Abstract
The mechanisms by which ethanol damages the developing and adult central nervous system (CNS) remain unclear. Activity-dependent neuroprotective protein (ADNP) is a glial protein that protects the CNS against a wide array of insults and is critical for CNS development. NAPVSIPQ (NAP), a potent active fragment of ADNP, potentiated axon outgrowth in cerebellar granule neurons by activating the sequential tyrosine phosphorylation of Fyn kinase and the scaffold protein Crk-associated substrate (Cas). Pharmacological inhibition of Fyn kinase or expression of a Fyn kinase siRNA abolished NAP-mediated axon outgrowth. Concentrations of ethanol attained after social drinking blocked NAP-mediated axon outgrowth (IC50 = 17 mM) by inhibiting NAP activation of Fyn kinase and Cas. These findings identify a mechanism for ADNP regulation of glial–neuronal interactions in developing cerebellum and a pathogenesis of ethanol neurotoxicity.
Keywords: cerebellum, Crk-associated substrate, fetal alcohol spectrum disorders, Fyn kinase
Alcohol exposure during pregnancy causes growth retardation, birth defects, and neurodevelopmental abnormalities, referred to as fetal alcohol spectrum disorder (FASD). Children with FASD show a spectrum of developmental abnormalities in different brain regions; among these, the cerebellum is particularly affected (1, 2). The prevention and treatment of FASD require a thorough understanding of its pathophysiology; however, the mechanisms underlying ethanol's developmental neurotoxicity remain unclear.
Prenatal ethanol exposure causes disordered neuronal migration, disruption of axonal and dendritic connectivity, death of neuronal and glial progenitors, and neuronal apoptosis (3–5). Numerous mechanisms of ethanol-induced neuronal death have been identified; however, one potentially important mechanism has not been sufficiently explored: cell death triggered by axonal injury. Axonal injury is sometimes an early event in neuronal death, and differential axon vulnerability might account for the region-specific damage observed in diverse neurological disorders (6), including those associated with alcoholism. Indeed, ethanol has been observed to inhibit neurite outgrowth before causing neuronal death (7).
The development of neuronal processes is regulated by a variety of molecular cues (8), many of which are derived from glial cells. Hence, ethanol might damage axons by disrupting the regulation of neuronal differentiation by glial-derived molecules. One such molecule is particularly worthy of study. Activity-dependent neuroprotective protein (ADNP) is an astrocyte-derived protein that is essential for brain development (9–11). ADNP is highly expressed in cerebellum, a brain region that is selectively vulnerable to ethanol toxicity during development and adult life (1, 12). The octapeptide NAPVSIPQ (NAP), an active fragment of ADNP, has been shown to potently protect the nervous system against a wide range of insults (11), including prenatal alcohol exposure (13). More recently, NAP has been shown to promote neurite outgrowth in several types of cultured neurons (11, 14) through mechanisms that are incompletely understood. To learn whether ethanol disrupts ADNP-stimulated neuronal differentiation, we first established that NAP stimulates axon outgrowth in cerebellar granule neurons (CGNs) and characterized the underlying signaling pathways. We then asked whether ethanol blocks NAP-mediated axon outgrowth by disrupting NAP signaling.
Results
Effects of NAP on Axon Outgrowth in Cerebellar Granule Neurons.
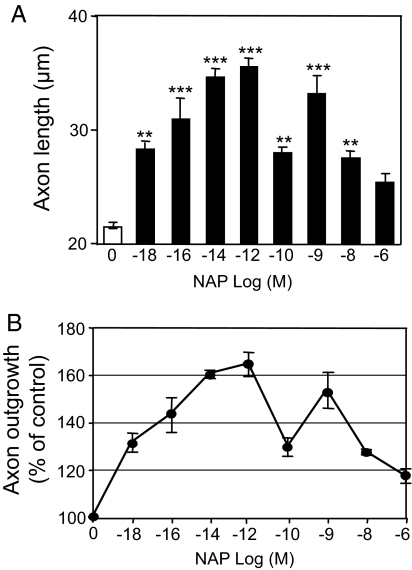
Although NAP has been shown to stimulate neurite outgrowth in a variety of cultured neurons, the effects of NAP on cerebellar neurons have not been investigated. Postnatal day 7 (PD7) rat CGNs were cultured for 20 h in serum-free medium supplemented with a range of concentrations of NAP (Fig. 1). At this time point, the majority (>95%) of CGNs gave rise to a single long process that stained positively with Tau-1 antibodies, identifying the process as an axon (15). NAP induced a bimodal, dose-dependent increase in axon length (Fig. 1) over a broad range of concentrations. The first limb of the dose–response curve showed a significant initial response at 10−18 M and a maximal increase in axon outgrowth of 64.8% at 10−12 M. The second limb showed a peak response at 10−9 M and a progressive decline at concentrations >10−8 M (Fig. 1B).
