Abstract
The regenerative process in the pancreas is of particular interest because diabetes results from an inadequate number of insulin-producing beta cells and pancreatic cancer may arise from the uncontrolled growth of progenitor/stem cells. Continued and substantial growth of islet tissue occurs after birth in rodents and humans, with additional compensatory growth in response to increased demand. In rodents there is clear evidence of pancreatic regeneration after some types of injury, with proliferation of preexisting differentiated cell types accounting for some replacement. Additionally, neogenesis or the budding of new islet cells from pancreatic ducts has been reported, but the existence and identity of a progenitor cell have been debated. We hypothesized that the progenitor cells are duct epithelial cells that after replication undergo a regression to a less differentiated state and then can form new endocrine and exocrine pancreas. To directly test whether ductal cells serve as pancreatic progenitors after birth and give rise to new islets, we generated transgenic mice expressing human carbonic anhydrase II (CAII) promoter: Cre recombinase (Cre) or inducible CreERTM to cross with ROSA26 loxP-Stop-loxP LacZ reporter mice. We show that CAII-expressing cells within the pancreas act as progenitors that give rise to both new islets and acini normally after birth and after injury (ductal ligation). This identification of a differentiated pancreatic cell type as an in vivo progenitor of all differentiated pancreatic cell types has implications for a potential expandable source for new islets for replenishment therapy for diabetes.
Keywords: diabetes, islets of Langerhans, lineage tracing
Regeneration studies in mammals have focused on tissue-specific stem and progenitor cells. The regenerative process in the pancreas is of particular interest because diabetes results from an inadequate number of insulin-producing beta cells (1) and pancreatic cancer may arise from the uncontrolled growth of progenitor/stem cells (2). Continued and substantial growth of islet tissue occurs after birth in rodents and humans, with additional compensatory growth in response to increased demand (3). In rodents there is clear evidence of pancreatic regeneration after some types of injury, with proliferation of preexisting differentiated cell types accounting for some replacement (4–7). The mechanisms thought to be responsible for beta cell growth are replication of preexisting beta cells and differentiation from precursor cells or neogenesis, defined as islet hormone-positive cells budding from ducts. For the latter, differentiated ductal cells have been hypothesized to act as pancreatic progenitor cells (3, 8). Whether adult stem cells contribute to this replacement is unclear.
In adult mice replication is the major mechanism for expanding the beta cell mass in pregnancy (9), obesity/insulin resistance (10), or normal growth and aging. Using inducible RIP-CreERTM mice to label the beta cells in adult mice, Dor et al. (11) confirmed the predominance of replication in the adult mouse and concluded that neogenesis does not occur after embryonic or early postnatal life and that solely self-duplication replenished beta cells. However, they neither examined the neonatal period nor clearly defined new lobes after partial pancreatectomy (12), both of which are reported in rats to have highly active neogenesis. Additionally, it is difficult to conclude that new islet formation does not occur by marking a small fraction of cells and attempting to show a reduction in that fraction. Recently, Xu et al. (13) reported multipotent islet progenitor cells of unknown origin within the adult pancreas by showing the induction of neurogenin 3 after ductal ligation, isolating these neurogenin 3-positive cells and showing that they could differentiate to islet cells. Additionally, increased neogenesis is reported in adult rodents given exendin-4 (14) or betacellulin (15), overexpressing IFN-γ (16) or TGF-α (17), or after partial pancreatectomy (12). The neogeneic pathway may be more important in adult humans for compensatory expansion of beta cell mass (1, 18, 19) because adult human beta cells have a very low replication (20).
