Abstract
Indole-3-carbinol (I3C), a naturally occurring component of Brassica vegetables, such as broccoli, cabbage, and Brussels sprouts, induces a G1 cell-cycle arrest of human breast cancer cells, although the direct cellular targets that mediate this process are unknown. Treatment of highly invasive MDA-MB-231 breast cancer cells with I3C shifted the stable accumulation of cyclin E protein from the hyperactive lower-molecular-mass 35-kDa form that is associated with cancer cell proliferation and poor clinical outcomes to the 50-kDa cyclin E form that typically is expressed in normal mammary tissue. An in vitro cyclin E processing assay, in combination with zymography, demonstrated that I3C, but not its natural dimer, 3,3′-diindolylmethane, disrupts proteolytic processing of the 50-kDa cyclin E into the lower-molecular-mass forms by direct inhibition of human neutrophil elastase enzymatic activity. Analysis of elastase enzyme kinetics using either cyclin E or N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanalide as substrates demonstrated that I3C acts as a noncompetitive inhibitor of elastase activity with an inhibitory constant of ≈12 μM. Finally, siRNA ablation of neutrophil elastase protein production in MDA-MB-231 cells mimicked the I3C-disrupted processing of the 50-kDa cyclin E protein and the indole-induced cell-cycle arrest. Taken together, our results demonstrate that elastase is the first identified specific target protein for I3C and that the direct I3C inhibition of elastase enzymatic activity implicates the potential use of this indole, or related compounds, in targeted therapies of human breast cancers where high elastase levels are correlated with poor prognosis.
A critical challenge in controlling breast cancer is the identification of therapeutic agents that can effectively control the growth of both estrogen-responsive and nonresponsive breast cancer cells with reduced side effects, especially during prolonged treatments. Epidemiological studies show that frequent consumption of certain vegetables is associated with a lower incidence of cancers at various sites (1). For example, the consumption of Brassica (cruciferous) vegetables, such as cabbage, broccoli, and Brussels sprouts, is directly associated with decreased risk of reproductive tissue cancers in humans (2, 3) and reduced tumor incidence in experimental animals (4). These studies implicate the existence of specific biologically active phytochemicals that represent a largely untapped source of potent chemotherapeutic agents. One such promising molecule is indole-3-carbinol (I3C), a natural compound derived from glycobrassicin in Brassica vegetables, which has been shown to exhibit potent anticarcinogenic properties in a wide range of cancers such as lung, liver, colon, cervical, endometrial, prostate, and breast cancer (5–7). In addition, out of broad spectrum of analyzed phytochemicals, I3C was 1 of the few that tested positive as a chemopreventative agent in a panel of short-term bioassays relevant to carcinogen-induced DNA damage, tumor initiation and promotion, and oxidative stress (8).
We have discovered that I3C induces a G1 cell-cycle arrest of both estrogen-responsive and unresponsive human breast cancer cells (9–12) that occurs with a concomitant inhibition of expression or activity of CDK6 and CDK2, respectively, and with a marked decrease in endogenous retinoblastoma (Rb) protein phosphorylation (9, 11, 12). I3C down-regulates CDK6 transcription by disrupting the interaction between Sp1 transcription factors with a Sp1-Ets composite DNA element in the CDK6 promoter (11). In estrogen-responsive breast cancer cells, I3C suppresses estrogen responsiveness (13, 14), down-regulates expression of estrogen receptor-α (13), and synergizes with the antiproliferative effects of tamoxifen, an anti-estrogen widely used in breast cancer therapies (10). In nontumorigenic human mammary epithelial cells, I3C can induce the ATM signaling pathway independent of DNA damage to stabilize an active p53 tumor suppressor protein (15). I3C can suppress invasion and migration of human breast cancer cells (16) and stimulate IFN-γ receptor production and IFN-γ responsiveness (17). In other types of human reproductive cancer cells, I3C has been shown to have strong antiproliferative effects (5, 6, 18–21), and it alters immune function in vivo (22). Despite compelling evidence for the potent anticancer properties of this indole, the direct cellular target(s) of I3C that play a central role in the striking cell-cycle effects of this phytochemical have not been uncovered.
Eukaryotic cellular growth relies on the activation of cyclin/cyclin-dependent kinase (CDK) protein complexes that function at specific stages of the cell cycle (23). Many breast tumors exhibit elevated levels of cyclin E and cyclin D, which implicate the loss of cell-cycle control by deregulation of the G1 phase of the cell cycle (24, 25). Both high-molecular-weight and lower-molecular-weight forms of cyclin E have been detected in mammalian cells. Interestingly, many highly proliferative tissues, such as that of metastatic breast cancer, predominantly express the lower-molecular-weight forms of cyclin E (26–28) whereas the corresponding normal tissue generally display a higher-molecular-weight form of cyclin E (26).
We previously reported that I3C treatment of MCF-7 human breast cancer cells caused the formation of an inactive, 200-kDa CDK2 protein complex as compared with an active, 90-kDa CDK2 protein complex observed in untreated growing cells (29). In the absence of I3C, lower-molecular-mass 35-/33-kDa forms of cyclin E are associated with CDK2, which have been shown to confer hyperactivity to the CDK2 protein complex and increase in cell proliferation (27). In contrast, after I3C treatment the predominant form of cyclin E is a higher-molecular-mass 50-kDa species that produces an inactive CDK2 protein complex (29). Thus, I3C treatment reestablishes control of the G1 phase of cell cycle in human breast cancer cells by promoting accumulation of the 50-kDa cyclin E instead of the lower-molecular-mass forms of cyclin E.