Fig. 1.
NAP enhances axon outgrowth of CGNs in a concentration-dependent manner. CGNs were cultured under serum-free conditions in the presence of the indicated concentrations of NAP. Axon length was measured 20 h after cell plating. (A) Shown is the mean ± SEM axon length determined from 3 independent experiments. F = 21.37; P < 0. 0001 for the main effect; **, P < 0.01; ***, P < 0.001 vs. control. (B) Percentage increase in axon outgrowth.
Signal Transduction Molecules Implicated in NAP-Mediated Axon Outgrowth.
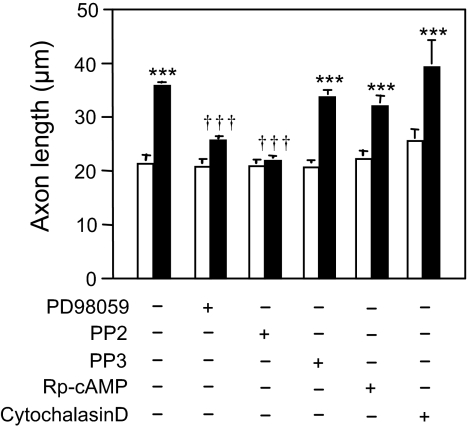
To determine the molecular mechanisms by which NAP potentiates axon outgrowth, we cultured CGNs in the absence and presence of NAP and concentrations of kinase inhibitors that selectively inhibit signal transduction in CGNs (16). None of the inhibitors significantly affected axon outgrowth in the absence of NAP (Fig. 2). NAP potentiation of axon outgrowth was abolished by 5 μM 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), an inhibitor of Src family kinases (SFKs) (17), but was not affected by 5 μM 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3), an inactive analog of PP2. NAP potentiation of axon outgrowth was significantly reduced by 10 μM 2′-amino-3′-methoxyflavone (PD98059), an inhibitor of mitogen-activated protein kinase kinase (MEK), but not by 100 μM adenosine 3′,5′-cyclic phosphorothioate-Rp isomer (Rp-cAMP), an inhibitor of protein kinase A (PKA), or cytochalasin D (100 nM), an inhibitor of F-actin polymerization. These data suggest that a SFK is a major intracellular mediator of NAP-stimulated axon outgrowth in CGNs, although other pathways may also be important.
Fig. 2.
Effects of signal transduction inhibitors on NAP-mediated axon outgrowth. CGNs were cultured as in Fig. 1. Signal transduction inhibitors were used in the absence (empty bars) or presence (filled bars) of 10−12 M NAP at the following concentrations: 10 μM PD98059, 5 μM PP2, 5 μM PP3, 100 μM Rp-cAMP, 100 nM cytochalasin D. Shown is the mean ± SEM axon length derived from 3 independent experiments. F = 31.43; P < 0. 0001 for the main effect; ***, P < 0.001 vs. control; †††, P < 0.001 vs. NAP-treated cells.
Knockdown of Fyn Kinase Expression Abolishes NAP-Mediated Axon Outgrowth.
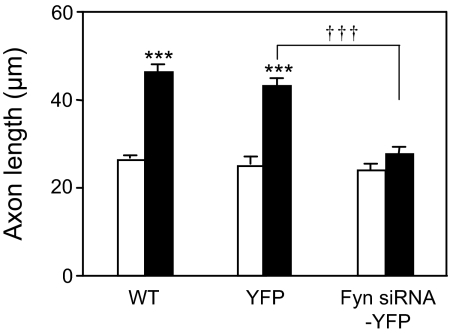
Tyrosine phosphorylation of Fyn kinase at Y416 is required for ligand induction of axon outgrowth in cortical neurons (18). To determine whether Fyn kinase is necessary for NAP-mediated axon outgrowth, we used Fyn siRNA coupled to a YFP reporter (Fyn siRNA-YFP) to knock down the expression of Fyn in CGNs. Fyn kinase expression and axon outgrowth were analyzed 48 h after transfection. Individual CGNs that expressed Fyn siRNA-YFP showed nearly absent immunostaining with an anti-Fyn kinase antibody (Fig. S1, arrow); in contrast, CGNs transfected with YFP alone showed normal levels of Fyn kinase (Fig. S1, arrowhead).
