Abstract
Decomposition is a critical source of plant nutrients, and drives the largest flux of terrestrial C to the atmosphere. Decomposing soil organic matter typically contains litter from multiple plant species, yet we lack a mechanistic understanding of how species diversity influences decomposition processes. Here, we show that soil C and N cycling during decomposition are controlled by the composition and diversity of chemical compounds within plant litter mixtures, rather than by simple metrics of plant species diversity. We amended native soils with litter mixtures containing up to 4 alpine plant species, and we used 9 litter chemical traits to evaluate the chemical composition (i.e., the identity and quantity of compounds) and chemical diversity of the litter mixtures. The chemical composition of the litter mixtures was the strongest predictor of soil respiration, net N mineralization, and microbial biomass N. Soil respiration and net N mineralization rates were also significantly correlated with the chemical diversity of the litter mixtures. In contrast, soil C and N cycling rates were poorly correlated with plant species richness, and there was no relationship between species richness and the chemical diversity of the litter mixtures. These results indicate that the composition and diversity of chemical compounds in litter are potentially important functional traits affecting decomposition, and simple metrics like plant species richness may fail to capture variation in these traits. Litter chemical traits therefore provide a mechanistic link between organisms, species diversity, and key components of below-ground ecosystem function.
Keywords: decomposition, species richness, chemical diversity, litter mixtures
Considerable effort has been invested to elucidate links between species composition, species diversity, and ecosystem function. Significant positive relationships have been found between plant species diversity and numerous above-ground ecosystem processes such as production (1–5). In contrast, the effect of plant species diversity on multitrophic processes such as decomposition has been much more difficult to predict (6, 7). The inability to predict the effect of plant species diversity on decomposition is problematic for multiple reasons. Decomposition of plant leaf and root litter is the most important process supplying nitrogen and phosphorus to plants (6), and decomposition releases 10× as much C into the atmosphere as fossil fuel combustion (8). Moreover, multiple species typically contribute to pools of plant litter, and researchers have reported strong positive and negative effects of diverse plant litter mixtures on litter mass loss, soil respiration, and soil N dynamics (e.g., refs. 9 and 10). It is therefore critical to develop a better mechanistic understanding of how plant litter diversity influences decomposition, and specifically, how plant diversity affects soil C and N dynamics.
Here, we demonstrate that a functional trait approach, which has been shown to link plant species, species diversity, and above-ground ecosystem processes (11–13), can also be used to explain the effects of plant species and plant species diversity on below-ground decomposition processes such as soil C and N cycling. Determining the functional diversity of species assemblages requires 2 steps: first, measurement of functional traits that are components of species' phenotypes known to influence ecosystem level processes, and second, use of functional trait data to compute a metric describing functional trait diversity (11). The first step in developing a functional diversity approach for predicting species effects on soil C and N cycling is to identify functional traits known to influence these processes.
Litter decomposition and soil C and N cycling are driven by microbial enzymes (14), and the production and activity of these enzymes should respond to the amount and type of substrates available within litter mixtures. Consequently, the use of litter chemical substrates as functional traits to predict variation in soil C and N cycling is appealing (15). The degree to which functional litter chemistry traits map onto plant species richness may explain why it has been difficult to predict the effects of plant species richness on soil processes. For example, increasing species richness may not affect decomposition if litter mixtures are composed of chemically similar species. In contrast, litter mixtures composed of chemically diverse species may show significant effects of species richness on decomposition, due to the positive correlation between plant species richness and increased functional diversity of litter compounds.