Production of the lower-molecular-weight forms of cyclin E correlate with the tumorigenic potential of breast tissues and can induce metastatic mammary carcinomas (26, 30), and as such cyclin E is considered 1 of the most accurate prognostic markers for breast cancer grade and outcome (25, 31). The majority of the lower-molecular-weight cyclin E forms are derived from the proteolysis of the full-length 50-kDa cyclin E by intracellular serine proteases, such as elastase, which are expressed in most tissue types (27). Of the 3 general classes of human elastase that have been characterized [polymorphonuclear neutrophil elastase or leukocyte elastase, metalloelastase (or MMP12), and pancreatic elastase], the neutrophil elastase is highly expressed in human breast cancers and has been implicated in the processes of tumorigenesis (32). Importantly, the level and enzymatic activity of neutrophil elastase are an independent prognostic marker for breast cancer survival and clinical outcome as well as for metastasis progression of various cancers (33–36). In this report, we demonstrate that the dietary phytochemical I3C is a noncompetitive inhibitor of human elastase enzymatic activity that directly accounts for the disrupted cyclin E processing in human breast cancer cells, and therefore we have identified the first functional cellular target protein for I3C that mediates the indole-induced G1 cell-cycle arrest of human cancer cells.
Results
I3C Directly Inhibits Human Elastase Activity and Processing of Cyclin E Protein.
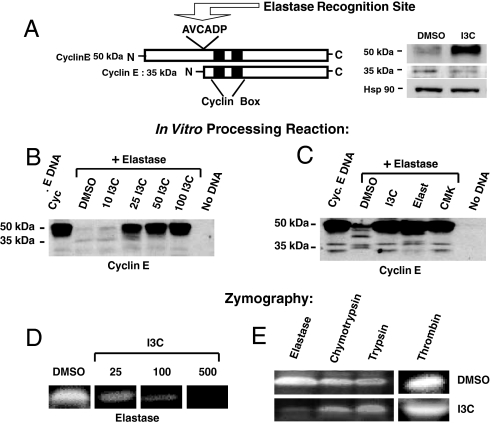
Treatment of MDA-MB-231 cells, a highly invasive and I3C-sensitive (9) human breast cell line that is representative of late-stage breast cancer, with 100 μM I3C causes the accumulation of the higher-molecular-mass 50-kDa cyclin E and loss of the lower-molecular-mass 35-kDa cyclin E (Fig. 1A, Western blot). Consistent with the known effects of the cyclin E protein forms on CDK2 (27), formation of the 50-kDa cyclin E/CDK2 protein in I3C-treated cells is associated with a significant reduction in CDK2 enzymatic activity whereas in the absence of indole treatment the CDK2 protein complex contains the lower-molecular-weight form of cyclin E and displays significant kinase activity [detailed with additional data in Fig. S1 in supporting information (SI) Text]. Cyclin E protein contains a AVCADP amino acid sequence from Ala-66 to Pro-71 that is followed by asparate–proline and embedded in a random coil, thereby forming a consensus sequence for the elastase class of serine proteases that generates the hyperactive 35-kDa cyclin E protein from the 50-kDa cyclin E (27) (see Fig. 1A, diagram). High levels of neutrophil elastase in human breast cancer cells are considered to be an important marker for disease progression (27, 28), and therefore an in vitro cyclin E protein processing assay was designed to directly test whether I3C can inhibit the elastase cleavage of human cyclin E protein. The unprocessed 50-kDa cyclin E was produced by a coupled in vitro transcription and in vitro translation system using rabbit reticulocyte lysates and then incubated with purified human neutrophil elastase in the presence of increasing concentrations of I3C. Western blot analysis of the in vitro reactions showed that production of the 50-kDa cyclin E protein depended on the inclusion of cyclin E cDNA in the in vitro assay (Fig. 1B: Cyc E DNA vs. No DNA lanes) and that adding elastase resulted in an almost quantitative loss of the 50-kDa cyclin E precursor protein (Fig. 1B: Cyc E DNA vs. DMSO + Elastase lane). As also shown in Fig. 1B, inclusion of I3C quantitatively inhibited elastase cleavage of the 50-kDa cyclin E protein with very detectable inhibitory activity occurring at 25 μM I3C and maximal inhibition observed by 50 μM I3C. In comparison to cells (Fig. 1A), the elastase-dependent in vitro processing of cyclin E generally does not yield a high level of stably accumulated 35-kDa protein, suggesting the existence of additional cleavage sites in vitro or perhaps the detection limitations of the assay. I3C also inhibited porcine pancreatic elastase activity (data not shown), suggesting that I3C is a new class of general elastase inhibitors.
Fig. 1.
