Abstract
Selection can alter predator-prey interactions. However, whether and how complex food-webs respond to selection remain largely unknown. We show in the field that antagonistic selection from predators and pathogens on prey body-size can be a primary driver of food-web functioning. In Windermere, U.K., pike (Esox lucius, the predator) selected against small perch (Perca fluviatilis, the prey), while a perch-specific pathogen selected against large perch. The strongest selective force drove perch trait change and ultimately determined the structure of trophic interactions. Before 1976, the strength of pike-induced selection overrode the strength of pathogen-induced selection and drove a change to larger, faster growing perch. Predation-driven increase in the proportion of large, infection-vulnerable perch presumably favored the pathogen since a peak in the predation pressure in 1976 coincided with pathogen expansion and a massive perch kill. After 1976, the strength of pathogen-induced selection overrode the strength of predator-induced selection and drove a rapid change to smaller, slower growing perch. These changes made perch easier prey for pike and weaker competitors against juvenile pike, ultimately increasing juvenile pike survival and total pike numbers. Therefore, although predators and pathogens exploited the same prey in Windermere, they did not operate competitively but synergistically by driving rapid prey trait change in opposite directions. Our study empirically demonstrates that a consideration of the relative strengths and directions of multiple selective pressures is needed to fully understand community functioning in nature.
Keywords: cost of immunity, intraguild predation, life history trade-offs, rapid evolution, trait-mediated indirect interactions
Interacting populations often show reciprocal phenotypic changes reflecting coadaptations. In turn, coadaptations alter the strength and even the nature of interactions (1–3). Therefore, community structure and functioning is driven by an interplay between demography and phenotypic change (4–7). Recently, there has been considerable interest in how prey adaptive responses to predators can drive community dynamics (5, 6, 8–11). At the same time, it has been shown that parasites and parasite-mediated trait changes can play a crucial role in food-web structuring (12, 13). However, despite the fact that organisms are often confronted with both predators and parasites (14), there have been few attempts to understand how adaptive response to joint predation and parasitism affects food-web functioning in nature. Here, we use a 50-year long time series from a whole-lake system (Windermere, U.K.) to show that simultaneous selection from both predators and pathogens may structure the food-web in a way that could not be predicted by considering each selective pressure separately.
Windermere is a glacial valley lake of the English Lake District, divided by shallows into north and south basins of different size and productivity (15, 16). The fish community of Windermere is size-structured, with only a few numerically dominant species interacting in a mixture of competition, predation, and cannibalism termed intraguild predation (IGP) (17, 18). Perch (Perca fluviatilis) are the most abundant fish and are preyed upon by pike (Esox lucius), the top predator of the system. Small perch below 16 cm body length (approximate age ≤ 2 years) feed entirely on zooplankton and macroinvertebrates, while large perch (above 16 cm body length) feed on macroinvertebrates and on their own fry (19–21). Small pike below 20 cm body length (approximately age ≤ 1.5 years) have the same diet as large perch (i.e., macroinvertebrates and small perch), while large pike feed exclusively on fish, mostly perch of 6–9 cm body length (22, 23). Based on these extensive diet data, we predicted that small perch were prey for pike in Windermere but that large perch were potentially strong competitors with pike (especially with small pike). Our results supported this prediction.
A long-term monitoring program for Windermere perch and pike was initiated in the early 1940s. Since 1944, pike have been gillnetted during winter (15, 16, 24, 25). Perch have been caught with traps set on their spawning grounds from the end of April to mid-June (26). On each lift of a trap, the whole catch or occasionally a random fraction of the catch has been sexed, measured for total body length, and opercular bones have been removed for age determination following a validated method (27). Bone density differs between summer and winter, producing narrow bands (“checks”) that are deposited on the opercular bones during the slow winter growth period. These checks then serve as an annual mark and, thus, allow the aging of individual fish (27). Pike were aged following the same method (28). The abundances of both perch and pike have been estimated annually for the 1944–1995 period, separately for each basin as well as for both small (i.e., age = 2) and large (i.e., age > 2) individuals (29) (Fig. 1 A and B). Together with these biological data, surface water temperatures were recorded on a near daily basis and were here averaged for each year. Finally, maximum phosphorus concentration between September/October in year y and February in year y + 1 was measured each year since 1945 in the north basin and since 1946 in the south basin and was here used as a proxy for Windermere primary productivity in year y + 1.