In this study, we tested the hypothesis that litter chemical composition and litter chemical diversity are important functional traits that explain the effect of diverse litter mixtures on soil C and N cycling. We used soil respiration, net N mineralization, and microbial biomass N measurements to describe soil C and N cycling. To assemble litter mixtures comprised of multiple compounds and spanning a range of chemical diversity, we used litter from 4 abundant, chemically distinct plant species that grow in alpine moist meadows on Niwot Ridge, CO (16, 17): Acomastylis rossii (Rosaceae); Artemisia scopulorum (Asteraceae); Bistorta bistortoides (Polygonaceae); and Deschampsia caespitosa (Poaceae). In a laboratory experiment, we incubated native soil with litter from each species, and litter mixtures comprised of all possible 2, 3, and 4 species combinations. We evaluated the chemical composition of litter mixtures by measuring concentrations of litter chemical traits known to have strong effects on soil C and N cycling (see Materials and Methods). The chemical diversity of litter mixtures was calculated using the proportional abundance of litter chemical traits and the Shannon diversity index (chemical diversity is abbreviated here as H′C, see Materials and Methods). Unlike dissimilarity metrics (11, 15), H′C accounts for the presence and abundance of compounds in litter samples, allowing H′C to describe the chemical diversity of single species and multispecies mixtures. We predicted that litter mixture chemical composition, including interactions among chemical traits, would be a strong predictor of soil C and N cycling rates. We also predicted litter chemical diversity would significantly influence soil C and N cycling, but to a lesser extent than litter chemical composition, because H′C cannot account for interactions among specific chemical traits. To compare our functional trait approach with more traditional metrics, we also evaluated relationships between plant species composition and richness and soil C and N cycling.
Results
Characterization of Study System.
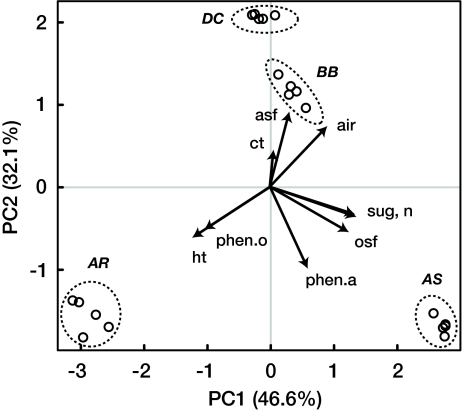
We measured 9 litter chemical traits that together accounted for 100% of litter dry mass, and enabled us to completely define the litter we used as a function of its chemistry. A principal components (PC) analysis based on these chemical traits showed that the 4 species we used werechemically distinct from one another [Fig. 1 and supporting information (SI) Tables S1 and S2]. The first two PC axes explained 78.7% of the variation in the chemical trait data, with PC1 strongly associated with sugar and N concentrations in the positive direction (Fig. 1). The second PC axis was strongly associated with the acid soluble fraction in the positive direction, and phenolic acids in the negative direction (Fig. 1). The third PC axis explained 20.2% of the variation in chemical trait data, and was positively correlated with condensed tannins (data not shown). Condensed tannins were detected only in B. bistortoides, and hydrolyzable tannins were found only in A. rossii (Table S1). The most chemically diverse litter treatment had H′C = 1.57 (A. rossii and B. bistortoides mixture), and the least chemically diverse had H′C = 1.07 (D. caespitosa litter alone). Chemical diversity scores were relatively evenly distributed between the two extremes (Fig. 2, Fig. S1 and Fig. S2).
Fig. 1.
Principal components plot of 9 litter chemistry traits measured for A. rossii (AR), A. scopulorum (AS), B. bistortoides (BB), and D. caespitosa (DC) (open circles). Species abbreviations are in bold italics. Arrows represent loading vectors for litter chemical traits. asf, acid soluble fraction; air, acid insoluble residue; ct, condensed tannins; ht, hydrolyzable tannins; n, nitrogen; osf, residual, unidentified organic soluble fraction (identified organic soluble compounds have been subtracted out); phen.a, phenolic acids (the sum of 4-hydroxybenzoic acid, caffeic acid, chlorogenic acid, ellagic acid, ferulic acid, and gallic acid concentrations); phen.o, “other” low-molecular-weight phenolics (the sum of anthocyanins, catechins, coumarins, and flavonoid glycoside concentrations); and sug, sugars (the sum of myo-inositol, glucose, fructose, sucrose, and raffinose concentrations).
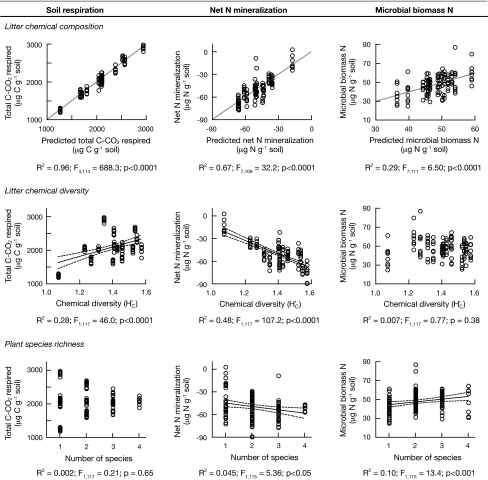
Fig. 2.