I3C inhibits elastase enzymatic activity and elastase-dependent processing of cyclin E. (A) Schematic representation of full-length cyclin E, highlighting the elastase consensus cleavage site (AVCADP). To assess endogenous cyclin protein forms, MDA-MB-231 cells were treated with or without 100 μM I3C for 48 h, and cell extracts were electrophoretically fractionated and analyzed by Western blots for the presence of the 50-kDa and hyperactive 35-kDa forms of cyclin E. (B) For the in vitro assay of elastase processing of cyclin E protein, full-length cyclin E was transcribed and translated in vitro followed by digestion of the cyclin E protein product by human neutrophil elastase in the presence of the indicated concentrations of I3C or DMSO. The “No DNA” sample was a reaction mixture without added cyclin E cDNA. Western blot analysis of the entire reaction mixtures was performed by using cyclin E-specific antibodies. (C) The in vitro elastase processing of cyclin E was assessed in the presence of the DMSO vehicle control, 50 μM I3C, and the known elastase inhibitors 25 μM elastatinal or 5 μM methoxysuccinyl-Ala-Ala-Pro-Val-chloromethylketone (CMK). Western blot analysis of the entire reaction mixture was performed by using cyclin E-specific antibodies. (D) For zymography, equal amounts of purified human elastase were electrophoretically resolved on a 10% polyacrylamide gel containing elastin as a substrate. After renaturation with 2.5% Triton X-100, each lane was cut out and placed in developing buffer containing either DMSO or the indicated concentrations of I3C for 25 h at 37 °C. Gel slices were stained with Coomassie blue followed by an overnight destain. Clear bands represent proteolytic degradation of substrates. (E) Purified human elastase, chymotrypsin, trypsin, or thrombin were electrophoretically resolved in gels containing gelatin (for evaluation of elastase, chymotrypsin, or trypsin) or fibronectin (for evaluation of thrombin), the enzymes were renatured in 2.5% Triton X-100, and individual gel slabs were developed in either DMSO or 500 μM I3C for 25 h at 37 °C.
Using the in vitro cyclin E processing assay, the inhibitory effects of I3C on human neutrophil elastase activity were compared with well characterized elastase inhibitors, elastatinal (Elast) and methoxysuccinyl-Ala-Ala-Pro-Val-chloromethylketone (CMK) (37, 38). As shown in Fig. 1C, I3C was as effective as either elastatinal or CMK in preventing the elastase-dependent processing of the 50-kDa cyclin E. In the absence of any elastase inhibitors, lower-molecular-weight forms of cyclin E can be detected in an elastase-dependent manner (Cyc E DNA vs. DMSO elastase lanes).
To further establish that I3C can directly inhibit human elastase enzymatic activity, purified elastase was assayed by zymography in which the enzyme was fractionated in nondenaturing polyacrylamide gels containing elastin (as a substrate) followed by incubation of the gel with or without I3C. As shown in Fig. 1D, in the absence of I3C (DMSO panels) a clear band appeared in areas where elastin was degraded (detectable as a white signal in the photograph) against a dark blue background of Coomassie blue staining where elastin is still present. Impregnation of several concentrations of I3C into the gel lane resulted in a dose-dependent inhibition of elastin degradation (Fig. 1D). The specificity of the I3C inhibition was assayed by zymography on several different serine proteases. Purified human elastase, trypsin, or chymotrypsin was fractionated in a nondenaturing polyacrylamide gel containing gelatin as a substrate, and, in a separate gel, purified thrombin was fractionated with fibrinogen as a substrate. As shown in Fig. 1E, although I3C significantly inhibited the activity of elastase, this indole has either a very minor or no inhibitory effects on the other tested serine proteases even at much longer incubation times. Thus, I3C is a specific inhibitor of elastase proteolytic activity.
Indole Specificity of Elastase Inhibition.
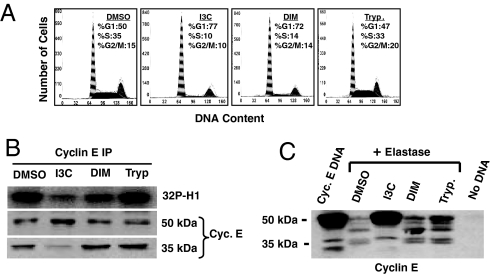
To determine the indole specificity of the I3C disruption in cyclin E processing and inhibition of elastase activity, the effects of I3C were compared with 3,3′-diindolylmethane (DIM), which is a natural bioactive dimerization product of I3C (12), and with tryptophol, an indole structurally similar to I3C but with no antiproliferative properties (9). MDA-MB-231 breast cancer cells were treated with 100 μM I3C, 30 μM DIM, 100 μM tryptophol, or the DMSO vehicle control for 48 h. One aliquot of cells was analyzed for their cell-cycle status by flow cytometry of propidium iodide-stained nuclei for DNA content (Fig. 2A). Cyclin E was immunoprecipitated from the other aliquot of cells, and the relative levels of the 50-kDa and 35-kDa forms of cyclin E protein were determined by Western blot analysis (Fig. 2B). As shown in Fig. 2 A and B, I3C and DIM induced a similar G1 cell-cycle arrest; however, the higher-molecular-mass 50-kDa cyclin E was the predominant form only in I3C-treated cells. The level of the 35-kDa cyclin E protein forms in DIM-treated cells was similar to that observed in growing vehicle control-treated cells (DMSO). The cell-cycle profile and cyclin E processing after tryptophol treatment were virtually identical to those observed in the vehicle control cells (Fig. 2 A and B). As also shown in Fig. 2B, the coprecipitating CDK2 protein kinase activity, assayed in vitro by using the histone H1 substrate, was strongly inhibited in the presence of I3C, but not with DIM or tryptophol. Thus, the I3C-mediated generation of the 50-kDa cyclin E and loss of the lower-molecular-weight forms of cyclin E are not indirect consequences of the cell-cycle arrest or nonspecific effects of exposing cells to an indole compound.