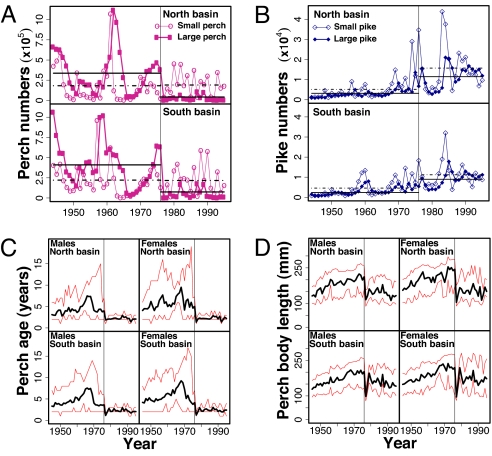
Fig. 1.
Background information for pike (E. lucius), perch (P. fluviatilis) and for expansion of a perch-specific pathogen in Windermere, United Kingdom. Vertical solid lines indicate the first massive perch kill from the pathogen in 1976. (A-B) Time series for population size of perch (A) and pike (B) in the north and south basins of the lake, separated into small (age-2 years) and large (age ≥ 3 years) individuals. Horizontal lines show mean abundances before and after pathogen invasion, separately for small (dashed and dotted lines) and large (solid lines) individuals. (C-D) Time series for perch mean age (C) and mean body length (D) with 95% confidence intervals, separated by sex and basin.
In 1976, a perch-specific pathogen severely impacted the perch population (Fig. 1 A, C, and D). Although the primary pathogenic agent remains unidentified, the disease is characterized by epidermal lesions associated with a wide variety of fungal and bacterial infections (30). The pathogen preferentially infects large, maturing (90–100% prevalence) perch over small, immature individuals (50–70% prevalence) and induced a 98% mortality of spawners during the 1976 reproductive period (30). By 1977, captured perch showed no external sign of disease (30), but the numbers of large perch have remained low since 1976 (Fig. 1A). Both the age structure (Fig. 1C) and mean body length (Fig. 1D) of the Windermere perch population remain severely truncated, suggesting that the pathogen is still present. The pathogen has dramatically reduced the potential for iteroparity in the perch population by significantly reducing the duration of the mature stage of the life cycle (Fig. 1C), setting the stage for increased investment into fewer reproductive bouts (31). Increased reproductive investment in perch is likely to have reduced somatic growth rate owing to the tradeoff between body growth and reproduction (32). Additionally, in immature perch, disease prevalence is much higher in fast-growers than in slow-growers (30), indicating a tradeoff between disease resistance and somatic growth (31).
Based on these observations, we predicted that pike (predator)-induced selection and pathogen-induced selection acted in opposite directions on perch body-size and somatic growth rate. Before pathogen invasion, perch somatic growth rate should have reflected the effect of increased predation due to an increase in the pike/perch ratio (Fig. 2A). After pathogen invasion, perch growth should have reflected the combined action of the two antagonistic selective forces (24). We have tested this prediction by estimating nonlinear changes in perch somatic growth rate (24). In our statistical analysis, we accounted for the effects of environmental variables known to affect perch growth plastically [i.e., primary productivity, water temperature, and perch density (26), see Material and Methods] and, by using a smooth term on the Year class effect, we removed any a priori expectation concerning the shape of the temporal trend. We performed separate analyses for each basin of Windermere because the two perch populations are considered distinct (33, 34), thus providing a natural replicate for hypothesis testing. Since life-history responses to pathogens may be sex-specific (31), we also performed separate analyses for each sex. In both basins of the lake, our results support the prediction that pike and pathogens induced selection in opposite directions on perch body-size.
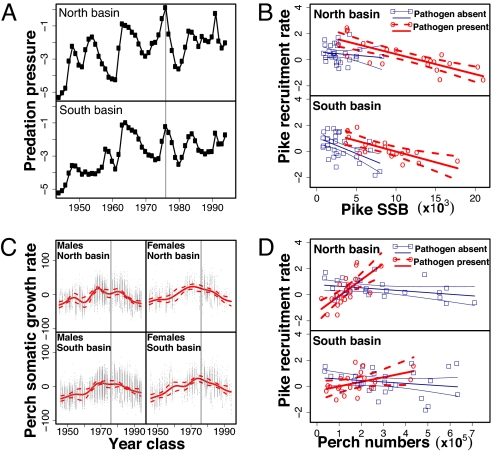
Fig. 2.