Axons associated with YFP-expressing and Fyn siRNA-YFP-expressing CGNs were identified by comparing bright-field and fluorescence images from the same cells. Axon length was then quantified from the bright-field images. Transfection of CGNs with Fyn siRNA-YFP or YFP had no significant effect on basal axon outgrowth (Fig. 3). Treatment with 10−12 M NAP produced a robust increase in axon outgrowth in WT and YFP-transfected CGNs, but had no effect in Fyn kinase siRNA-YFP-transfected CGNs (Fig. 3). These results indicate that Fyn kinase is necessary for NAP-mediated axon outgrowth in CGNs.
Fig. 3.
Effect of Fyn kinase knockdown on NAP-mediated axon outgrowth. CGNs were transfected with YFP or Fyn siRNA-YFP plasmids by using nucleofection. Twenty-four hours after transfection, the medium was supplemented with 0 (empty bars) or 10−12 M NAP (filled bars). The cells were cultured for an additional 24 h and then fixed for immunostaining and analysis of axon outgrowth. Altogether, 23% of CGNs expressed Fyn siRNA-YFP. Shown is the mean ± SEM axon length of nontransfected (WT), YFP-transfected (YFP), or Fyn siRNA-YFP-transfected (Fyn siRNA-YFP) CGNs. F = 54.32; P < 0. 0001 for the main effect; ***, P < 0.001 between control and NAP-treated CGNs; †††, P < 0.001 between NAP-treated YFP and NAP-treated Fyn siRNA-YFP CGNs.
NAP Activates Fyn Kinase.
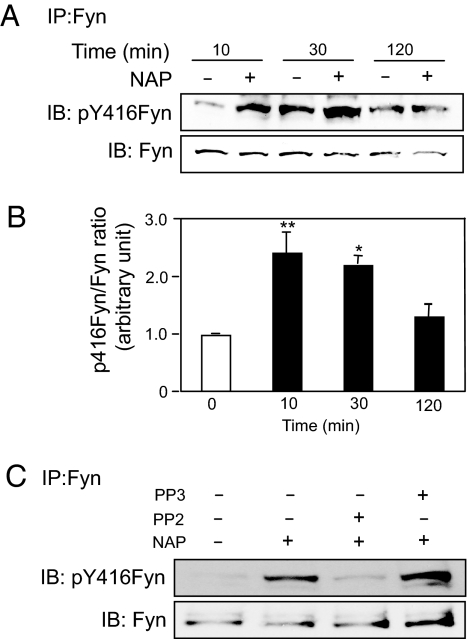
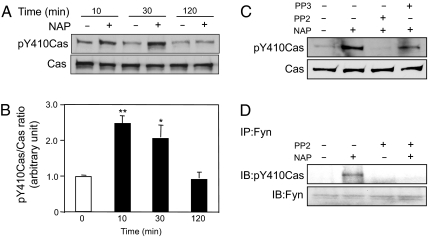
To determine whether NAP activates Fyn kinase, we measured Y416 phosphorylation in immunoprecipitates of Fyn kinase from control and NAP-treated CGNs. Incubation of CGNs for 10 min with 10−12 M NAP caused a significant increase in Y416 phosphorylation of Fyn kinase (Fig. 4). Levels of Y416 phosphorylation remained significantly increased 30 min after NAP treatment and declined to control levels by 2 h (Fig. 4 A and B). NAP-induced Y416 phosphorylation of Fyn kinase was inhibited by PP2, but not by PP3 (Fig. 4C). These results indicate that NAP activates Fyn kinase in CGNs.
Fig. 4.
NAP activation of Fyn kinase in CGNs. CGNs at day 1 in vitro (DIV1) were incubated for the indicated times in the absence or presence of 10−12 M NAP. Cell lysates were immunoprecipitated (IP) with polyclonal anti-Fyn antibodies. (A and C) The resulting immunocomplexes were subjected to immunoblotting (IB) using antibody against pY416 of SFK (Upper) and reprobed with monoclonal anti-Fyn antibody (Lower). (A) Time course for NAP activation of tyrosine phosphorylation of Fyn kinase at Y416. Shown is a representative gel from 1 of 3 experiments. (B) Densitometry of gels described in A. Shown is the mean ± SEM ratio of pY416Fyn to total Fyn in CGNs treated without (empty bar) or with (filled bars) NAP 10−12 M for the indicated times. F = 11.63; P = 0. 0027 for the main effect; *, P < 0.05; **; P < 0.01 vs. non-NAP-treated cells. (C) A representative gel showing NAP-induced phosphorylation of Fyn kinase at Y416 of CGNs in the absence and presence of 5 μM PP2 (n = 3) or 5 μM PP3 (n = 1).