Fig. 2.
Indole specificity of the I3C inhibition of elastase enzymatic activity. (A) Flow cytometry analysis of MDA-MB-231 cells treated for 72 h with the DMSO vehicle control, 100 μM I3C, 30 μM DIM, or 100 μM tryptophol. (B) MDA-MB-231 cells were treated with 100 μM I3C, 30 μM DIM, 100 μM tryptophol, or the DMSO vehicle control for 72 h. Half of the immunoprecipitated cyclin E was assessed for the associated CDK2 kinase activity using Histone H1 as the in vitro substrate, and the other half was analyzed for cyclin E form by Western blots. (C) The effects of indoles on human neutrophil elastase processing of cyclin E was analyzed by using an in vitro transcription–translation system as described. The indicated reaction mixtures contained the DMSO vehicle control, 50 μM I3C, 30 μM DIM, or 50 μM tryptophol.
The in vitro cyclin E protein processing assay using purified elastase was used to assess the indole selectively of elastase inhibition. As shown in Fig. 2C, I3C inhibited the elastase-mediated in vitro processing of cyclin E in that the level of the remaining 50-kDa cyclin E was similar to that detected in the absence of any added elastase (Cyc E DNA lane). In contrast, in the presence of DIM or tryptophol the elastase-mediated processing of the 50-kDa cyclin E was similar to the vehicle control in vitro processing reactions, which demonstrates that elastase activity was not inhibited by either molecule. Zymography of purified elastase confirmed these results, in that I3C, but not DIM or tryptophol, directly inhibited elastase activity (data not shown).
I3C Acts as a Noncompetitive Inhibitor of Human Elastase Enzymatic Activity.
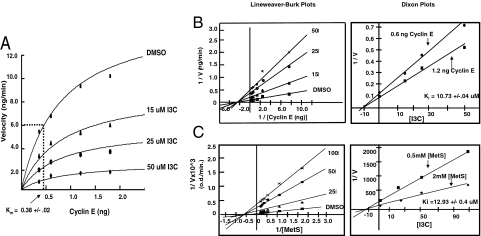
To assess the mechanism of I3C inhibition human elastase activity, the potential effects of I3C on the Michaelis constant (Km) and/or the maximal velocity of the reaction (Vmax) was determined by increasing concentrations of the 50-kDa cyclin E substrate and of I3C in the in vitro cyclin E processing reactions. Elastase activity was quantified by densitometric analysis of the amount of the 50-kDa cyclin E protein detected by Western blots that remained in each reaction mixture compared with the starting amount of added cyclin E protein. As shown in Fig. 3A, in the absence of I3C the Vmax was calculated to be ≈11.95 ± 0.05 ng/min using nonlinear regression analysis. Increasing concentrations of I3C inhibited the maximal velocity of the elastase reactions, whereas the calculated Km for cyclin E remained at ≈0.38 ± 0.02 ng in each dose of I3C. Furthermore, Lineweaver–Burk double reciprocal plots of 1/velocity versus 1/substrate at the various I3C concentrations revealed the same −1/Km on the abscissa at each indole concentration (Fig. 3B Left). These results demonstrate that I3C is a noncompetitive elastase inhibitor. Dixon plotting of 1/V versus the concentration of I3C at 2 different substrate concentrations (0.6 ng and 1.2 ng of cyclin E protein per reaction) showed that the Ki for the I3C-induced inhibition of cyclin E processing by human elastase is 10.73 ± 0.04 μM (Fig. 3B Right).
Fig. 3.
Kinetic analysis of the indole inhibition of elastase activity. (A) The in vitro elastase cyclin E processing assay was used to determine the effects of I3C on the Km and Vmax for human neutrophil elastase enzymatic activity. Human elastase, the indicated cyclin E substrate concentrations, and indicated concentration of I3C were all mixed to a total volume of 20 μL and incubated for 5 min at 37 °C followed by Western blot analysis of cyclin E. The reaction velocities were determined as a function of the loss of the 50-kDa cyclin E protein and were graphed versus the cyclin E concentration. (B) Lineweaver–Burk and Dixon plot analysis of the I3C inhibition of elastase processing of cyclin E using the in vitro transcription–translation reactions. Double reciprocal plotting of 1/velocity versus 1/substrate yields the Lineweaver–Burk plot. For the Dixon plots, the ratio 1/V is plotted against the different concentrations of I3C at 2 different substrate concentrations (0.6 and 1.2 ng), and the Ki values were graphically determined as the intercept with the abscissa. (C) Lineweaver–Burk and Dixon plot analysis of the I3C inhibition of human elastase hydrolysis of the chromogenic substrate methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide (MetS).
A potential limitation of generating cyclin E protein in an in vitro transcription–translation system is the presence of other proteins during the elastase reactions that could potentially alter the enzyme kinetics. As a complementary confirmation of the noncompetitive I3C inhibition of elastase activity using pure elastase and pure substrate, the hydrolysis of the chromogenic elastase substrate N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanalide (MetS) (39) was measured at different substrate and I3C concentrations by spectrophotometry. As shown in Fig. 3C Left, the double reciprocal plots at various I3C concentrations all displayed the same −1/Km on the abscissa, which further confirms that I3C acts as a noncompetitive elastase inhibitor. The calculated Ki value of enzyme inhibition using pure elastase in the presence of the MetS substrate was 12.93 ± 0.4 μM (Fig. 3C Right), which is a value that is similar to that calculated value using cyclin E protein as a substrate (Fig. 3B Right).
siRNA Knockdown of Cellular Elastase Levels Mimics the I3C-Mediated Inhibition of Cyclin E Protein Processing and of the G1 Cell-Cycle Arrest of Breast Cancer Cells.