Effects of predator (pike, E. lucius)- and pathogen-induced selection on perch (P. fluviatilis) trait-change and resultant impacts on pike-perch-pathogen interactions in Windermere, U.K. Vertical solid lines indicate the first massive perch kill from the pathogen in 1976. (A) Time series for the predation pressure from pike on perch in each basin of Windermere. Note that a peak in the predation pressure coincided with the perch kill. (B) Effect of the perch pathogen on the link between number of pike spawners (SSB) and pike recruitment rate (i.e., natural log of number of age-2 recruits per spawner) in each basin of the lake (see also Table 1). Circles and squares represent observed data and lines represent predicted values with 95% confidence intervals. (C) Nonlinear temporal trends for perch somatic growth rate (in partial residuals units) with 95% bootstrap confidence intervals, accounting for the effects of environmental variation in growth conditions. Gray points represent the partial residuals for the smooth term (i.e., residuals that would be obtained by dropping the focal term from the model while leaving all other estimates fixed). Trends are provided separately for each sex and basin of the lake. (D) Effect of the perch pathogen on the link between perch density and pike recruitment rate (see also Table 1). Circles and squares represent observed data and lines represent predicted values with 95% confidence intervals.
Results and Discussion
Before 1976, perch somatic growth rate generally increased in both basins and in both male and female perch (Fig. 2C), in parallel with an overall increase in predation pressure (Fig. 2A). Short-term variations in predation pressure (Fig. 2A) were in remarkably close match with similar changes in perch growth in both basins (especially in males, Fig. 2C), supporting the prediction that pike selected for increased somatic growth in Windermere perch. A correlation analysis revealed that predation pressure had a statistically significant (P < 0.05) positive effect on perch somatic growth at lags ranging from 0 to 9 years, with the highest correlation at a 5-year lag. This lag corresponds roughly to 1.25 to 5 perch generations since male perch in Windermere may mature at age 1, but mean age of mature fish in the catch was approximately age 4. Interestingly, female perch responded less closely than male perch to variation in the predation pressure (Fig. 2C), presumably because females reached a size refuge faster than males (Sex effect in Table 1). Indeed, fast immature growth generally lasts longer in female than in male fish because females mature at an older age (32). Note that perch density had a negative effect on perch somatic growth (Table 1), suggesting that the negative effect of competition overwhelmed the positive effect of cannibalism. After outbreak of the pathogen in 1976, Windermere perch somatic growth decreased rapidly in both basins and for both sexes (Fig. 2C) despite the fact that predation pressure remained high (Fig. 2A). This result supports the prediction that the pathogen selected for slow somatic growth in perch, and further suggests that the strength of pathogen-induced selection overrode the strength of pike-induced selection (24, 25). Finally, perch somatic growth rate in 1995 decreased to 1940s values in the north but not in the south basin (Fig. 2C), in accordance with raw data observation on perch numbers (Fig. 1A), age (Fig. 1C) and size (Fig. 1D) suggesting that the infection was more severe in the north than in the south basin.
Table 1.