Soil respiration, net N mineralization, and microbial biomass N as a function of litter chemical composition (Top), litter chemical diversity (H′C) (Middle), and plant species richness (Bottom). (Top) Predicted values were derived from multiple regression models that used PC axis scores and their interactions as independent variables. Results for net N mineralization and microbial biomass N multiple regressions were obtained using natural log-transformed response variables, but untransformed data are graphed for comparison purposes. Gray lines represent the hypothetical 1:1 relationship between observed values and predicted values from the models. (Middle) The linear regression of soil respiration as a function of litter chemical diversity was performed using natural log-transformed response data, but untransformed data are graphed. (Bottom) For linear regressions of soil respiration and net N mineralization as a function of plant species richness, response variables were natural log-transformed, but untransformed data are graphed. Dashed lines in Middle and Bottom represent the 95% confidence intervals of the regression lines.
Litter mixtures enhanced soil respiration and microbial biomass N, and decreased net N mineralization rates relative to the no-litter treatment to varying degrees (Fig. S1). These data show that the single species litter and litter mixtures we used spanned a range of chemical traits and chemical diversity, and provided a suitable system for testing the influence of litter chemical composition and litter chemical diversity on observed variation in soil C and N cycling. The system also allowed us to evaluate relationships between species composition and chemical composition, and species richness and chemical diversity.
Plant Species Composition and Litter Chemical Composition.
Plant species composition was a strong predictor of litter mixture effects on soil respiration (R2 = 0.96; F3,114 = 884.3; P < 0.0001) and net N mineralization rates (R2 = 0.59; F1,117 = 166.7; P < 0.0001), and was a significant but weak predictor of litter mixture effects on microbial biomass N (R2 = 0.064; F1,117 = 7.97; P < 0.01). Notably, increasing quantitites of A. scopulorum within litter mixtures was positively correlated with soil respiration, and negatively correlated with net N mineralization rates (Table S3). In contrast, increasing quantities of D. caespitosa within litter mixtures was negatively correlated with soil respiration and positively correlated with net N mineralization rates (Table S3). These results show that different species within litter mixtures influenced soil processes in opposing directions. However, these results do not mechanistically explain why plant species composition affected soil C and N cycling.
We used litter chemical composition data to better understand how plant species composition controlled litter mixture effects on soil processes. The first three axes of a PC analysis explained 98.9% of the total variance in litter chemical traits, and scores from these axes were used to describe the chemical composition of litter from single species and multispecies litter mixtures (see Materials and Methods). Compared with plant species composition, litter chemical composition (i.e., the identity and amount of specific compounds in litter) was an equal or better predictor of observed soil respiration rates, net N mineralization rates, and microbial biomass N (Fig. 2 Top). Moreover, different chemical traits influenced soil C and N dynamics in opposing directions (Table 1). For example, chemical traits associated with PC2 were positively correlated with net N mineralization rates, whereas PC3 was negatively correlated with net N mineralization rates (Table 1). The association of chemical traits with the PC axes suggests that the acid soluble fraction was positively correlated with net N mineralization rates, whereas phenolic acids and condensed tannins were negatively correlated with net N mineralization rates (Fig. 1 and data not shown).
Table 1.
Multiple regression analysis of soil C and N responses as a function of the first 3 principal components (PC) axes that describe litter chemical composition
| Soil response | PC axis | Slope (SE) | R2 | t | P |
|---|---|---|---|---|---|
| Total CO2 respired | PC1 | 163.7 (5.9) | 0.262 | 27.7 | <0.0001 |
| PC2 | −292.7 (7.1) | 0.609 | −41.2 | <0.0001 | |
| PC3 | 134.1 (8.9) | 0.087 | 15.1 | <0.0001 | |
| Net N mineralization | PC1 | 0.02 (0.03) | 0.008 | 0.74 | 0.46 |
| PC2 | 0.25 (0.02) | 0.275 | 10.8 | <0.0001 | |
| PC3 | −0.42 (0.04) | 0.223 | −10.8 | <0.0001 | |
| Microbial biomass N | PC1 | 0.02 (0.02) | 0.066 | 0.68 | 0.50 |
| PC2 | 0.04 (0.02) | 0.007 | 2.3 | <0.05 | |
| PC3 | −0.04 (0.03) | 0.001 | −1.3 | 0.19 |
Multiple regression models contained scores from the first 3 PC axes, and significant interaction terms among PC axes, which are not shown here. Data for total CO2 respired were not transformed, and net N mineralization and microbial biomass N data were natural log-transformed prior to analysis.