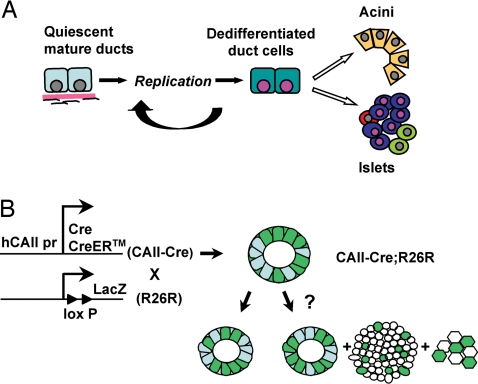
We hypothesize that the progenitors of these new islets were differentiated pancreatic ductal cells that regressed to a less differentiated phenotype after replication and then functioned as progenitors (Fig. 1A) (3, 8). To test this hypothesis we took a direct approach of genetically marking ductal cells by generating transgenic (Tg) mice in which the human carbonic anhydrase II (CAII) promoter drives expression of Cre recombinase (CAII-Cre) or tamoxifen-inducible CAII-CreERTM (Fig. 1B). CAII has been used previously as a marker of differentiated ductal cells (21), which are distinct from the embryonic pancreatic progenitors. Starting late in gestation (embryonic day 18.5), CAII protein is expressed throughout the ductal tree in adult mice but not in the beta cells (22). The human promoter was chosen to provide specificity for tracing the progeny of ductal cells because in human pancreas CAII is expressed only throughout the ductal tree (23), whereas in rodents glucagon-expressing alpha cells also express CAII (22). Thus, in these transgenic mice, if the transgene expression is faithful to that of the promoter, Cre recombinase should be expressed only in the ducts and not the islets. Here, we show that the CAII-expressing cells act as pancreatic progenitors that give rise to both new islets and acini after birth and after injury.
Fig. 1.
Experimental approach to test the hypothesis of ductal cells as pancreatic progenitors. (A) Our hypothesis is that with replication, mature duct cells regress to a less differentiated phenotype and then act as pancreatic progenitors to form new acini, islets, and ducts. (B) Tg mice in which human CAII promoter drives Cre or CreERTM expression were generated (CAII-Cre and CAII-CreERTM) and crossed with a Cre-mediated recombination reporter strain, R26R. In the double-Tg mice (CAII-Cre; R26R), if CAII-expressing ductal cells serve as pancreatic progenitors after birth, we should find β-galactosidase-positive cells not only in ducts but also in islets and acini; however, if duct cells do not act as progenitors, only ducts should be labeled.
Results
We generated Tg mice in which the CAII promoter drives expression of CAII-Cre or inducible CAII-CreERTM (Fig. 1B). Upon mating these transgenic mice with a reporter strain, ROSA26 loxP-Stop-loxP LacZ (R26R) (24), Cre-mediated recombination should result in the permanent marking of CAII-expressing cells and their progeny. Therefore, if the CAII transgene is specifically expressed only in ductal cells and they serve as pancreatic progenitors after birth, we should find β-galactosidase-positive cells not only in ducts but also in islets and acini in double-Tg mice; however, if they do not act as progenitors, only ducts should be labeled (Fig. 1B).
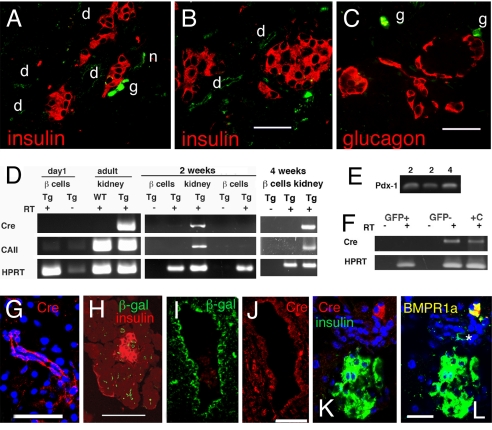
To determine whether the transgene expression was faithful to that of the human promoter, we undertook several assessments. In double-heterozygous CAII-R26R mice, β-galactosidase immunostaining was seen throughout the ducts at birth (Fig. 2 A–C and H) and only rarely at embryonic day 18.5 [supporting information (SI) Fig. S1]; these structures were confirmed as ducts by morphology and by immunostaining (Fig. S2). At birth β-galactosidase expression in pancreatic cell types was restricted to the ducts: its expression was not found in insulin-positive beta cells (Fig. 2 A and B and Figs. S3 and S4), glucagon-positive alpha cells (Fig. 2C and Fig. S5), or acini (Fig. 2 A–C and Figs. S3–S5). CAII is expressed in both the peripheral and central nervous systems (25) but is irrelevant to the current analysis, and CAII neural expression serves as positive control (Fig. 2 A and B). However, because ganglia are intimately associated with islets in the mouse (26), we could not use RNA from isolated islets to exclude the possibility of Cre expression in beta cells of our mice. Therefore, we crossed our Tg mice with MIP-GFP mice (27), dispersed the excised pancreas, and FACS purified GFP+ beta cells from 1-day-old, 2-week-old, 4-week-old, and 8-week-old CAII-Cre:MIP-GFP mice (Fig. S6). In FACS-purified beta cells, no CAII or Cre mRNA was found, even after 40 cycles of PCR amplification, but this mRNA was found in both Tg kidneys (22) and GFP− pancreatic cells (Fig. 2 D and F). The RNA integrity of these samples was verified by probing for pdx1 at 28 cycles (Fig. 2E). To further confirm the specificity of transgene expression, we immunostained for Cre recombinase in pancreas from CAII-Cre and CAII-CreERTM mice: the only Cre-positive cells were in ducts (Fig. 2 G–J) and ganglia (Fig. 2 K and L). Thus, transgene expression appears appropriately restricted.