Model parameter estimates and their statistical significance (df: degrees of freedom, edf: estimated degrees of freedom of smooth term)
| Response | Effects | Estimate* | df (linear effect) or edf (smooth term) | F value | P† |
|---|---|---|---|---|---|
| Perch body length (n = 67,457) | f(Age) | none | 1.992; 67,445.17 | 53593 | <0.0001 |
| Basin (south relative to north) | 2.987 | 1; 67,445.17 | 249.97 | <0.0001 | |
| Sex (females relative to males) | 5.297 | 1; 67,445.17 | 566.25 | <0.0001 | |
| Temperature | 1.210 | 1; 67,441 | 2366.4 | <0.0001 | |
| Phosphorus | −8.071 | 1; 67,441 | 919.3 | <0.0001 | |
| Perch density | −3.688 e-5 | 1; 67,441 | 4277.9 | <0.0001 | |
| Phosphorus × Temperature | 7.016 | 1; 67,445.17 | 999.74 | <0.0001 | |
| Perch density × Temperature | −6.223e−06 | 1; 67,445.17 | 134.01 | <0.0001 | |
| f(Year class) | none | 4.749; 67,445.17 | 3877 | <0.0001 | |
| Ln(Pike recruits/SSB), North basin (n = 50) | SSB (spawning stock biomass) | −1.517e−04 | 1,45 | 20.4 | <0.0001 |
| Temperature | 2.860e−01 | 1,45 | 4.8 | 0.0344 | |
| Perch density | −7.819e−07 | 1,45 | 1.2 | 0.1259 | |
| Pathogen (presence/absence) | 8.704e−01 | 1,45 | 8.7 | 0.0051 | |
| SSB × Pathogen | −8.831e−05 | 1,44 | 1.1 | 0.3016 | |
| Perch density × Pathogen | 7.934e−06 | 1,44 | 7.7 | 0.0080 | |
| Ln(Pike recruits/SSB), South basin (n = 50) | SSB (spawning stock biomass) | −1.994e−04 | 1,45 | 16.6 | <0.0002 |
| Temperature | 4.532e−01 | 1,45 | 6.1 | 0.0172 | |
| Perch density | −9.134e−07 | 1,45 | 4.2 | 0.0458 | |
| Pathogen (presence/absence) | 7.247e−01 | 1,45 | 5.6 | 0.0220 | |
| SSB × Pathogen | 7.568e−05 | 1,44 | 0.9 | 0.3577 | |
| Perch density × Pathogen | 1.273e−06 | 1,44 | 0.5 | 0.4985 |
*Parameter estimates for main effects are from models without interaction terms.
†Sequentially tested in case of stock-recruitment models.
Antagonistic selection from multiple consumers on their joint prey may result in counterintuitive demographic effects. Indeed, while linear density-dependence predicts a negative impact of multiple consumers on each other (i.e., exploitative competition), antagonistic selection on a joint resource can make consumers mutually beneficial foragers (5, 6). In Windermere, observations are consistent with the predictions that the effects of antagonistic selection overrode the effects of exploitative competition and made pike and the pathogen mutually beneficial foragers. Indeed, signs of an externally similar disease on perch were reported as early as 1963 (30), but the spread of the pathogen and massive perch kill in 1976 coincided with a peak in predation pressure in both basins (Fig. 2A). Additionally, predation pressure was higher in the north than in the south basin both before and after the spread of the pathogen (Fig. 2A), and the infection was more severe in the north than in the south basin (see above). Therefore, by increasing the proportion of large, fast-growing perch which were more sensitive to infection, pike may have facilitated the spread of the pathogen. Then, by selecting against slow somatic growth in perch, pike may have prevented perch from maximizing energy allocation to disease resistance (31) and may have favored the maintenance of high levels of pathogen prevalence.
In turn, by preventing perch from reaching a size refuge, the pathogen may have made perch become easier prey for and weaker competitors with pike (17). Examination of trends in Windermere pike numbers supports this hypothesis. Indeed, at odds with a linear density-dependent effect, pike numbers increased markedly after invasion of the perch pathogen in Windermere (Fig. 1B). We predicted that juvenile pike should have most strongly benefited from invasion of the perch pathogen because (i) juvenile pike were shown from diet data to be more directly in potential competition with large perch (20, 21, 23) and (ii) juvenile pike eat at a higher rate than large pike and are thus more susceptible to competition for food (23). To test this prediction, we used pike stock-recruitment models which explored the relationship between parental stock size in year y and the number of age-2 pike in year y + 2 (see Materials and Methods). These models allowed us to estimate the effects of pathogen-induced trait changes in perch on the pike-perch interaction, while controlling for the effects of temperature, perch numbers, and pike numbers (Table 1). As emphasized above, perch populations in the north and south basins of Windermere should be considered distinct and only about 20% of pike disperse between the two basins (15, 16). We therefore analyzed pike recruitment separately for the north and south basins. Our results clearly show that pathogen-induced trait changes in perch increased juvenile pike survival by changing perch from being mainly a competitor to being mainly a prey for pike.