Multiple regression models allowed us to determine whether interactions among litter chemical traits (i.e., interactions among PC axis scores) were important in predicting soil C and N cycling dynamics. Interactions among litter compounds played a minor role in influencing soil respiration rates (Rinter2 = 0.002, where Rinter2 is the amount of variation in the data explained by interactions among litter compounds, see Materials and Methods). For net N mineralization rates, all four possible interactions among the PC axes (PC1*PC2, PC1*PC3, PC2*PC3 and PC1*PC2*PC3) were significant in the model (Rinter2 = 0.17), and all four interaction terms were also significant predictors of microbial biomass N (Rinter2 = 0.22). Combined with the sensitivity of microbial biomass N to litter compounds with low abundance (see Materials and Methods), these results indicate rare litter compounds were involved in interactions that influenced the microbial biomass N response. Taken together, the results show that interactions among litter compounds had noticeably stronger effects on soil N cycling than on soil C cycling.
Plant Species Richness and Litter Chemical Diversity.
Soil respiration rates were not correlated with plant species richness alone (Fig. 2 Bottom), but after controlling for plant species composition, there was a weakly positive correlation between soil respiration and plant species richness (R2 = 0.002; F1,113 = 5.41; P < 0.05). However, reduced net N mineralization rates were correlated with increasing plant species richness (Fig. 2 Bottom), and microbial biomass N was positively correlated with plant species richness (Fig. 2 Bottom). Controlling for plant species composition did not statistically change the effect of plant species richness on either net N mineralization or microbial biomass N (i.e., no change in slope, R2, or P value associated with plant species richness, data not shown).
Litter chemical diversity was positively correlated with soil respiration, and negatively correlated with net N mineralization, and, although R2 was relatively low for these regressions (< 0.5), in both cases H′C was a much stronger predictor than plant species richness (Fig. 2 Middle and Bottom). Microbial biomass N was not correlated with H′C (Fig. 2 Middle). After controlling for litter chemical composition, the correlation between soil respiration and H′C was still positive, but weaker and marginally significant (R2 = 0.001; F1,113 = 3.68; P = 0.057). Similarly, the correlation between net N mineralization and H′C remained significantly negative, but was weaker after controlling for litter chemical composition (R2 = 0.076; F1,112 = 31.9; P < 0.0001). In contrast, we observed a significant positive correlation between microbial biomass N and H′C (R2 = 0.075; F1,112 = 9.72; P < 0.01) after variation attributable to litter chemical composition was removed.
Comparing traditional metrics used to describe litter mixtures (i.e., species composition and richness) with the functional trait metrics presented here (chemical composition and diversity), we found species richness to be the poorest predictor of soil respiration and net N mineralization rates (Fig. 2). Regressions between plant species richness and PC axis scores were all nonsignificant (R2 = 0.00, P > 0.99 for all PC axes), indicating that plant species richness was not correlated with the chemical composition of litter. Plant species richness was also not correlated with H′C (R2 = 0.09, P = 0.28) (Fig. S2). As such, variation in plant species richness did not capture variation in the functional chemical traits (i.e., litter chemical composition and diversity) that significantly influenced soil C and N cycling.
Discussion
Plant litter chemical composition is an important functional trait explaining the strong effects of plant litter mixtures on below-ground ecosystem function. Variation in soil C and N cycling was also significantly correlated with the functional diversity of litter chemical traits. These results support the idea that key soil processes are governed by interactions between plant litter chemical traits and the microbial enzymes that catalyze decomposition reactions. Moreover, functional litter chemical traits were not related to the species richness of the litter mixtures. The lack of a strong relationship between plant species richness and litter chemical composition and diversity may explain why plant species richness was a poor predictor of soil C and N cycling in our study and others (6, 9, 10, 15, 18, 19).