NAP Activation of Fyn Kinase Leads to Phosphorylation of Crk-Associated Substrate (Cas).
Fyn kinase induces tyrosine phosphorylation of Cas, a scaffold protein that links Fyn kinase signaling to axon elongation in CGNs (19). Therefore, we asked whether NAP potentiation of axon outgrowth is associated with increased phosphorylation of Cas. CGNs were treated with 10−12 M NAP for varying lengths of time and cell lysates were immunoblotted with an antibody against pY410Cas. Phosphorylation of Cas increased within 10 min of NAP treatment, was sustained at 30 min, and decreased to control levels by 2 h (Fig. 5 A and B), a time course similar to that observed for NAP activation of Fyn kinase (Fig. 4). Indeed, NAP activation of Cas phosphorylation was eliminated after treatment with PP2, but not PP3 (Fig. 5C). These data suggest that NAP induces the phosphorylation of Cas through the activation of Fyn kinase.
Fig. 5.
NAP activation of Cas. CGNs at DIV1 were incubated for the indicated times in the absence and presence of 10−12 M NAP, 5 μM PP2, or 5 μM PP3. Cell lysates or Fyn kinase immunoprecipitates from cell lysates were subjected to immunoblotting, as indicated. (A) Time course of tyrosine phosphorylation of Cas at Y410 in response to NAP. Immunoblots of whole-cell lysates were probed with anti-phospho-Cas (Y410) antibody and reprobed with anti-Cas antibody. Shown is a representative gel from 1 of 3 experiments. (B) Densitometry of gels described in A. Shown is the mean ± SEM ratio of pY410Cas to total Cas in CGNs treated without (empty bar) or with (filled bars) NAP 10−12 M for the indicated times. F = 14.33; P = 0. 0014 for the main effect; *, P < 0.05; **, P < 0.01 vs. non-NAP-treated cells. (C) A representative immunoblot from 1 of 3 experiments shows inhibition of NAP-induced Cas phosphorylation by PP2 but not PP3. (D) Whole-cell lysates were immunoprecipitated with a polyclonal anti-Fyn antibody (IP) and analyzed by immunoblot (IB) using an anti-pY410Cas antibody. A representative gel from 1 of 2 experiments shows that PP2 prevents the association of activated Cas with Fyn in NAP-treated cells.
We also asked whether activated Cas and Fyn are physically associated in NAP-treated CGNs. Cell lysates from CGNs were immunoprecipitated with a Fyn antibody and immunoblotted with an antibody against pY410Cas. Treatment with 10−12 M NAP increased levels of phosphorylated Cas in Fyn antibody immunoprecipitates, and PP2 blocked this effect of NAP (Fig. 5D). These results indicate that activated Cas and activated Fyn kinase are physically associated in NAP-treated CGNs.
Ethanol Inhibits NAP-Mediated Axon Outgrowth.
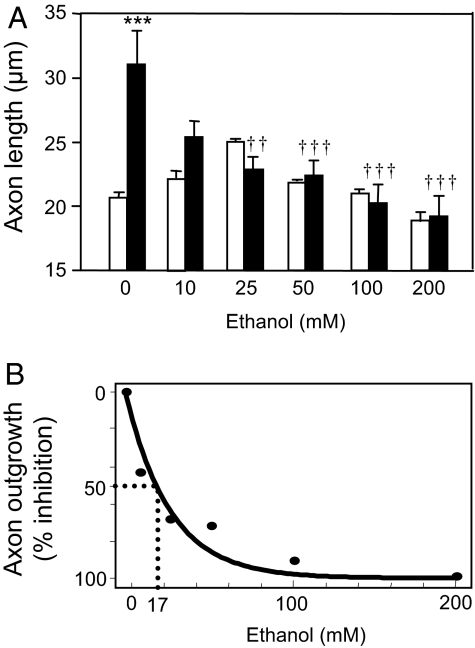
To test the hypothesis that ethanol disrupts the actions of ADNP, we determined the effects of ethanol on NAP-mediated axon outgrowth. CGNs were incubated for 20 h with 10−12 M NAP in the absence and presence of increasing concentrations of ethanol. Ethanol alone had no significant effect on basal levels of axon outgrowth (Fig. 6A). In contrast, ethanol caused a dose-dependent reduction in NAP-mediated axon outgrowth, with significant effects at concentrations as low as 10 mM and half-maximal inhibition at a concentration of 17 mM (Fig. 6B). These findings demonstrate that concentrations of ethanol attained during moderate drinking reduce NAP-mediated axon outgrowth in developing CGNs.