Pike recruitment rate (i.e., number of recruits per spawner) increased significantly in both basins after invasion of the pathogen (pathogen effects in Table 1, intercepts in Fig. 2B). This increase was not the result of a higher number of eggs produced by female pike because female pike reproductive investment decreased from 1963 to 1995 (24). Increased pike recruitment rate was also not due to a relaxation of density dependence (competition and cannibalism) in the pike population because the strength of density dependence did not change significantly (SSB*pathogen interactions in Table 1, slopes almost unchanged in Fig. 2B). Therefore, increased pike recruitment rate most likely reflected increased survival of small pike due to pathogen-induced trait changes in perch. Modeling the effect of perch on pike recruitment rate supported this hypothesis. Pathogen invasion changed the effect of perch from negative to positive (perch*pathogen interactions in Table 1, slopes changing from negative to positive in Fig. 2D), indicating that the pike-perch link was changed from a mixture of predation and competition dominated by perch toward a simpler predator-prey relationship dominated by pike. Interestingly, perch traits were more severely shifted by the pathogen in the north than in the south basin (see above), driving a locally higher increase in pike recruitment and steeper change in the effect of perch on pike survival (Fig, 2D, Table 1). These results suggest that antagonistic selection from predators and pathogens on Windermere perch body-size generated a mechanism similar to the so-called “synergy” [i.e., synergistic foraging rates (5, 6, 11)] which has been modeled to arise among multiple predators when there is a tradeoff in the prey for behavioral avoidance of the predators (5, 6). To our knowledge, our results provide the first empirical example of this synergistic effect acting through selection on prey life-history.
Conclusions
Our study highlights the immense scientific value of long-term monitoring of ecological systems. Although we cannot discount possible effects of other unmeasured variables, we have been able to analyze trait values, environmental factors (primary productivity, temperature), and population sizes within an integrated treatment. Our results support the view that food-web structure and functioning cannot be accurately predicted without considering the directions and strengths of selective pressures operating within the web. This is in line with recent theoretical literature showing that antagonistic selection from multiple predators promotes species coexistence and food-web stability (5, 6, 35). Our data further expand these conclusions to parasitism, which has been shown to constitute a large part of food-web links (12, 18). Taken together, these results depict food-webs where positive indirect interactions due to antagonistic selection on common resources—synergism—tend to dominate the negative effects of resource competition. In a world replete with synergistic interactions, loss or gain of species may induce cascading effects that depend on the direction and strength with which species select on one another. Hence, improvement of conservation strategies will probably necessitate managing eco-evolutionary dynamics at the community scale (7, 36, 37). So far, the effects of selection remain largely overlooked when considering large threats on the natural world such as overharvesting, species coextinctions, and the biodiversity crisis.
Materials and Methods
Perch Growth Modeling.
Perch traps used for sampling were unselective for individuals ranging from 9 to 30 cm body length and thus captured both fast and slow growers for ages ranging from 2 to 6 years (26). However, age 5 and 6 perch became rare after the invasion of the pathogen in Windermere. Therefore, to rule out confidently possible effects of sampling bias we restricted our growth analysis to perch caught from age 2 to age 4. We modeled temporal changes in Windermere perch somatic growth rate using a generalized additive model (mgcv library of R; ref. 38) of the form:
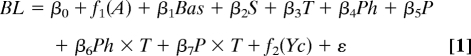 |
where BL stands for body length of individual i and year class Yc (n = 67,457), A is the individual's age at capture, Bas is the basin in which the individual was captured, S is the individual's sex, T, Ph, and P are mean temperature, mean phosphorus concentration and mean perch density (small + large), respectively, experienced by the individual (i.e., from year Yc to year Yc + A), βs are slopes of the linear effects, β0 is an intercept, ε is an error term, and f1 and f2 are nonparametric smoothing functions (natural cubic splines fitted by generalized cross validation; ref. 38). We grouped small and large perch into a single P covariate because both had a negative effect on perch somatic growth rate. In the model, interactions between temperature and the other biological covariates accounted for the thermal dependence of primary productivity and competitive interactions. Plots in Fig. 2C were produced with basin- and sex-specific models as described in Eq. 1 but in which Bas and S were dropped (north basin: n = 17,321 males and n = 3,279 females; south basin: n = 40,904 males and n = 5,953 females); 95% confidence limits around the Yc effect in Fig. 2C were computed using a modified wild bootstrap approach (39). Briefly, the bootstrap distribution for the effect estimate was obtained by randomly inverting the signs of the errors from the model, adding these to the fitted values, and refitting the model (repeated 500 times). To account for intra year-class correlation, all errors from a given year-class in a given bootstrap sample were either inverted or not with probability 0.5. Estimates of the main effects of T, Ph, and P in Table 1 were obtained from a model in which the interaction terms were omitted from Eq. 1. We calculated predation pressure from pike on perch as the natural log of the ratio of the numbers of all (age ≥ 2) pike on the number of small (age-2) perch because pike target mainly small perch in Windermere (23). Ratio-dependent predation was assumed because it has been shown that pike somatic growth rate in Windermere depends on both perch and pike numbers (24). Finally, we tested for the link between predation pressure and perch somatic growth using correlations between the fitted Yc effect (from 4 basin- and sex-specific models as in Fig. 2C) and predation pressure from pike on perch in year class Yc-t where t varied from 0 to 16 years.