Influence of Litter Chemical Composition.
We found that the chemical composition of litter amendments was the best overall predictor of soil processes compared with plant species composition, plant species richness, and litter chemical diversity (Fig. 2 and Results). Litter chemical traits appear to be the most important controls on the soil C and N cycling rates we measured. Based on our results, we suggest three nonmutually exclusive mechanisms that explain why litter chemical composition was the most successful predictor of soil C and N cycling. First, chemical traits influenced soil C and N mineralization in opposing directions, and some traits influenced these processes far more than others (Table 1). Accounting for specific compounds and their unique effects was critical for predicting soil C and N mineralization rates. This result is consistent with other studies showing that soil C and N dynamics are differentially affected by the identity and specific structure of chemical substrates entering soil (20–23).
Second, interactions among chemical traits (i.e., PC axis scores) explained a significant percentage of the variation in the N cycling data associated with litter chemical composition (25.4% and 74.2% for net N mineralization and microbial biomass N, respectively). Although we could not specifically identify which compounds interacted to control soil N cycling, the concept that N mineralization rates are linked to the amount and type of available C in litter is well established (24, 25). For example, because tannins bind to N-containing substrates (20, 21), N mineralization and microbial biomass N accumulation could depend on interactions between litter N content and litter tannin concentrations.
Finally, we found significantly more variation in microbial biomass N could be explained with multiple regression models, using PC axis scores derived from scaled litter chemistry measurements than with unscaled litter chemistry measurements (see Materials and Methods). Scaling the variance to one before performing the PC analysis makes the analysis more sensitive to compounds with low concentration. Combined with the fact that interactions among compounds are significant predictors of microbial biomass N (discussed above), our results indicate that litter compounds with low concentration influence microbial biomass N via interactions with other compounds. For example, litter contains relatively low concentrations of both N and labile C (e.g., sugars and LMW phenolics), which can interact to influence microbial biomass N (26).
Influence of Litter Chemical Diversity.
Soil respiration and net N mineralization rates were also significantly correlated with the diversity of functional litter chemical traits (Fig. 2 and Results). After the effect of litter chemical composition was removed, increasing microbial biomass N was positively correlated with litter chemical diversity. Mechanistically, litter chemical diversity may have influenced soil C and N mineralization because of the increased probability of chemically diverse litter mixtures containing compounds with strong effects on these processes—i.e., the sampling effect (27). Chemical diversity may also have affected soil C and N cycling because chemically diverse litter mixtures would increase the probability of different chemical groups reacting with each other and with soil microbes in nonadditive ways—i.e., the complementarity effect (27). For example, chemically diverse litter mixtures would contain a more even representation of both labile and recalcitrant compounds. Increasing litter chemical diversity could therefore stimulate soil respiration via “priming” of recalcitrant C-substrates. Priming, the process by which labile C or N stimulates mineralization of recalcitrant C (28, 29), could take place more frequently in chemically diverse litter mixtures because the cooccurrence of labile and recalcitrant compounds would be more likely. Chemically diverse litter mixtures could similarly influence net N mineralization. Interactions among N-containing substrates and C-based compounds that have strong positive or negative effects on N cycling would be more likely in chemically diverse mixtures (6).
Our results also show that, although litter chemical diversity had a significant effect on key soil processes, it was unable to explain a high proportion of variation in the data (R2 was relatively low for H′C in most instances). One likely explanation is that H′C cannot explicitly account for litter chemical traits that influence soil processes in opposing directions (shown to occur in Table 1). Another reason for low R2 values associated with litter chemical diversity is that H′C cannot incorporate either the presence or strength of interactions among chemical traits, and interactions were clearly important for predicting soil N cycling.
Finally, the lowest level of H′C was represented by only one species (D. caespitosa), and the unique chemical composition of D. caespitosa litter was therefore a confounding factor at the lowest level of H′C. Although litter chemical diversity remained a significant predictor of soil C and N dynamics after controlling for the effects of litter chemical composition, it is unclear whether other species with low chemical diversity would show similar results to those we obtained with D. caespitosa litter.