Fig. 2.
Characterization of transgene expression. (A–C) At birth β-galactosidase immunostaining (green) was seen in ducts (d) and nerve ganglion (g) but not in insulin-positive beta cells (red; A and B), glucagon-positive cells (red; C) or acini of CAII-Cre mice. (D) GFP-expressing beta cells from CAII-Cre:MIP-GFP mice FACS purified for RNA analysis. At day 0 and at 2 and 4 weeks of age, the GFP+ sorted beta cells showed no band for Cre or CAII, even with 40 cycles of amplification. Kidneys from the transgenic (Tg) and non-Tg (WT) animals were positive and negative controls; RT − indicates controls for genomic contamination. (E) The 2- and 4-week RNA samples shown in D were probed for the transcription factor Pdx-1 at 28 cycles to show the integrity of RNA. (F) RNA from 10,000 pancreatic cells, either GFP+ beta cells or GFP− cells from an 8-week-old CAII-Cre:MIP-GFP mouse, was similarly probed for Cre at 40 cycles. +C indicates positive control. (G) Cre immunostaining found in the cytoplasm and nuclei of small ducts (here 4 weeks) in CAII-Cre mice. (H) Expression of β-galactosidase in day 0 small ducts in CAII-Cre mice. (I and J) In CAII-CreERTM mice killed 3 weeks after the end of tamoxifen treatment, Cre and β-galactosidase immunostaining was found in larger ducts (I and J, adjacent sections). Cre staining here was cytoplasmic but not nuclear, and was only in some ductal cells. (K) The only other cells expressing Cre protein were ganglia (insulin, green; Cre, red). (L) The same section as shown in K with overlay of anti-BMPR1A also in green; ganglionic cells expressing BMPR1A are now yellow if expressing Cre or green (*) if not immunostained in previous image. (Scale bars, 50 μm in A–C, G, and I–L and 100 μm in H.)
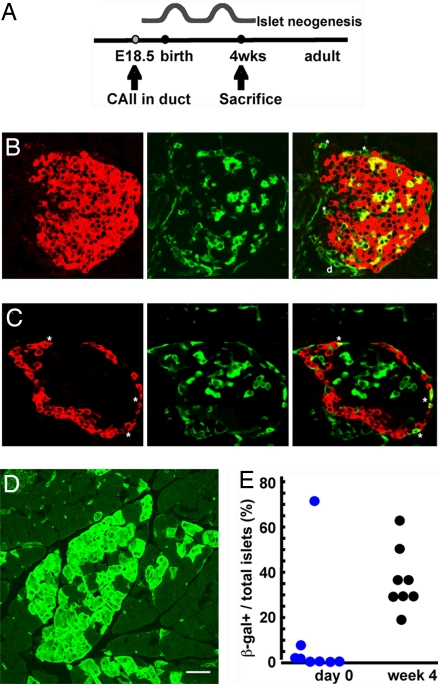
Pancreatic weight increases about 15-fold between birth and 4 weeks of age (28). Using data from our longitudinal study of beta cell mass and its determinants (28), we estimated that more than 30% of the new beta cells seen at day 31 did not arise from replication of preexisting beta cells (3). Therefore, pancreases of double-heterozygous CAII-Cre:R26R mice were analyzed at day 0 and at 4 weeks to determine the contribution of new islets or lobes during normal postnatal development (Fig. 3A). At 4 weeks β-galactosidase expression was found in many ducts, patches of acinar cells, and some islets (Fig. 3 B–D). Within marked islets, both beta and alpha cells were β-galactosidase-positive (Fig. 3 B and C). Islets are formed by coalescence of endocrine cells and are polyclonal in origin (29), so it was expected that islets would have various proportions of β-galactosidase-positive cells. Positive acinar cells were scattered as single cells or small clusters, with few, if any, in some lobes, and as many as 50% in others (Fig. 3D). CAII expression is initiated in ducts only about embryonic day 18, when a large founder population of islets and acini have already been specified; thus, only a fraction of islets and acini are expected to be duct-derived after birth.
