Abstract
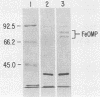
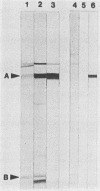
Escherichia coli septicemia is a common disease of young poultry and several species of mammals. Rabbit antiserum was prepared against iron-regulated outer membrane proteins of E. coli. Eighteen-day-old turkeys were passively immunized with antiserum and challenged by air sac inoculation of 1 X 10(6) to 2 X 10(6) CFU of E. coli O78:K80:H9. Turkeys injected with normal rabbit serum or saline solution before challenge served as controls. Fatalities (8 of 51 turkeys inoculated) occurred only in groups given saline solution or normal rabbit serum. The remaining turkeys were necropsied 96 h after challenge. Passive immunization with antiserum significantly (P less than 0.05) reduced the frequency of bacteremia at 96 h after challenge, the frequency of recovery of E. coli from air sacs, and the severity of gross lesions in inoculated birds as compared with birds given normal rabbit serum or saline solution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolin C. A. Effects of exogenous iron on Escherichia coli septicemia of turkeys. Am J Vet Res. 1986 Aug;47(8):1813–1816. [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Frank M. M. The role of complement in host resistance to bacteria. Springer Semin Immunopathol. 1983;6(4):349–360. doi: 10.1007/BF02116279. [DOI] [PubMed] [Google Scholar]
- Chart H., Griffiths E. Antigenic and molecular homology of the ferric enterobactin receptor protein of Escherichia coli. J Gen Microbiol. 1985 Jun;131(6):1503–1509. doi: 10.1099/00221287-131-6-1503. [DOI] [PubMed] [Google Scholar]
- Cheville N. F., Arp L. H. Comparative pathologic findings of Escherichia coli infection in birds. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):584–587. [PubMed] [Google Scholar]
- Coulton J. W. The ferrichrome-iron receptor of Escherichia coli K-12. Antigenicity of the fhuA protein. Biochim Biophys Acta. 1982 Jul 16;717(1):154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- Deb J. R., Harry E. G. Laboratory trials with inactivated vaccines against Escherichia coli (O78 K80) infection in fowls. Res Vet Sci. 1976 Mar;20(2):131–138. [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Grewal K. K., Warner P. J., Williams P. H. An inducible outer membrane protein involved in aerobactin-mediated iron transport by co1V strains of Escherichia coli. FEBS Lett. 1982 Apr 5;140(1):27–30. doi: 10.1016/0014-5793(82)80513-9. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Isolation of enterochelin from the peritoneal washings of guinea pigs lethally infected with Escherichia coli. Infect Immun. 1980 Apr;28(1):286–289. doi: 10.1128/iai.28.1.286-289.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Thorpe R., Chart H. Naturally occurring antibodies in human sera that react with the iron-regulated outer membrane proteins of Escherichia coli. Infect Immun. 1985 Mar;47(3):808–813. doi: 10.1128/iai.47.3.808-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Characterization of antibody to the ferripyochelin-binding protein of Pseudomonas aeruginosa. Infect Immun. 1986 Mar;51(3):896–900. doi: 10.1128/iai.51.3.896-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Iron-suppressible production of hydroxamate by Escherichia coli isolates. Infect Immun. 1982 Jun;36(3):870–875. doi: 10.1128/iai.36.3.870-875.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]