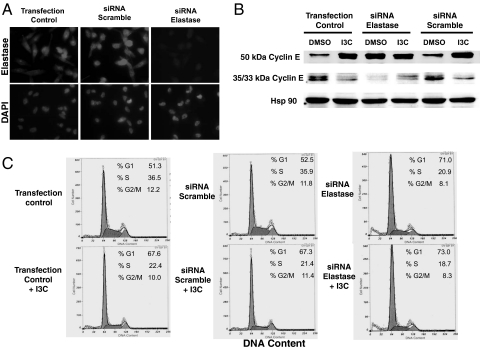
A key prediction for elastase being a direct I3C target in human breast cancer cells is that the ablation of elastase protein production by siRNA should mimic the I3C effects on cyclin E protein processing and cell-cycle control. MDA-MB-231 cells were transfected with a human neutrophil elastase-specific siRNA for 48 h, or with a scrambled siRNA sequence, and the level of elastase protein was monitored in siRNA-transfected, scrambled siRNA-transfected, and control-transfected cells by indirect immunofluorescence using anti-human elastase-specific primary antibodies and Texas red-conjugated fluorescent secondary antibodies. Elastase production was substantially reduced in cells exposed to the elastase-specific siRNA (Fig. 4A Upper Right) compared with the transfection and scrambled siRNA controls (Fig. 4A Upper Left), showing that the siRNA markedly knocked down elastase production in human breast cancer cells. Fig. 4A Lower shows DAPI staining of cell nuclei from the siRNA and control-transfected cells.
Fig. 4.
siRNA ablation of elastase production mimics the effects of I3C on cyclin E protein processing and the cell-cycle arrest. (A) MDA-MB-231 breast cancer cells were transfected with or without human neutrophil elastase-specific siRNA or with scrambled siRNA sequences for 24 h, and resulting levels of cellular elastase were monitored by indirect immunofluorescence. DAPI staining of nuclear DNA was used as a control for the presence of cells in siRNA-treated and untreated samples. (B) siRNA (elastase or scrambled)-transfected and control-transfected cells were then treated with or without 100 μM I3C for 48 h, and electrophoretically fractionated total cell extracts were examined for the levels of the 50-kDa and the 35-/33-kDa cyclin E by Western blot analysis. The level of hsp90 was used a gel loading control. (C) siRNA (elastase or scrambled)-transfected and control-transfected cells were then treated with or without 100 μM I3C for 48 h, and cell proliferation was monitored by flow cytometry for DNA content of propidium iodide-stained nuclei.
Production of the 50-kDa cyclin E protein and lower-molecular-mass 35-kDa cyclin E proteins was analyzed in MDA-MB-231 cells transfected with or without the elastase or scrambled siRNA sequences and then incubated in the absence or the presence of 100 μM I3C for 48 h. Western blot analysis revealed that siRNA disruption of elastase expression prevented cyclin E processing to the same extent as treatment with I3C. As shown in Fig. 4B, control (DMSO) cells predominantly express the lower-molecular-weight cyclin E proteins whereas the 50-kDa cyclin E was the predominant form in both the elastase-ablated (siRNA) and I3C-treated cells. Interestingly, when the elastase siRNA-transfected cells were treated with I3C, there appeared to be an enhanced effect on level of the 50-kDa cyclin E protein (Fig. 4B, I3C + siRNA lane). Flow cytometry analysis revealed that siRNA ablation of elastase expression induced a cell-cycle arrest comparable to cells incubated with 100 μM I3C (Fig. 4C). Taken together, these results demonstrate that the knockdown of elastase protein is sufficient to growth-arrest and disrupt cyclin E protein processing in human breast cancer cells and that the I3C inhibition of elastase activity plays a crucial role in the indole-mediated cell-cycle effects.
Discussion
We have identified elastase as the first specific target protein of the phytochemical I3C that mediates the indole-induced cell-cycle arrest and disruption of cyclin E processing in human breast cancer cells. I3C acts as a specific and potent noncompetitive enzymatic inhibitor of human neutrophil elastase activity, which is highly expressed in breast cancer cells and has been shown to be a prognostic marker for reduced survival rates of primary breast cancer patients (34–36). Intracellular elastase selectively cleaves the 50-kDa cyclin E to generate several lower-molecular-weight forms of cyclin E ranging from 33 kDa to 43 kDa in size. Although all forms of cyclin E are capable of binding to the CDK2 protein complex, the lower-molecular-weight forms are hyperactive in that they exert significantly higher CDK2 activity as compared with the 50-kDa cyclin E (27, 28). The direct cellular consequences of the I3C inhibition of elastase activity are the disruption in cyclin E processing and subsequent association of the 50-kDa cyclin E with CDK2 protein complex that down-regulates CDK2 kinase activity and induces a cell-cycle arrest of human breast cancer cells. A key functional test of this pathway was the demonstration that siRNA ablation of elastase production mimicked the cell-cycle response of I3C, demonstrating that the loss of elastase activity is sufficient to growth-arrest human breast cancer cells.