Fig. 3.
Testing hypothesis of progenitors in normal neonatal development. (A) CAII starts being expressed at embryonic day 18.5. To test whether neogenesis occurs after birth, double-Tg mice were killed at 4 weeks of age after the expected waves of neogenesis (immediately after birth and around weaning). (B and C) At 4 weeks of age, some islets have costaining of β-galactosidase (green) and insulin (B), or glucagon (C) in red. Double-positive cells are shown as yellow in merged panels. (D) At 4 weeks of age, marked (β-galactosidase-positive) acinar cells are found as single scattered cells or in clusters localized to a lobe, suggesting new lobe formation since birth. (E) Quantification of positive islets/total islets, with each circle representing a single animal. At 4 weeks β-galactosidase+ insulin-positive cells were 16.7% ± 3.0% of all insulin-positive cells (mean: 345/2,268 insulin-positive cells per animal; 8 animals). (Scale bar, 50 μm.)
The β-galactosidase-positive islets as a proportion of total islets showed a substantial increase in the number of marked islets from day 0 to 4 weeks of age (Fig. 3E). At birth few marked islets were seen. Of 189 scored islets from 8 double-Tg mice, 14 islets had β-galactosidase-positive cells; of these, 11 were in 1 animal. We consider this mouse an outlier because there were numerous marked islets (with multiple β-galactosidase-positive insulin or glucagon-positive cells), ducts, and acinar cells (data not shown), indicating an earlier CAII expression; excluding this animal, only 1.7% of islets were marked at day 0. However, at 4 weeks, 143 of 383 islets from 8 animals (37.8%; range, 30–64%) were marked. Thus, formation of new islets and acini from ductal progenitors occurs during the neonatal period. Although we cannot definitively rule out a transient spike of Cre expression from this promoter in pancreatic cell types other than ductal, neither Cre mRNA nor protein was seen in beta cells (Fig. 2 F–L), and such expression in 3 different pancreatic cell types (acinar, beta, and alpha) would be unlikely.
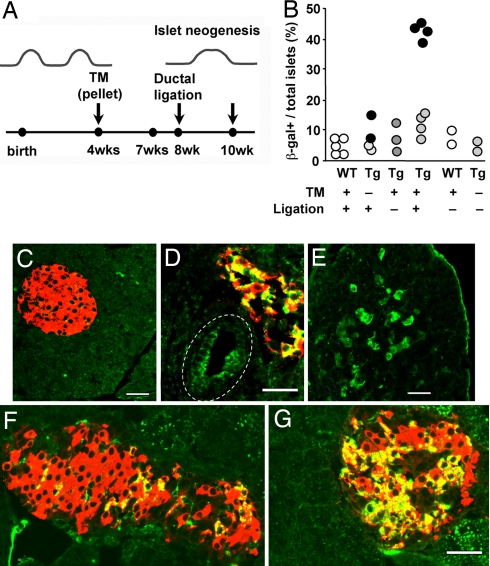
To test the ability of adult pancreatic ductal cells as pancreatic progenitors, we used inducible CAII-CreERTM mice in a regeneration model (Fig. 1B). In CreERTM mice, Cre-mediated recombination is tamoxifen-dependent, providing a pulse–chase type of analysis. Both acinar formation and neogenesis are reported in various regeneration models—for example, partial pancreatectomy, cellophane wrapping (30), and duct ligation (4, 13). Ductal ligation has the advantage of having regeneration localized to the ligated (distal) portion with little to none in the nonligated (proximal) portion (4), allowing comparison of labeling in regenerated and nonregenerated tissue within the same pancreas. To avoid marking the neonatal neogenesis, we implanted tamoxifen pellets in double-CAII-CreERTM:R26R mice at 4 weeks of age for 3-week labeling. Animals underwent ductal ligation at 8 weeks and were killed at 10 weeks (Fig. 4A).