Conclusions
There is an inherent disconnect between functional trait composition and diversity, which are known to influence ecosystem processes, and taxonomic diversity (e.g., species richness), which is often experimentally measured and manipulated but may be unrelated to functional traits (3, 12). This disconnect is obvious in the literature dealing with the effects of plant species richness on litter decomposition and soil C and N cycling (6, 15, 18), with researchers reporting positive, negative, neutral and stochastic effects of plant species richness on these processes (9, 10, 19, 30). In this study, we experimentally demonstrated that litter chemical composition and diversity are significant functional traits influencing soil C and N cycling, and these functional traits were not correlated with the species richness of plant litter mixtures. In combination with other recent studies (15, 23), our results contribute to a new mechanistic framework linking functional traits, plant species diversity, and key components of below-ground ecosystem function.
Materials and Methods
Soil and Litter Incubation Experiment.
We examined soil C and N cycling with a laboratory incubation experiment. We mixed native alpine soil with litter from 4 abundant, alpine moist meadow species: A. rossii, A. scopulorum, B. bistortoides, and D. caespitosa. Litter was collected from an alpine moist meadow on Niwot Ridge, CO (a National Science Fountation Long Term Ecological Research site at 40°03′N, 105°35′W, 3,500 m elevation), in September 2004. Litter was freeze-dried without prefreezing, and dried litter was cut into 1-cm pieces and stored in paper bags at room temperature before experimental use. Soil was collected after snow-melt in early June 2006 from an alpine moist meadow on Niwot Ridge, and was then sieved (2-mm mesh), homogenized, and stored in sealed containers at 4 °C before mixing with the litter. Based on field studies of soil microclimate and microbial community composition on Niwot Ridge (31), these conditions did not limit microbial taxa from growing under the experimental conditions.
Immediately before we amended soils with plant litter, we measured initial total inorganic N (TIN) concentrations and initial microbial biomass N on the bulked soil (10 subsamples per analysis) (ref. 32 and SI Methods). To construct litter treatments, we mixed soil with litter from each of the 4 species, and all 2, 3, and 4 species combinations, and a soil only control (16 total treatments, 8 replicates per treatment). We mixed 0.75 g of litter with 50 g of dry-weight soil in 120-mL polypropylene specimen cups. The amount of litter used was based on estimates of litter inputs for moist meadows at our field site. Litter mixtures contained equal mass of all component species. Litter/soil treatments were maintained between 50% and 60% gravimetric moisture and were kept at 8 °C in a growth chamber for 6 weeks (mean growing season temperature, ref. 17).
We assessed litter effects on short-term soil C dynamics by measuring soil respiration daily with an infra-red gas analyzer (LI-COR 6200) for the first 9 days, and then either every other day or every third day for the remainder of the 6 wk experiment. The total CO2 respired was calculated by plotting respiration rate versus time and obtaining the area under the curve (PrismGraph software, version 4.0). Final TIN and microbial biomass N were also determined (SI Methods).
Litter Chemistry Analyses.
We quantified functional litter chemistry traits for A. rossii, A. scopulorum, B. bistortoides, and D. caespitosa by measuring percentages of N, percentage of condensed tannins (CT), percentage of hydrolyzable tannins (HT), low-molecular-weight (LMW) phenolic acid concentrations, other LMW phenolic concentrations, and the percentage litter mass associated with standard proximate C fractions [an organic soluble fraction (OSF), an acid soluble fraction (ASF), and the acid insoluble residue (AIR)] (see Table S1). These functional chemical traits were chosen because of their demonstrated effects on soil C and N dynamics (20, 21, 23, 24, 33, 34). Chemical trait data for the single species were then used to determine the litter chemical composition and chemical diversity of the 2, 3, and 4 species mixtures.
Plant litters were analyzed for %N with a Shimadzu elemental analyzer. Litter sugar extractions (myo-inositol, glucose, fructose, sucrose, and raffinose) were performed as described in ref. 17. Sugars were then quantified using high-performance anion-exchange chromatography-PAD (35). Low molecular-weight phenolic and hydrolyzable tannin concentrations in litter were quantified via high pressure liquid chromatography (HPLC), as described in ref. 36. We compared spectra and retention times from unknown phenolic peaks with those of external standards to assign unknown peaks to representative LMW phenolic and tannin classes (36). Extractable condensed tannins (CT) and cell-wall bound CT were quantified using the acid-butanol assay (37 and SI Methods). To account for soluble compounds we could not identify, and to quantify recalcitrant C (e.g., lignin and cellulose), we performed a proximate C fractionation on litter from each plant species (34 and SI Methods).