Of the 3 general classes of human elastases that have been characterized (pancreatic, polymorphonuclear neutrophil, and metalloelastase), high levels of neutrophil elastase have been implicated in playing a critical role in the process of tumorigenesis of human breast cancers (32, 33) and other cancers including non-small-cell lung carcinoma (40) and bladder cancer (41). In patients, high levels of elastase expression and/or activity in breast cancer tissue can be associated with poor clinical outcome, reduced survival rates (35, 36), decreased responsiveness to chemotherapy (42), and reduced responsiveness to endocrine treatment (33, 34). The level of immunoreactive elastase in tumors has been shown to be an independent prognostic factor for reduced survival in patients with breast cancer who have undergone curative surgery (36). Elastase expression in primary breast cancers was correlated with production of the hyperactive lower-molecular-weight forms of cyclin E (35), suggesting a clinically relevant direct link between elastase activity and tumor growth. There are significant variations of elastase levels in the general population and in specific tissues (36). Conceivably individual differences in elastase expression and/or activity may directly contribute to variations in correlation between the intake of cruciferous vegetables and cancer risk (3) as well as susceptibility of patients to the potential therapeutic use of I3C.
We have previously shown that I3C synergizes with tamoxifen, an anti-estrogen that is currently used for treating hormone-dependent breast cancers, to more stringently induce a G1 cell-cycle arrest through the additive inhibition of CDK2 kinase activity with the combined drug treatments (10). Because high elastase levels correlate with tamoxifen failure in aggressive breast cancers (33), it is tempting to consider that the I3C inhibition of elastase enzymatic activity could potentially prevent and/or attenuate the process leading to tamoxifen resistance in breast cancers and thus would prolong the effectiveness of tamoxifen treatment in aggressive breast cancers. The concentration of I3C required to inhibit elastase activity in vitro has relevance to the dietary intake of this indole. In a typical western diet (European or American) an individual ingests ≈10 mg of I3C per day whereas in a typical Asian diet intake is ≈10-fold higher. Clinical studies have established that ingestion of 400 mg of I3C twice daily is the maximum tolerated dose of this indole that alters estrogen metabolism and other cellular pathways (3). Importantly, under these conditions, the concentration of indole metabolites in the plasma was reduced to 15 ng/mL after 12 h (the lower limit of detection), strongly suggesting that because I3C is rapidly cleared from the system it is likely to be active at target sites at much lower concentrations that are closer to the observed concentrations needed to inhibit elastase activity.
We propose that I3C represents a new class of natural nonpeptide elastase enzymatic inhibitors that is a highly potent with Ki values within the 10–15 μM range using either cyclin E or N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanalide as substrates. This indole is relatively selective for the elastase family of serine proteases in that several other serine proteases were either not inhibited (such as trypsin and thrombin) or only very weakly inhibited (such as chymotrypsin) by I3C. Furthermore, I3C acts as a general inhibitor of elastases because both porcine pancreatic elastase and human neutrophil elastase activity were equivalently inhibited by I3C using several different substrates (data not shown). The noncompetitive inhibition of elastase suggests that I3C does not bind to the active site of elastase but may act as an allosteric inhibitor at a distinct site in the elastase molecule. Our results strongly suggest that I3C directly binds to elastase because I3C was able to inhibit elastase enzymatic activity in zymographic assays, which requires that the inhibitor permeates through the gel matrix and directly inhibits proteolytic activity. Structural studies are currently underway to elucidate the precise interaction site where I3C binds to the elastase protein.
The tissue-specific expression, stable accumulation, and activity of intracellular forms of elastase regulate the relative levels of the 50-kDa cyclin E and formation of its hyperactive lower-molecular-weight forms that associate with CDK2 protein complex. The lower-molecular-weight cyclin E forms have been detected in nearly all metastatic breast cancer cell lines and tissues tested and are not generally present in nontumorigenic breast epithelium (26). A key cellular consequence of the I3C inhibition of elastase activity in human breast cancer cells is the shift from a hyperactive cyclin E/CDK2 protein complex that promotes cell-cycle progression to a more “normal-like” cellular context in which CDK2 associates with the 50-kDa cyclin E to produce a relatively inactive complex. In this regard, the direct inhibitory effect of I3C on elastase enzymatic activity suggests that this indole could be developed as a targeted therapeutic for the clinical management of breast cancers that exhibit high expression levels of elastase. I3C disrupts cyclin E protein processing in different classes of human breast cancer cells including estrogen-responsive MCF-7 human breast cancer cells (29) as well as the hormone-unresponsive and highly metastasizing MDA-MB-231 human breast cancer cells used in this current study. Furthermore, elastase acts on a variety of other intracellular and extracellular substrates, such as elastin and other extracellular matrix components, and the direct I3C inhibition of elastase activity implicates this natural phytochemical as a potential therapeutic not only for certain cancers but also for other physiological disorders associated with alterations in the levels of elastase activity.
Materials and Methods
Materials, Cell Culture, Flow Cytometry, Western Blots, Immunoprecipitation, and CDK Enzymatic Assay.
This information is detailed in SI Text. The flow cytometry was performed as we previously described (15), and the Western blots, cyclin E immunoprecipitations, and assays of CDK2 enzymatic activity using histone H1 as a substrate were carried out as described (29).
Assay of Human Elastase Enzymatic Digestion of in Vitro Transcribed/Translated Cyclin E Protein.