Fig. 4.
Testing hypothesis of progenitors in adult pancreatic regeneration in response to injury. (A) To determine whether new islets are formed in response to injury from duct cells of adult animals, the tamoxifen-inducible CAII-CreERTM double-Tg mice were implanted with 3-week tamoxifen pellets (TM) at 4 weeks of age, had washout period for about 1 week, had duct ligation at 8 weeks, and were killed 2 weeks later. (B) Quantification of positive islets/total islets, with each solid circle representing the mean of a single animal; for ligated Tg, values for pancreas distal to the ligation (dark circles) and for nonligated portion (light circles) are given. The last 2 columns are WT and double Tg at 6 months age, showing the stability of transgene without tamoxifen. In controls or the nonligated portion of the Tg with tamoxifen and ligation (C), there was little β-galactosidase (green) positivity, but distal to ligation in experimentals, some areas had β-galactosidase-marked ducts (D), acini (E), and islets (D, F, and G). In ligated portions, β-galactosidase+ insulin-positive cells were 23.6% ± 2.2% of all insulin-positive cells (mean: 673/2,997 insulin-positive cells; 4 animals), nonligated portions of the same animals were 5.5% ± 2.0% (mean: 110/821 insulin-positive), and in controls were 1.3% ± 1.2% (mean: 11/1,055 cells, 3 WT animals) and 0.9% ± 0.5% (mean: 17/1,785 cells, 4 Tg animals). (D) In this main duct (dashed line), Cre recombination occurred in about half the cells. (E) Expression of β-galactosidase was seen in acinar cells, often localized as to suggest new lobe formation. (F and G) The proportion of positive cells within the marked islets varied as seen in these islets. Shown are expression of β-galactosidase (green), insulin (red), and newly formed beta cells (yellow). (Scale bar, 50 μm.)
Wild-type (10-week-old) animals (Fig. 4B) had a few, low-intensity, β-galactosidase-positive beta cells (5.1% ± 1.2% marked islets, five animals; 1.3% ± 1.2% positive beta cells, 3 animals), with no differences between ligated and nonligated portions. We interpret these to be false-positives independent of CAII promoter expression and resulting from antibody binding to the low level of endogenous β-galactosidase expressed in adult beta cells (31). Similarly low levels of marked islets in 6-month-old wild-type or untreated double-Tg mice further support that false-positives are independent of CAII promoter expression (Fig. 4B). Tg controls (with tamoxifen but no ligation or without tamoxifen but with ligation) (7.4% ± 1.8% islets, n = 5; 0.9% ± 0.5% positive beta cells, 4 animals) did not differ from wild-type animals. The nonligated portions (Fig. 4C) of tamoxifen-treated double-Tg mice had duct cells and an occasional islet or acinus expressing β-galactosidase (Tg: 12.1% ± 1.9% positive islets, P < 0.01 vs. wild type; 5.5% ± 2.0% positive beta cells, n = 4 animals; Fig. 4B); the percentage of marked islets differed from wild type, possibly reflecting a low amount of neogenesis that occurred since the start of the tamoxifen treatment. In contrast, in the ligated portions we found considerably more β-galactosidase-marked islets and acini (42.4% ± 1.2% positive islets in the distal, regenerated pancreas compared with nonligated pancreas of the same animals (P < 0.002, n = 4); the ligated portion had 23.6% ± 2.2% marked beta cells (Fig. 4 D, F, and G). Occasionally, a lobe had many marked acinar cells, suggesting a new lobe (Fig. 4E). No more than 30–50% of total ductal cells (Fig. 4D) were marked, as frequently seen in other Cre recombinase driver lines, thus decreasing the chance of seeing every duct-to-endocrine transition.
