLA-4YF protein was carried out as previously described (25).
In Vitro T Cell Activation, Proliferation, and Cell Cycle Analysis.
Plates were prepared by coating with 0.1–0.25 μg of anti-CD3 antibody (eBioscience) per well and incubating for 1 h at 37 °C. Splenocytes were incubated with 0.5 μg of anti-CD28 antibody (eBioscience) on ice for 20 min. Cells were added to the anti-CD3 antibody-coated wells and cultured for 1–3 days. After stimulation for 3 days, 20 μl of WST-8 dye (Dojindo) or 1 μCi of thymidine were added to 200 μl of culture media. Live cells were detected by reading wells at 450 nm on a microplate reader (BioRad) and incorporated radioactivity was counted by a Liquid Scintillation Counter (Perkin-Elmer). For NFAT and AP-1 promoter activity, a SEAP assay was performed in accordance with the manufacturer's instructions (BD Biosciences). For cell cycle analysis, cells were harvested, fixed with 70% EtOH, and incubated with PI solution including 0.1% Triton X-100 and RNase for 30 min at room temperature and analyzed by FACS.
Generation of Human CTL and CTL Assay.
Human allo-CTL against HUVEC was generated as previously described (39). HUVEC were isolated by collagenase treatment of human umbilical veins under a protocol approved by the Yale Human Investigation Committee.
Murine CIA Model.
All of the animal procedures were approved by the Experimental Animal Commission of the Institute of Traditional Medicine and Bioscience at Daejeon University. Male DBA/1J mice (7–9 weeks) received 200 μg of bovine type II collagen (Sigma) in Freund's complete adjuvant (Sigma) by intradermal injection at the base of the tail on day 0 and a booster injection on day 21 (40). Mice were monitored daily for signs of arthritis, and each paw was scored individually as follows: 0, normal; 1, slight erythema and edema; 2, increased edema with loss of landmarks; 3, marked edema; and 4, marked edema with ankylosis on flexion. Each mouse was assigned an arthritis score (articular index) that equaled the sum of the scores of all four paws. The incidence of arthritis was indicated as a percentage of the number of arthritic paws per four paws of each mouse. Mice were injected intravenously with Hph-1-ctCTLA-4 (40 ng or 4 μg) every 3 days from day 7 to day 28 and monitored for disease incidence and the severity of arthritis up to day 30. For transdermal administration, Hph-1-ctCTLA-4 protein in an ointment mixture was rubbed on joint skin of CIA mice 5 days per week for the following 3 weeks.
Cytokine and Antibody Assays.
Culture supernatants from the stimulated splenocytes or the sera from the CIA mice were assayed by ELISA for murine IL-1β, IL-6, TNF-α, and IL-17A, using paired antibodies in accordance with the manufacturer's instructions (eBioscience). The total serum IgG, IgM, and anti-CII antibody concentration was determined using a mouse antibody isotyping kit (Pierce).
Statistical Analysis.
Statistical analysis of group differences was examined using the nonparametric Mann–Whitney U test. *P < 0.05 and **P < 0.01 were considered to be significant.
Supplementary Material
Acknowledgments.
We thank all members of the Immune Cell Engineering Laboratory, Dept. of Biotechnology, Yonsei University for their spiritual help. This work was supported in part by research grants to S.K.L. from the Korea Science and Engineering Foundation (R11-2007-040-02005-0, 2008-01224), Brain Korea 21, Seoul Development Institute (11112), and Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs (A085136), Republic of Korea. This work was also supported in part by research grant to J.-S. L. from Oriental Medicine R&D Project. Ministry of Health & Welfare, Republic of Korea (0405–OD00–0815–0010) and by NIH grant (HL51448) to A.L.M.B. This work was also supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2006-E00010) to J.-M.C.
Footnotes
Conflict of interest statement: The authors have pending patent applications.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805198105/DCSupplemental.