The TNT coupled reticulocyte lysate system (Promega) was used to in vitro transcribe and translate the cyclin E-flag construct in a pCDNA 3.1 vector (generous gift of Dirk Bohmann, University of Rochester, Rochester, NY). All reactions were carried out according to the manufacturer's instruction. Briefly, 1 μg of pCDNA3.1 plasmid containing either the cyclin E-Flag insert or no insert (empty vector) was added to rabbit reticulocyte lysate in the presence of T7 RNA polymerase and 1 mM complete amino acid mixture in a total volume of 50 μL and incubated at 30 °C for 90 min. Where appropriate, the final levels of cyclin E protein were quantified by determining the final protein concentrations in the reaction mixtures before and after in vitro synthesis of the cyclin E protein. To assess human elastase activity, a total of 5 × 10−5 units of human neutrophil elastase were mixed with 0.5 μL of the TNT reaction mixture (5 μL of a 1:10 dilution) in the elastase reaction buffer (50 mM Tris, pH 8.5/250 mM NaCl) for 5 min at 30 °C (total reaction volume was 20 μL). In the appropriate experiments, in vitro elastase reaction mixtures contained final concentrations of I3C (10–100 μM as indicated), 30 μM DIM, 100 μM tryptophol, 25 μM elastatinal, or 5 μM MCK were incubated with the DMSO vehicle control. The reactions were stopped by the addition of 2× protein loading buffer, and 3 μL of each reaction mixture was subjected to SDS/PAGE and Western blot analysis of cyclin E.
Elastase Enzyme Kinetics, Use of Chromogenic Substrates, and Modified Zymographic Analysis.
Description of the I3C effects on elastase enzyme kinetics, use of cyclin E protein or a chromogenic substrate, double reciprocal and Dixon plot analyses of I3C inhibition of elastase enzymatic activity, and the modified zymographic analysis of enzyme activity (43) are detailed in SI Text.
siRNA Knockdown of Cellular Elastase Levels.
GenomeWide siRNA for human neutrophil elastase and the scramble siRNA were obtained from Qiagen. MDA-MB-231 cells were seeded at 60% confluency on 6-well plates in full-growth media on the day of the transfection. A total of 150 ng of siRNA was mixed with 100 μL of growth media not containing serum and antibiotics. Hiperfect siRNA transfection reagent (Qiagen) was added to the siRNA media solution and mixed by vortexing, and the resulting siRNA suspensions were incubated at room temperature for 10 min. The siRNA mixtures were then added to the cells in a dropwise manner and mixed in by plate agitation to assure even siRNA treatment distribution. The cells were incubated at 37 °C for 24 h, and then the media were aspirated and replaced with full-growth media with or without 100 μM I3C for 48 h. The efficiency of siRNA ablation of elastase levels was determined by indirect immunofluorescence with cells plated on glass chamber slides (Nunc) at 60% confluency. The indirect immunofluorescence using elastase primary antibodies and Texas red-conjugated secondary antibodies and the DAPI staining of cell nuclei were carried out as previously described (16).
Supplementary Material
Acknowledgments.
We thank the members of the G.L.F. laboratory for their helpful suggestions during the course of the work. This study was supported by National Institute of Health Public Service Grant CA102360 from the National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806581105/DCSupplemental.
References
- 1.Feldman EB. Dietary intervention and chemoprevention—1992 perspective. Prev Med. 1993;22:661–666. doi: 10.1006/pmed.1993.1059. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Otin C, Diamandis EP. Breast and prostate cancer: An analysis of common epidemiological, genetic, and biochemical features. Endocr Rev. 1998;19:365–396. doi: 10.1210/edrv.19.4.0337. [DOI] [PubMed] [Google Scholar]
- 3.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubbs CJ, et al. Chemoprevention of chemically-induced mammary carcinogenesis by indole-3-carbinol. Anticancer Res. 1995;15:709–716. [PubMed] [Google Scholar]
- 5.Aggarwal BB, Ichikawa H. Molecular targets and anti-cancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 6.Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Stutzman JD, Kelloff GJ, Steele VE. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. 1994;54:5848–5855. [PubMed] [Google Scholar]
- 9.Cover CM, et al. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 10.Cover CM, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res. 1999;59:1244–1251. [PubMed] [Google Scholar]
- 11.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem. 2001;276:22332–22340. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 12.Firestone GL, Bjeldanes LF. Indole-3-carbinol (I3C) and 3–3′diindolylmethane (DIM) anti-proliferative signaling pathways control cell cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 tanscription factor interactions. J Nutr. 2003;133:2448S–2455S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 13.Sundar SN, et al. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol. 2006;20:3070–3082. doi: 10.1210/me.2005-0263. [DOI] [PubMed] [Google Scholar]
- 14.Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Brew CT, et al. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int J Cancer. 2006;118:857–868. doi: 10.1002/ijc.21445. [DOI] [PubMed] [Google Scholar]
- 16.Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3- carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147–152. doi: 10.1023/a:1006495824158. [DOI] [PubMed] [Google Scholar]