Characterizing Litter Chemical Composition and Chemical Diversity.
We used litter chemistry measurements to calculate the chemical composition and chemical diversity of the litter amendments. The composition of functional chemical traits within single species and litter mixtures was calculated using scores from the first three axes of a principal components analysis (Fig. 1 and SI Methods). We used a principal components approach rather than concentrations of the chemical traits themselves because some chemical traits covaried with each other, which prevented the use of multiple regression models. Linear combinations of the single species scores for PC1, PC2, and PC3 were used to calculate axis scores for all litter mixtures.
Chemical trait diversity (H′C) was calculated for each litter treatment using the equation for the Shannon diversity index:
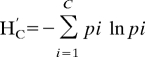 |
As defined, C is the total number of chemical traits present in a given litter treatment, and pi is the proportion of the total mixture dry weight for chemical trait i.
Statistical Analyses.
The soil C and N responses to the litter treatments were modeled as a function of litter chemical composition, litter chemical diversity (H′C), and plant species richness. To model the soil C and N response as a function of litter chemical composition, we used PC scores as independent variables, and a model containing 3 PC axis scores and all possible interactions was constructed (for a total of 3 primary terms and 4 interaction terms). We then used a stepwise forward and backward elimination procedure and the hierarchy principle to arrive at the most parsimonious model that fit the data. For analyses employing PC scores as independent variables, P < 0.10 was used as the cutoff for including terms in the model. To determine the amount of variation explained by interaction terms (Rinter2), sums of squares for all significant interaction terms were added and the total interaction sum of squares was then divided by the total sum of squares for the entire model (including residual sum of squares).
For all analyses, the response variables were natural log-transformed as necessary to meet the assumptions of parametric statistics. Quantile-quantile plots were used to assess the normality of residuals, and data were tested for homogeneity of variance with fitted versus residual plots. A priori contrasts with a Bonferroni corrected alpha were used to determine whether soil respiration, net N mineralization, and microbial biomass N responses to the litter treatments were significantly different from the controls. Statistical analyses were performed with R software, version 2.6.2.
Supplementary Material
Acknowledgments.
We thank John Murgel, Melissa Maxa, Ken Keefover-Ring, Volker Ebbert, Dan Fernandez, Kim Lohnas, Jia Hu, Noah Fierer, and Laura Scott-Denton for field and laboratory assistance. This work was supported by the Andrew W. Mellon Foundation, the National Science Foundation-sponsored Niwot Ridge Long-Term Ecological Research program, the University of Colorado Mountain Research Station, the John Marr Memorial Ecology Fund, and a University of Colorado Department of Ecology and Evolutionary Biology grant (to C.L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805600105/DCSupplemental.
References
- 1.Hector A, et al. Biodiversity and ecosystem functioning: Reconciling the results of experimental and observational studies. Funct Ecol. 2007;21:998–1002. [Google Scholar]
- 2.Fargione JE, Tilman D. Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett. 2005;8:604–611. [Google Scholar]
- 3.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 4.Loreau M, et al. Ecology - Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 5.Fargione J, et al. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc R Soc B-Biol Sci. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hättenschwiler S, Tiunov AV, Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol S. 2005;36:191–218. [Google Scholar]
- 7.Scherer-Lorenzen M. Functional diversity affects decomposition processes in experimental grasslands. Funct Ecol. 2008;22:547–555. [Google Scholar]
- 8.Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Global Change Biol. 2000;6:751–765. [Google Scholar]
- 9.Wardle DA, Bonner KI, Nicholson KS. Biodiversity and plant litter: Experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos. 1997;79:247–258. [Google Scholar]
- 10.Bardgett RD, Shine A. Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol Biochem. 1999;31:317–321. [Google Scholar]