Fig. 6.
Effect of ethanol on NAP-mediated axon outgrowth of CGNs. CGNs were cultured under serum-free conditions in the absence and presence of 10−12 M NAP and the indicated concentrations of ethanol. Axon length was measured 20 h after treatment. (A) Shown is the mean ± SEM axon length of CGNs treated with increasing concentrations of ethanol in the absence (empty bar) or presence (filled bar) of NAP. F = 8.580; P < 0. 0001 for the main effect; ***, P < 0.001 vs. control; ††, P < 0.01; †††, P < 0.001 vs. NAP-treated cells; n = 3–5. (B) Percentage inhibition by ethanol of NAP-mediated axon outgrowth (IC50 = 17 mM).
Ethanol Inhibits NAP Activation of Fyn Kinase and Phosphorylation of Cas.
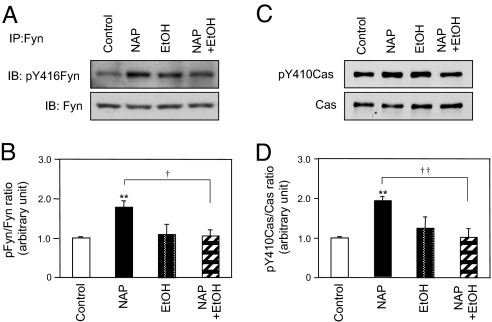
We next asked whether ethanol decreases NAP-mediated axon outgrowth by disrupting NAP activation of Fyn kinase and Cas. CGNs were incubated for 30 min with 10−12 M NAP in the absence and presence of 25 mM ethanol. Ethanol alone did not significantly increase Y416 phosphorylation in Fyn kinase (Fig. 7 A and B) and Cas phosphorylation at Y410 (Fig. 7 C and D). However, ethanol significantly decreased NAP-induced phosphorylation of Fyn kinase (101.5 ± 19.5% reduction; n = 4; P < 0.05) and Cas (107.0 ± 16.9% reduction; n = 4; P < 0.01) (Fig. 7). Taken together, these data demonstrate that ethanol inhibits NAP-mediated axon outgrowth in CGNs by blocking NAP activation of the Fyn kinase–Cas signaling pathway.
Fig. 7.
Effect of ethanol on NAP-induced activation of Fyn kinase and Cas. CGNs at DIV1 were treated for 30 min with 10−12 M NAP and 25 mM ethanol, as indicated. (A) Whole-cell lysates were immunoprecipitated (IP) with a polyclonal anti-Fyn antibody; immunoprecipitates were immunoblotted (IB) with antibody against pY416 of the SFK and reprobed with a monoclonal anti-Fyn antibody. Shown is a representative immunoblot from 4 independent experiments. (B) Densitometric analysis of Fyn kinase activation from A. Shown is the mean ± SEM ratio of pY416Fyn to total Fyn. F = 7.462; P = 0. 0044 for the main effect; **, P < 0.01 vs. control; †, P < 0.05 NAP vs. NAP plus ethanol (EtOH). (C) Whole-cell lysates were immunoblotted with anti-phospho-Cas (Y410) antibody and reprobed with anti-Cas antibody. Shown is a representative immunoblot from 1 of 4 independent experiments. (D) Densitometric analysis of Cas phosphorylation from C. Shown is the mean ± SEM ratio of pY416Cas to total Cas. F = 8.117; P = 0. 0032 for the main effect; **, P < 0.01 vs. control; ††, P < 0.01 NAP vs. NAP plus ethanol.
Discussion
The major finding of this article is that ethanol inhibits axon outgrowth in CGNs by disrupting NAP activation of SFK signaling. We used NAP, an extremely potent octapeptide fragment of ADNP, to model ADNP signaling and function. Subfemtomolar concentrations of NAP stimulated axon outgrowth in CGNs through the sequential activation of Fyn kinase and the scaffold protein Cas. Importantly, ethanol blocked this NAP signaling pathway and inhibited NAP-mediated axon outgrowth at concentrations that are readily attained during social drinking. Our findings suggest that ethanol might cause FASD partly by disrupting the actions of ADNP that support the differentiation and survival of CGNs.