Discussion
In these studies we show that the CAII-expressing pancreatic cells act as pancreatic progenitors in neonates and adults. Neural tissue was marked, and so the unlikely possibility that neuronal cells, whether differentiated ones of the enteric nervous system or undifferentiated Phox2b-expressing neural precursors from the neural crest, act as the pancreatic progenitors cannot be completely ruled out. Nonetheless, ductal cells are likely to be the progenitors because: (i) they express CAII in both rodent and human pancreas and are the only pancreatic-specific cells expressing the transgene at birth; (ii) mature ductal cells partially dedifferentiate after replication (3, 8); (iii) many ductal genes are expressed in the newly formed beta cells after partial pancreatectomy and at birth (3); (iv) islet cells form from human pancreatic ductal cells in vitro and in grafts (32, 33). Our data provide strong support to the concept of a shared lineage of ductal, acinar, and islet cells after birth. Although the true progenitors may be only a subpopulation within the ductal epithelium, the transient expression of PDX1 protein, a marker of embryonic pancreatic progenitors, in all replicating duct cells (8) is consistent with most, if not all, having the potential. A similar paradigm for replenishment of cells by dedifferentiation/redifferentiation of a mature phenotype to other organ-specific phenotypes has been reported in the liver (34) and kidney (35).
In summary, we showed by direct lineage tracing that pancreatic CAII-expressing cells give rise to new islet and acinar cells normally after birth and after injury. This identification of a differentiated pancreatic cell type as an in vivo progenitor of all differentiated pancreatic cell types has implications for a potential expandable source for new islets for replenishment therapy for diabetes. Although our emphasis has been on the endocrine pancreas, the implications of our findings extend to pancreatic cancer, which may arise from the uncontrolled growth of progenitor/stem cells.
Methods
Generation of Transgenic Animals.
Carbonic anhydrase II (CAII)Cre and CreERTM constructs were generated with 1.6-kb human CAII promoter from pCAIIXbaI-TtH111I (a gift from T. Takeya, Nara Institute of Science and Technology, Nara, Japan) as described previously (31), and Cre or CreERTM coding sequence from pPDXPB CreERTM (36). Plasmid construction was confirmed by restriction mapping and sequencing of critical regions. DNA was digested with KpnI and AflII, and the CAII-Cre or CreERTM transgenes were isolated and microinjected into the fertilized eggs of C57BL/6 × C57BL/6 mice (Joslin/Brigham and Women's Hospital Transgenic Core). Potential founder animals were screened by PCR and by Southern blotting analysis by using EcoRI digestion and a Cre-specific probe. For PCR, mouse tail DNA was amplified by 32 cycles by using 2 independent sets of Cre-specific primers: 5′-TGATGGACATGTTCAGGGATCG-3′ and 5′-ACCGTCAGTACGTGAGATATCTT-3′; 5′-ACCTGAAGATGTTCGCGATTATCT-3′ and 5′-GATCATCAGCTACACCAGAGAC-3′. All procedures with mice were approved by the Joslin Diabetes Center's Institutional Animal Care and Use Committee. CAII Cre or CreERTM transgenic mice were crossed to ROSA26 reporter mice (R26R) (24). The sequences of the primers to detect R26R alleles were as published (24).
For genetic marking experiments, CreERTM-R26R double-Tg mice had 3-week slow-release (1–2 mg/day) tamoxifen (TM) pellet (Innovative Research of America) implanted. At 4 weeks after implant (washout period of 8 days), these animals had duct ligation. As controls, both double-Tg and R26R-only littermates received tamoxifen without duct ligation, no tamoxifen with duct ligation, or neither tamoxifen nor duct ligation. Two weeks after ligation, animals were killed. Pancreases were excised, fixed in cold 4% paraformaldehyde for 2 h, incubated in 30% sucrose overnight at 4°C, embedded in Tissue-Tek OCT compound (Sakura Finetechnical), and cut into 7- to 10-μm sections.
Sorting of Beta Cells for Verification of Cre Recombinase Expression.