- 11.Petchey OL, Gaston KJ. Functional diversity: Back to basics and looking forward. Ecol Lett. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 12.Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: Deriving solutions to a seemingly insurmountable problem. Ecol Lett. 2003;6:567–579. [Google Scholar]
- 13.Spehn EM, et al. Ecosystem effects of biodiversity manipulations in European grasslands. Ecol Monogr. 2005;75:37–63. [Google Scholar]
- 14.Sinsabaugh RL, Carreiro MM, Repert DA. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry. 2002;60:1–24. [Google Scholar]
- 15.Epps KY, Comerford NB, Reeves JB, Cropper WP, Araujo QR. Chemical diversity - highlighting a species richness and ecosystem function disconnect. Oikos. 2007;116:1831–1840. [Google Scholar]
- 16.Dearing MD. In: Structure and Function of an Alpine Ecosystem. Bowman WD, Seastedt TR, editors. Oxford: Oxford Univ Press; 2001. pp. 266–282. [Google Scholar]
- 17.Bowman WD, Steltzer H, Rosenstiel TN, Cleveland CC, Meier CL. Litter effects of two co-occurring alpine species on plant growth, microbial activity and immobilization of nitrogen. Oikos. 2004;104:336–344. [Google Scholar]
- 18.Gartner TB, Cardon ZG. Decomposition dynamics in mixed-species leaf litter. Oikos. 2004;104:230–246. [Google Scholar]
- 19.Spehn EM, Joshi J, Schmid B, Alphei J, Korner C. Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant Soil. 2000;224:217–230. [Google Scholar]
- 20.Fierer N, Schimel JP, Cates RG, Zou JP. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem. 2001;33:1827–1839. [Google Scholar]
- 21.Kraus TEC, Zasoski RJ, Dahlgren RA, Horwath WR, Preston CM. Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem. 2004;36:309–321. [Google Scholar]
- 22.Nierop KGJ, Preston CM, Verstraten JM. Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol Biochem. 2006;38:2794–2802. [Google Scholar]
- 23.Orwin KH, Wardle DA, Greenfield LG. Ecological consequences of carbon substrate identity and diversity in a laboratory study. Ecology. 2006;87:580–593. doi: 10.1890/05-0383. [DOI] [PubMed] [Google Scholar]
- 24.Palm CA, Sanchez PA. Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic contents. Soil Biol Biochem. 1991;23:83–88. [Google Scholar]
- 25.Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA. A comparative-analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol Monogr. 1982;52:155–177. [Google Scholar]
- 26.Gallardo A, Schlesinger WH. Factors determining soil microbial biomass and nutrient immobilization in desert soils. Biogeochemistry. 1995;28:55–68. [Google Scholar]
- 27.Cardinale BJ, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA. 2007;104:18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamer U, Marschner B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol Biochem. 2005;37:445–454. [Google Scholar]
- 29.Fontaine S, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007;450:277–U210. doi: 10.1038/nature06275. [DOI] [PubMed] [Google Scholar]
- 30.Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH. Consequences of the reduction of plant diversity for litter decomposition: Effects through litter quality and microenvironment. Oikos. 2000;90:357–371. [Google Scholar]
- 31.Lipson DA, Schadt CW, Schmidt SK. Changes in soil microbial community structure and function in an alpine dry meadow following spring snow melt. Microbial Ecol. 2002;43:307–314. doi: 10.1007/s00248-001-1057-x. [DOI] [PubMed] [Google Scholar]
- 32.Fierer N, Schimel JP. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J. 2003;67:798–805. [Google Scholar]
- 33.Parton W, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science. 2007;315:361–364. doi: 10.1126/science.1134853. [DOI] [PubMed] [Google Scholar]
- 34.Valachovic YS, Caldwell BA, Cromack K, Griffiths RP. Leaf litter chemistry controls on decomposition of Pacific Northwest trees and woody shrubs. Can J Forest Res. 2004;34:2131–2147. [Google Scholar]
- 35.Moore BD, Palmquist DE, Seemann JR. Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol. 1997;115:241–248. doi: 10.1104/pp.115.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier CL, Suding KN, Bowman WD. Carbon flux from plants to soil: Roots are a below-ground source of phenolic secondary compounds in an alpine ecosystem. J Ecol. 2008;96:421–430. [Google Scholar]
- 37.Hagerman AE. Tanin Chemistry Handbook. Oxford, OH: Miami University; 2002. Available at www.users.muohio.edu/hagermae/tannin.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.