ADNP is a glial-derived protein that is critical for brain development. In mouse, ADNP mRNA is expressed during embryogenesis, peaks during neural tube closure, and persists into adulthood, with the highest levels in cerebellum and hippocampus (9, 20). In adult human brain, ADNP expression is greatest in cerebellum and cerebral cortex (21). Deletion of the mouse ADNP gene results in failure of closure of the neural tube and death by embryonic days 8.5–9 (10). Hence intermittent disruption of ADNP signaling by ethanol could significantly disrupt brain development.
Recent studies have explored the mechanisms by which ADNP regulates brain development. NAP causes a concentration-dependent stimulation of neurite outgrowth in hippocampal, cortical, and retinal ganglion neurons and in PC-12 pheochromocytoma cells (14, 22–24). NAP binds tubulin and facilitates microtubule assembly in astrocytes (25) and stimulates AKT, MAPK, and adenylate cyclase in cortical neurons (14). NAP also increases the polyADP-ribosylation of histone H1 in PC12 cells, and inhibitors of PARP-1 diminished NAP-induced neurite outgrowth (24).
Our study examined the effects of NAP on axon outgrowth in neurons derived from the cerebellum, the brain region richest in ADNP. Our findings establish that NAP activation of Fyn kinase is necessary for NAP potentiation of axon outgrowth in CGNs. Axon outgrowth was abolished by PP2, an SFK inhibitor, or by transfection with a Fyn kinase siRNA. NAP potentiation of axon outgrowth was partially reduced by inhibitors of MAPK, suggesting that additional signaling pathways contribute to the actions of NAP. Consistent with this observation, Pascual and Guerri (14) recently reported that inhibitors of MAPK and PI3-kinase/AKT reduced NAP-mediated increases in MAP-2 staining in cortical neurons. It remains to be determined whether MAPK is activated upstream or downstream of Fyn kinase and Cas.
Fyn kinase is a member of the Src family of nonreceptor tyrosine kinases. Fyn kinase is developmentally regulated and is highly expressed in the growth cones and axons of cerebellar neurons. Fyn kinase is necessary for axon outgrowth stimulated by diverse axon guidance molecules, including neural cell adhesion molecule, prion protein, semaphorin-3A, contactin, glial cell line-derived neurotrophic factor, and netrin-1 (18, 26–30). Our results indicate that ADNP is a member of the family of molecules that regulate axon outgrowth by activating Fyn kinase. Because neuronal differentiation and axon guidance are important components of normal brain development, activation of Fyn kinase by ADNP may contribute to its critical role in development.
The scaffold protein Cas plays an essential role in intracellular signaling events by coordinating the interaction of multiple cellular proteins involved in cell adhesion, migration, proliferation, and survival (31). Cas is enriched in the cerebellum and is colocalized with Fyn kinase. Cas is concentrated in neurites and growth cones and is required for axon extension in CGNs (19) and netrin-mediated commissural axon guidance (32). Our results demonstrate that NAP potentiates axon outgrowth in CGNs by the sequential activation of Fyn kinase and Cas, suggesting that ADNP might regulate neuronal differentiation by activating this signal transduction pathway.
NAP-mediated axon outgrowth was particularly sensitive to ethanol, with significant inhibition occurring at concentrations attained after only 1 or 2 drinks. Our studies indicate that ethanol inhibits NAP activation of Fyn kinase and Cas, 2 critical signaling events in NAP-mediated axon outgrowth. These observations suggest that ethanol might disrupt neuronal differentiation partly by blocking ADNP signaling. Interestingly, prenatal ethanol exposure decreased the expression of ADNP mRNA in cerebral cortex and astrocytes and reduced astrocyte induction of neurite outgrowth and synaptogenesis in cortical neurons (14). Hence, ethanol may disrupt brain development by disrupting both the expression and signaling of ADNP. Because ADNP is expressed in the adult nervous system, ethanol disruption of ADNP signaling might also contribute to alcoholic cerebellar degeneration, a common disorder in alcoholics.
NAP is protective against a variety of brain insults, including prenatal ethanol exposure (13, 33). We showed previously that NAP antagonizes ethanol inhibition of L1 adhesion and prevents ethanol teratogenesis in mouse embryos (33, 34). Whereas NAP effectively antagonizes some actions of ethanol, particularly during embryogenesis, our current data indicate that ethanol also blocks some actions of NAP, at least during the period of brain growth and neuronal differentiation. Our findings provide insights into the pathophysiology of FASD and clarify the potential role for NAP as a therapeutic agent for preventing FASD.