Pike Recruitment Modeling.
We modeled pathogen-associated change in pike recruitment using linear stock-recruitment models (40) of the form:
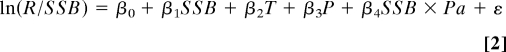 |
where R stands for the number of pike recruits (i.e., age-2 pike) in year y and basin Bas (n = 50 for each basin), SSB is pike spawning stock biomass (i.e., number of spawners) in year y-2 and basin Bas, T, and P are mean water temperature and mean perch density (small + large) experienced by the recruits from year y − 2 to year y, Pa is the pathogen (i.e., presence or absence), βs are slopes of the effects, β0 is an intercept, and ε is an error term. Note that the positive effect of perch on pike recruitment was likely underestimated in our models since age-0 and age-1 perch were not sampled. We modeled changes in the pike-perch interaction using a model similar to Eq. 2 except that P and SSB were inverted in Eq. 2. In our models, the response (natural log of the R/SSB ratio) measured recruitment rate, i.e., the number of recruits per spawner (40). The SSB effect in the right hand side of Eq. 2 captured cannibalism and competition (density-dependence) in the pike population (40), and the SSB*Pa interaction tested for an effect of the perch pathogen on density dependence in the pike population. The P effect captured predation and competition between perch and juvenile pike, while the P*Pa interaction tested for an effect of pathogen-induced trait changes in perch on the pike-perch trophic interactions. Estimation of the main effects of T, P, SSB, and Pa in Table 1 were obtained from a model in which the interaction term was omitted from Eq. 2. Predicted values in Fig. 2 B and D were computed from 2 different models as in Eq. 2 but in which only the focal terms (SSB and Pa in Fig. 2B; P and Pa in Fig. 2D) were kept.
Acknowledgments.
We are grateful to the many individuals who have participated in the Windermere data collection over the years. We thank Stephanie M. Carlson and Leif C. Stige for comments on an earlier version of the manuscript; Leif C. Stige also provided statistical advice. Two anonymous reviewers provided very useful comments for which we are very thankful. We also thank the Freshwater Biological Association for their joint stewardship of these invaluable data. E.E. received support from the Research Council of Norway and T.B.A. received support from a Marie Curie PhD fellowship awarded to CEES. Support from the Natural Environment Research Council to the Centre for Ecology and Hydrology is also acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Agrawal AA. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- 2.Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- 3.Bolker B, Holyoak M, Krivan V, Rowe L, Schmitz O. Connecting theoretical and empirical studies of trait-mediated interactions. Ecology. 2003;84:1101–1114. [Google Scholar]
- 4.Loeuille N, Loreau M. Evolutionary emergence of size-structured food webs. Proc Natl Acad Sci USA. 2005;102:5761–5766. doi: 10.1073/pnas.0408424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxel GR. Antagonistic and synergistic interactions among predators. B Math Biol. 2007;69:2093–2104. doi: 10.1007/s11538-007-9214-0. [DOI] [PubMed] [Google Scholar]
- 6.Kondoh M. Anti-predator defence and the complexity-stability relationship of food webs. Proc R Soc Lond B. 2007;274:1617–1624. doi: 10.1098/rspb.2007.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21:465–477. [Google Scholar]
- 8.Matsuda H, Abrams PA, Hori M. The effect of adaptive antipredator behavior on exploitive competition and mutualism between predators. Oikos. 1993;68:549–559. [Google Scholar]
- 9.Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, et al. Cryptic population dynamics: rapid evolution masks trophic interactions. Plos Biol. 2007;5:1868–1879. doi: 10.1371/journal.pbio.0050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG. Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz OJ, Krivan V, Ovadia O. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett. 2004;7:153–163. [Google Scholar]