References
- 1.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91(18):8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85(3):307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 4.Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177(5):3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 5.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 6.Bresnihan B, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41(12):2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Choy EH, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46(12):3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 8.Talwalkar SC, Grennan DM, Gray J, Johnson P, Hayton MJ. Tumour necrosis factor alpha antagonists and early postoperative complications in patients with inflammatory joint disease undergoing elective orthopaedic surgery. Ann Rheum Dis. 2005;64(4):650–651. doi: 10.1136/ard.2004.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldbach-Mansky R, Lipsky PE. New concepts in the treatment of rheumatoid arthritis. Annu Rev Med. 2003;54:197–216. doi: 10.1146/annurev.med.54.101601.152342. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Luggen M. T cell costimulation: a rational target in the therapeutic armamentarium for autoimmune diseases and transplantation. Annu Rev Med. 2007;58:347–358. doi: 10.1146/annurev.med.58.080205.154004. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 12.Hueber AJ, et al. CTLA-4 lacking the cytoplasmic domain costimulates IL-2 production in T-cell hybridomas. Immunol Cell Biol. 2006;84(1):51–58. doi: 10.1111/j.1440-1711.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- 13.Ling V, et al. Complete sequence determination of the mouse and human CTLA4 gene loci: cross-species DNA sequence similarity beyond exon borders. Genomics. 1999;60(3):341–355. doi: 10.1006/geno.1999.5930. [DOI] [PubMed] [Google Scholar]
- 14.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 15.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 16.Fawell S, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88(2):223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 18.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara H, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4(12):1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 20.Fan YF, Lu CZ, Xie J, Zhao YX, Yang GY. Apoptosis inhibition in ischemic brain by intraperitoneal PTD-BIR3-RING (XIAP) Neurochem Int. 2006;48(1):50–59. doi: 10.1016/j.neuint.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee KC, et al. Fusion of the HSV-1 tegument protein vp22 to cytosine deaminase confers enhanced bystander effect and increased therapeutic benefit. Gene Ther. 2006;13(2):127–137. doi: 10.1038/sj.gt.3302631. [DOI] [PubMed] [Google Scholar]
- 22.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11(8):892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi H, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004;10(3):305–309. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 24.Rothbard JB, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6(11):1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 25.Choi JM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006;12(5):574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- 26.Greenwald RJ, et al. CTLA-4 regulates cell cycle progression during a primary immune response. Eur J Immunol. 2002;32(2):366–373. doi: 10.1002/1521-4141(200202)32:2<366::AID-IMMU366>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev. 2008;8(2):153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 28.Lee KM, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 29.Schneider H, da Rocha Dias S, Hu H, Rudd CE. A regulatory role for cytoplasmic YVKM motif in CTLA-4 inhibition of TCR signaling. Eur J Immunol. 2001;31(7):2042–2050. doi: 10.1002/1521-4141(200107)31:7<2042::aid-immu2042>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Carreno BM, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165(3):1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 31.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164(10):5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 32.Vijayakrishnan L, et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20(5):563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 33.Chikuma S, Abbas AK, Bluestone JA. B7-independent inhibition of T cells by CTLA-4. J Immunol. 2005;175(1):177–181. doi: 10.4049/jimmunol.175.1.177. [DOI] [PubMed] [Google Scholar]
- 34.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 35.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annual Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Brennan FM, Foey AD, Feldmann M. The importance of T cell interactions with macrophages in rheumatoid cytokine production. Curr Top Microbiol Immunol. 2006;305:177–194. doi: 10.1007/3-540-29714-6_9. [DOI] [PubMed] [Google Scholar]
- 37.Yi LA, Hajialiasgar S, Chuang E. Tyrosine-mediated inhibitory signals contribute to CTLA-4 function in vivo. Int Immunol. 2004;16(4):539–547. doi: 10.1093/intimm/dxh055. [DOI] [PubMed] [Google Scholar]
- 38.Schneider H, Rudd CE. Tyrosine phosphatase SHP-2 binding to CTLA-4: absence of direct YVKM/YFIP motif recognition. Biochem Biophys Res Commun. 2000;269(1):279–283. doi: 10.1006/bbrc.2000.2234. [DOI] [PubMed] [Google Scholar]
- 39.Zheng L, Gibson TF, Schechner JS, Pober JS, Bothwell AL. Bcl-2 transduction protects human endothelial cell synthetic microvessel grafts from allogeneic T cells in vivo. J Immunol. 2004;173(5):3020–3026. doi: 10.4049/jimmunol.173.5.3020. [DOI] [PubMed] [Google Scholar]
- 40.Lee SI, et al. Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product library. Arthritis Rheum. 2005;52(1):345–353. doi: 10.1002/art.20715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.