CAII-Cre mice were crossed with homozygous MIP–GFP mice on C7BL/6 background. Under anesthesia, the pancreases of mice at 0, 2, 4, and 8 weeks of age were excised, minced, and incubated in collagenase solution (Liberase RI; Roche) for 20 min at 37°C. After washing in PBS, digested tissue was dispersed by exposure to 1 mg/mL bovine pancreas trypsin (Sigma) and 30 μg/mL DNase I (Roche) in PBS for 15 min at 37°C. After wash with RPMI 1640 plus 10% FBS, they were spun down and resuspended in less than 1 mL of PBS. Cells were passed through a 40-μm filter and stained with propidium iodide (Sigma). All samples were analyzed on a MoFlo cell sorter with Summit software (Cytomation) with gates for live cells (propidium iodide) and beta cells (GFP).
RNA Isolation and Reverse Transcription-PCR.
Total RNA was extracted from freshly sorted beta cells and whole kidneys of the same animals by using an RNAeasy Micro kit (Invitrogen) and TRIzol (GIBCO), respectively. Beta cell cDNA was synthesized by a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and kidney cDNA by SuperScript reverse transcriptase (Life Technologies). The cDNAs were amplified by PCR using specific oligonucleotides for hypoxanthine phosphoribosyltransferase (HPRT), insulin, Cre, and CAII. The primer sequences were: HPRT, 5′-GGGGGCTATAAGTTCTTTGC-3′ and 5′-TCCAACACTTCGAGAGGTCC-3′; insulin, 5′-GACCAGCTATAATCAGAGACC-3′ and 5′-AGTTGCAGTAGTTCTCCAGCT-3′, the first set for Cre given above, and CAII (22). Total RNA without reverse transcription served as a negative control.
Immunohistochemistry.
Tyramide signal amplification system (Renaissance; Perkin–Elmer) was used for anti-β-galactosidase antibody (1:8,000; Cappel); biotin-SP-conjugated donkey anti-rabbit IgG serum (1:400; Jackson ImmunoResearch), streptavidin-horseradish peroxidase (1:100; Perkin–Elmer), and fluorescein-tyramide (1:50) were sequentially applied. Subsequently, guinea pig anti-insulin antibody (1:200; Linco Research), guinea pig anti-glucagon antibody (1:3,000; a gift from M. Appel, University of Massachusetts Medical School, Worcester, MA), or mouse anti-pan-cytokeratin was incubated overnight at 4°C and visualized with a secondary antibody conjugated to Texas red fluorochrome; mouse anti-pan-cytokeratin was incubated overnight, followed by biotinylated goat anti-mouse Ig (1:800) and Cy3-strepavidin (1:800). For Cre localization on adjacent sections, rabbit anti-Cre recombinase (1:800; Novagen) was incubated overnight, followed by biotinylated goat anti-rabbit IgG (1:800) and Cy3-strepavidin (1:800). For identifying ganglia (37), goat anti-BMPR1A (1:100; Santa Cruz Biotechnology) was applied to sections previously stained for Cre and visualized with FITC-conjugated anti-goat IgG (1:200) or on sections stained for β-galactosidase and hormone visualized with 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated anti-goat IgG (1:200). In some slides DAPI was used to identify the nuclei. Confocal images were captured on a Zeiss LSM 410 microscope; images of 1 experiment were taken at the same time under identical settings and handled in Adobe Photoshop similarly across all images.
Quantification of Marked Islets.
Islets (at least 6 insulin-positive cells in cross-section) marked with β-galactosidase were quantified; the slides were coded until after quantification. At day 0 all insulin-positive cells on 1 section were photographed. At 4 weeks of age, all islets on 1 section (8–28 islets per animal) for 5 mice and 2 sections at least 150 μm apart (63–154 total islets per animal) for 3 mice were counted from the slide directly. For duct ligation experiments, at least 2 sections (at least 150 μm apart) from both portions (distal and proximal to the ligation) were scored directly for 4 double-Tg mice and various controls (39–170 total islets per animal). Unpaired 2-tailed Student's t test was used for statistics. The proportions of marked beta cells per total insulin-positive cells were counted on the same slides.
Supplementary Material
Acknowledgments.