Materials and Methods
Reagents and Animals.
NAP was purchased from New England Peptides. For other reagents see SI Text. All animal procedures in this report were approved by the Animal Care and Use Committee of the Veterans Affairs Boston Healthcare System and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council.
Cell Culture and Axon Outgrowth Assay.
CGNs were prepared from the cerebellum of 6- to 7-day-old rats and cultured as described (35) (see SI Text). NAP, ethanol, and/or inhibitors of signal transduction pathways were added to culture medium 2 h after cell plating. For quantification of axon outgrowth, CGNs were cultured for 20 h, fixed with 4% paraformaldehyde and stained with toluidine blue to visualize cells and axons. Axon length was measured with Openlab software (Improvision). In each experiment, at least 25 cells in each of 3 wells were analyzed.
Knockdown of Fyn Kinase Expression in CGNs with siRNA.
Plasmid pSUPER Fyn siRNA-YFP and the control plasmid pSUPER YFP were generously provided by H. Colognato (State University of New York, Stony Brook). The following target sequence was used as Fyn siRNA: 5′-GCAGGACAGAAGATGACCT-3′. The introduction of Fyn kinase siRNA to CGNs was achieved by nucleofection using Nucleofector and the Rat Neuron Nucleofector Kit (Amaxa) with modification of the manufacturer's instructions and published procedures (36, 37). In brief, 5 × 106 CGNs in a freshly prepared, dissociated suspension were mixed with 3 μg of plasmid DNA and electroporated with program G-13 using the Nucleofector device. The nucleofected cells were plated in RPMI medium 1640 containing 10% horse serum; the medium was replaced with serum-free medium (SI Text) 4–6 h after plating. The transfection efficiency was evaluated based on the expression of YFP. NAP was added to the treatment groups 20 h after transfection. Forty-eight hours after transfection, cells were processed for Fyn kinase immunostaining to verify the reduction in Fyn kinase expression and to measure axon outgrowth. Total levels of Fyn kinase were determined from lysates of CGNs by immunoblotting with a Fyn kinase mAb.
Immunostaining.
CGNs cultured on glass coverslips were fixed with 4% paraformaldehyde for 30 min, permeabilized with cold methanol for 5 min, blocked with 3% BSA, and incubated with anti-Fyn kinase antibody followed by Cy3-conjugated secondary antibody. Nuclei were visualized with Hoechst dye. Images were acquired with a Nikon fluorescence microscope using a digital camera (Hamamatsu; model C4742–95) and imaging software (OpenLab).
Immunoprecipitation and Immunoblotting.
Immunoprecipitation was performed by using cell lysates from CGNs and an anti-Fyn kinase polyclonal antibody as described (SI Text). Immunoprecipitated proteins were separated with 4–15% gradient SDS/PAGE, transferred to nitrocellulose membranes, and probed with the following antibodies: rabbit anti-phospho-Src family (Tyr-416) to measure phospho-Fyn kinase; mouse monoclonal anti-Fyn kinase antibody to measure total Fyn kinase, and an anti-phospho-Cas (Tyr-410) antibody to measure phospho-Cas. Whole-cell lysates were subjected to immunoblotting by using antibodies against phospho-Cas and total Cas. The densitometric analysis of Western blots was performed with TINA Image software (version 2.0).
Statistical Analysis.
Data are presented as the mean ± SEM from at least 3 independent experiments performed in triplicate in axon outgrowth studies and at least 3 independent experiments in quantification of images of Western blot analysis. Statistical analysis of the data was performed by using ANOVA and the Tukey test for post hoc comparisons of means.
Supplementary Material
Acknowledgments.
We thank D. Arambula and C. Menkari for excellent technical assistance, Dr. M. Wilkemeyer for helpful discussions during the initial period of this study, and Dr. H. Colognato (State University of New York, Stony Brook, NY) for pSUPER/YFP/FYN siRNA and pSUPER/YFP plasmids. This study was supported in part by National Institute on Alcohol Abuse and Alcoholism Grant R37AA12974 (to M.E.C.), the Medical Research Service, Department of Veterans Affairs (M.E.C), and the William Randolph Hearst Fund (S.C).
Footnotes
Conflict of interest statement: M.E.C. is on the scientific advisory board of and holds stock in Allon Therapeutics, which is developing NAP as a therapeutic agent.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807758105/DCSupplemental.