- 17.Chatterji U, et al. Indole-3-carbinol stimulates transcription of the interferon gamma receptor 1 gene and augments interferon responsiveness in human breast cancer cells. Carcinogenesis. 2004;25:1119–1128. doi: 10.1093/carcin/bgh121. [DOI] [PubMed] [Google Scholar]
- 18.Chen DZ, Qi M, Auborn KJ, Carter TH. Indole-3-carbinol and diindolylmethane induce apoptosis of human cervical cancer cells and in murine HPV16- transgenic preneoplastic cervical epithelium. J Nutr. 2001;131:3294–3302. doi: 10.1093/jn/131.12.3294. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JC, et al. Indole-3-carbinol mediated cell cycle arrest of LNCaP human prostate cancer cells requires the induced production of activated p53 tumor suppressor protein. Biochem Pharmacol. 2006;72:1714–1723. doi: 10.1016/j.bcp.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Hsu JC, Kinseth MA, Bjeldanes LF, Firestone GL. Indole-3-carbinol (I3C) induces a G1 cell cycle arrest of human LNCaP prostate cancer cells and inhibits expression of prostate specific antigen. Cancer. 2003;98:2511–2520. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]
- 21.Souli E, Machluf M, Morgenstern A, Sabo E, Yannai S. Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem Toxicol. 2008;46:863–870. doi: 10.1016/j.fct.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Exon JH, South EH. Dietary indole-3-carbinol alters immune functions in rats. J Toxicol Environ Health A. 2000;59:271–279. doi: 10.1080/009841000156934. [DOI] [PubMed] [Google Scholar]
- 23.Sherr CJ. The Pezcoller lecture: Cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 24.Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem. 2006;97:261–274. doi: 10.1002/jcb.20690. [DOI] [PubMed] [Google Scholar]
- 25.Hunt KK, Keyomarsi K. Cyclin E as a prognostic and predictive marker in breast cancer. Semin Cancer Biol. 2005;15:319–326. doi: 10.1016/j.semcancer.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Harwell RM, Porter DC, Danes C, Keyomarsi K. Processing of cyclin E differs between normal and tumor breast cells. Cancer Res. 2000;60:481–489. [PubMed] [Google Scholar]
- 27.Porter DC, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akli S, Keyomarsi K. Cyclin E and its low molecular weight forms in human cancer and as targets for cancer therapy. Cancer Biol Ther. 2003;2:S38–S47. [PubMed] [Google Scholar]
- 29.Garcia HH, Brar GA, Nguyen DH, Bjeldanes LF, Firestone GL. Indole-3-carbinol (I3C) inhibits cyclin-dependent kinase-2 function in human breast cancer cells by regulating the size distribution, associated cyclin E forms, and subcellular localization of the CDK2 protein complex. J Biol Chem. 2005;280:8756–8764. doi: 10.1074/jbc.M407957200. [DOI] [PubMed] [Google Scholar]
- 30.Akli S, et al. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–7222. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland RL, Musgrove EA. Cyclin E and prognosis in patients with breast cancer. N Engl J Med. 2002;347:1546–1547. doi: 10.1056/NEJMNEJMp020124. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, et al. Neutrophil elastase and cancer. Surg Oncol. 2006;15:217–222. doi: 10.1016/j.suronc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Foekens JA, et al. Elevated expression of polymorphonuclear leukocyte elastase in breast cancer tissue is associated with tamoxifen failure in patients with advanced disease. Br J Cancer. 2003;88:1084–1090. doi: 10.1038/sj.bjc.6600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foekens JA, et al. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63:337–341. [PubMed] [Google Scholar]
- 35.Desmedt C, et al. Impact of cyclins E, neutrophil elastase and proteinase 3 expression levels on clinical outcome in primary breast cancer patients. Int J Cancer. 2006;119:2539–2545. doi: 10.1002/ijc.22149. [DOI] [PubMed] [Google Scholar]
- 36.Akizuki M, et al. Prognostic significance of immunoreactive neutrophil elastase in human breast cancer: Long-term follow-up results in 313 patients. Neoplasia. 2007;9:260–264. doi: 10.1593/neo.06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molla A, Hellen CU, Wimmer E. Inhibition of proteolytic activity of poliovirus and rhinovirus 2A proteinases by elastase-specific inhibitors. J Virol. 1993;67:4688–4695. doi: 10.1128/jvi.67.8.4688-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein RL, Trainor DA. Mechanism of inactivation of human leukocyte elastase by a chloromethyl ketone: Kinetic and solvent isotope effect studies. Biochemistry. 1986;25:5414–5419. doi: 10.1021/bi00367a011. [DOI] [PubMed] [Google Scholar]
- 39.Bjornland K, et al. Polymorphonuclear elastase in human colorectal carcinoma. Int J Oncol. 1998;12:535–540. doi: 10.3892/ijo.12.3.535. [DOI] [PubMed] [Google Scholar]
- 40.Guner G, Kirkali G, Baskin Y, Karlikaya C, Akkoclu A. Elastase levels in small and non-small cell lung carcinoma. Biochem Soc Trans. 1993;21:305S. doi: 10.1042/bst021305s. [DOI] [PubMed] [Google Scholar]
- 41.Nemoto S, Koiso K, Aoyagi K, Tojo S. A study of invasive factor in bladder cancer-elastase-like activity as a marker of bladder cancer invasiveness. Nippon Hinyokika Gakkai Zasshi. 1984;75:1391–1400. doi: 10.5980/jpnjurol1928.75.9_1391. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita JI, et al. Production of immunoreactive polymorphonuclear leucocyte elastase in human breast cancer cells: Possible role of polymorphonuclear leucocyte elastase in the progression of human breast cancer. Br J Cancer. 1994;69:72–76. doi: 10.1038/bjc.1994.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.