We thank Dr. Tatsuo Takeya (Nara, Japan) for providing the human CAII promoter plasmid; Dr. Philippe Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA) for the R26R reporter mice; Dr. Jun-ichi Miyazaki (Osaka, Japan) for the LoxP flanked-reporter plasmid; Dr. Tomoyuki Akashi for help with implantation of tamoxifen pellets; Jennifer Lock, Chris Cahill, Alevtina Pinkhasov, and Maria Petruzzelli for technical assistance; and Drs. Gordon Weir (Joslin Diabetes Center, Boston, MA), and Chris Wright (Vanderbilt University, Nashville, TN) for many helpful discussions. This study was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants to S.B.-W. and to the Joslin Diabetes Center (Diabetes and Endocrinology Research Center Advanced Microscopy and Flow Cytometry Cores), a Juvenile Diabetes Research Foundation grant (S.B.-W.), the Diabetes and Wellness Foundation, and generous private donors. A.I. was a recipient of a Mary Iaccoca Visiting Scientist Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805803105/DCSupplemental.
References
- 1.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2005;5:15–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 5.Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988;37:232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- 6.Jensen JN, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Desai BM, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant human growth hormone-expressing transgenic, and pituitary dwarf mice: Effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 10.Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52:1716–1722. doi: 10.2337/diabetes.52.7.1716. [DOI] [PubMed] [Google Scholar]
- 11.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 12.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of the adult exocrine and endocrine pancreas: A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K, et al. Recombinant human betacellulin promotes the neogenesis of beta-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes. 2000;49:2021–2027. doi: 10.2337/diabetes.49.12.2021. [DOI] [PubMed] [Google Scholar]
- 16.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-gamma transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Wang TC, et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest. 1993;92:1349–1356. doi: 10.1172/JCI116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 19.Yoon KH, et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 20.Tyrberg B, Ustinov J, Otonkoski T, Andersson A. Stimulated endocrine cell proliferation and differentiation in transplanted human pancreatic islets: Effects of the ob gene and compensatory growth of the implantation organ. Diabetes. 2001;50:301–307. doi: 10.2337/diabetes.50.2.301. [DOI] [PubMed] [Google Scholar]
- 21.Hale MA, et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286:225–237. doi: 10.1016/j.ydbio.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Inada A, Nienaber C, Fonseca S, Bonner-Weir S. Timing and expression pattern of carbonic anhydrase II in pancreas. Dev Dyn. 2006;235:1571–1577. doi: 10.1002/dvdy.20754. [DOI] [PubMed] [Google Scholar]
- 23.Kivela AJ, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 24.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 25.Cammer W, Tansey FA. Immunocytochemical localization of carbonic anhydrase in myelinated fibers in peripheral nerves of rat and mouse. J Histochem Cytochem. 1987;35:865–870. doi: 10.1177/35.8.3110266. [DOI] [PubMed] [Google Scholar]
- 26.Ushiki T, Watanabe S. Distribution and ultrastructure of the autonomic nerves in the mouse pancreas. Microsc Res Tech. 1997;37:399–406. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<399::AID-JEMT4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Hara M, et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta-cells. Am J Physiol. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 28.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 29.Deltour L, et al. Polyclonal origin of pancreatic islets in aggregation mouse chimaeras. Development. 1991;112:1115–1121. doi: 10.1242/dev.112.4.1115. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg LA, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidoblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983;35:63–72. doi: 10.1016/0022-4804(83)90127-0. [DOI] [PubMed] [Google Scholar]
- 31.Inada A, Nienaber C, Bonner-Weir S. Endogenous beta-galactosidase expression in murine pancreatic islets. Diabetologia. 2006;49:1120–1122. doi: 10.1007/s00125-006-0186-7. [DOI] [PubMed] [Google Scholar]
- 32.Yatoh S, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 33.Hao E, et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 34.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Fujitani Y, Wright CV, Gannon M. Efficient recombination in pancreatic islets by a tamoxifen-inducible Cre-recombinase. Genesis. 2005;42:210–217. doi: 10.1002/gene.20137. [DOI] [PubMed] [Google Scholar]
- 37.Brewer KC, Mwizerva O, Goldstein AM. BMPRIA is a promising marker for evaluating ganglion cells in the enteric nervous system—A pilot study. Hum Pathol. 2005;36:1120–1126. doi: 10.1016/j.humpath.2005.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.