- 12.Lafferty KD, Dobson AP, Kuris AM. Parasites dominate food web links. Proc Natl Acad Sci USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, et al. Parasites alter community structure. Proc Natl Acad Sci USA. 2007;104:9335–9339. doi: 10.1073/pnas.0700062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigby MC, Jokela J. Predator avoidance and immune defence: Costs and trade-offs in snails. Proc R Soc Lond B. 2000;267:171–176. doi: 10.1098/rspb.2000.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haugen TO, Winfield IJ, Vøllestad LA, Fletcher JM, James JB, et al. The ideal free pike: 50 years of fitness-maximizing dispersal in Windermere. Proc R Soc Lond B. 2006;273:2917–2924. doi: 10.1098/rspb.2006.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haugen TO, Winfield IJ, Vøllestad LA, Fletcher JM, James JB, et al. Density dependence and density independence in the demography and dispersal of pike over four decades. Ecol Monogr. 2007;77:483–502. [Google Scholar]
- 17.Holt RD, Polis GA. A theoretical framework for intraguild predation. Am Nat. 1997;149:745–764. [Google Scholar]
- 18.Hatcher MJ, Dick JTA, Dunn AM. How parasites affect interactions between competitors and predators. Ecol Lett. 2006;9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 19.Allen KR. The food and migration of perch (Perca fluviatilis) in Windermere. J Anim Ecol. 1935;4:264–273. [Google Scholar]
- 20.McCormack JC. Observations on food of perch (Perca fluviatilis L. ) in Windermere. J Anim Ecol. 1970;39:255–267. [Google Scholar]
- 21.Craig JF. A study of the food and feeding of perch, Perca fluviatilis L, in Windermere. Freshwater Biol. 1978;8:59–68. [Google Scholar]
- 22.Allen KR. A note on the food of pike (Esox lucius) in Windermere. J Anim Ecol. 1939;8:72–75. [Google Scholar]
- 23.Frost WE. The food of pike, Esox lucius L, in Windermere. J Anim Ecol. 1954;23:339–360. [Google Scholar]
- 24.Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proc Natl Acad Sci USA. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius) Ecol Lett. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 26.Le Cren ED. Observations on the growth of perch (Perca fluviatilis L. ) over twenty-two years with special reference to the effects of temperature and changes in population density. J Anim Ecol. 1958;27:287–334. [Google Scholar]
- 27.Le Cren ED. The determination of the age and growth of the perch (Perca fluviatilis) from the opercular bone. J Anim Ecol. 1947;16:188–204. [Google Scholar]
- 28.Frost WE, Kipling C. The determination of the age and growth of pike (Esox lucius) from scales and opercular bones. J Cons Int Explor Mer. 1959;24:314–341. [Google Scholar]
- 29.des Clers S, et al. Technical Report No. WI/T11050d5/4. London: Ministry of Agriculture, Fisheries and Food; 1994. [Google Scholar]
- 30.Bucke D, Cawley GD, Craig JF, Pickering AD, Willoughby LG. Further studies of an epizootic of perch, Perca fluviatilis L, of uncertain aetiology. J Fish Dis. 1979;2:297–311. [Google Scholar]
- 31.Zuk M, Stoehr AM. Immune defense and host life history. Am Nat. 2002;160:S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]
- 32.Roff DA. The Evolution of Life Histories: Theory and Analysis. New York: Chapman and Hall; 1992. [Google Scholar]
- 33.Kipling C, Le Cren ED. Mark-recapture experiments on fish in Windermere, 1943–1982. J Fish Biol. 1984;24:395–414. [Google Scholar]
- 34.Bodaly RA, Ward RD, Mills CA. A genetic stock study of perch, Perca fluviatilis L, in Windermere. J Fish Biol. 1989;34:965–967. [Google Scholar]
- 35.De Roos AM, Schellekens T, Van Kooten T, Persson L. Stage-specific predator species help each other to persist while competing for a single prey. Proc Natl Acad Sci USA. 2008;105:13930–13935. doi: 10.1073/pnas.0803834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends Ecol Evol. 2003;18:94–101. [Google Scholar]
- 37.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21:444–454. [Google Scholar]
- 38.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 39.Stige LC, Ottersen G, Brander K, Chan KS, Stenseth NC. Cod and climate: Effect of the North Atlantic Oscillation on recruitment in the North Atlantic. Mar Ecol Prog Ser. 2006;325:227–241. [Google Scholar]
- 40.Ricker WE. Stock and recruitment. J Fish Res Board Can. 1954;11:559–623. [Google Scholar]