References
- 1.Sowell ER, et al. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 2.Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: A decade of brain imaging. Am J Med Genet. 2004;127C:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- 3.Miller MW. Mechanisms of ethanol induced neuronal death during development: From the molecule to behavior. Alcohol Clin Exp Res. 1996;20:128A–132A. doi: 10.1111/j.1530-0277.1996.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: Mechanisms of damage and strategies for intervention. Exp Biol Med. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- 5.Olney JW. Fetal alcohol syndrome at the cellular level. Addict Biol. 2004;9:137–149. doi: 10.1080/13556210410001717006. and discussion 151. [DOI] [PubMed] [Google Scholar]
- 6.Conforti L, Adalbert R, Coleman MP. Neuronal death: Where does the end begin? Trends Neurosci. 2007;30:159–166. doi: 10.1016/j.tins.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Dow KE, Riopelle RJ. Ethanol neurotoxicity: Effects on neurite formation and neurotrophic factor production in vitro. Science. 1985;228:591–593. doi: 10.1126/science.3983644. [DOI] [PubMed] [Google Scholar]
- 8.Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16. doi: 10.1007/978-0-387-76715-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Bassan M, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- 10.Pinhasov A, et al. Activity-dependent neuroprotective protein: A novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 11.Gozes I. Activity-dependent neuroprotective protein: From gene to drug candidate. Pharmacol Ther. 2007;114:146–154. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Charness ME, Simon RP, Greenberg DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–454. doi: 10.1056/NEJM198908173210706. [DOI] [PubMed] [Google Scholar]
- 13.Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–779. [PubMed] [Google Scholar]
- 14.Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways and protects neurons cocultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:567–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- 15.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loers G, Chen S, Grumet M, Schachner M. Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J Neurochem. 2005;92:1463–1476. doi: 10.1111/j.1471-4159.2004.02983.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanke JH, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Sakai R, Furuichi T. The docking protein Cas links tyrosine phosphorylation signaling to elongation of cerebellar granule cell axons. Mol Biol Cell. 2006;17:3187–3196. doi: 10.1091/mbc.E05-12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gennet N, Herden C, Bubb VJ, Quinn JP, Kipar A. Expression of activity-dependent neuroprotective protein in the brain of adult rats. Histol Histopathol. 2008;23:309–317. doi: 10.14670/HH-23.309. [DOI] [PubMed] [Google Scholar]
- 21.Zamostiano R, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- 22.Lagreze WA, et al. The peptides ADNF-9 and NAP increase survival and neurite outgrowth of rat retinal ganglion cells in vitro. Invest Ophthalmol Visual Sci. 2005;46:93393–93398. doi: 10.1167/iovs.04-0766. [DOI] [PubMed] [Google Scholar]
- 23.Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR. Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci. 2005;25:225–238. doi: 10.1385/JMN:25:3:225. [DOI] [PubMed] [Google Scholar]
- 24.Visochek L, et al. PolyADP-ribosylation is involved in neurotrophic activity. J Neurosci. 2005;25:7420–7428. doi: 10.1523/JNEUROSCI.0333-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem. 2004;279:28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- 26.Santuccione A, Sytnyk V, Leshchyns'ka I, Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J Cell Biol. 2005;169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Mange A, Dong L, Lehmann S, Schachner M. Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol Cell Neurosci. 2003;22:227–233. doi: 10.1016/s1044-7431(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 28.Beggs HE, Soriano P, Maness PF. NCAM-dependent neurite outgrowth is inhibited in neurons from Fyn-minus mice. J Cell Biol. 1994;127:825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meriane M, et al. Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J Cell Biol. 2004;167:687–698. doi: 10.1083/jcb.200405053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita A, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Defilippi P, Di Stefano P, Cabodi S. p130Cas: A versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, et al. p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkemeyer MF, et al. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci USA. 2003;100:8543–8548. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkemeyer MF, Menkari CE, Spong CY, Charness ME. Peptide antagonists of ethanol inhibition of l1-mediated cell–cell adhesion. J Pharmacol Exp Ther. 2002;303:110–116. doi: 10.1124/jpet.102.036277. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Mantei N, Dong L, Schachner M. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J Neurobiol. 1999;38:428–439. doi: 10.1002/(sici)1097-4695(19990215)38:3<428::aid-neu10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Dityateva G, et al. Rapid and efficient electroporation-based gene transfer into primary dissociated neurons. J Neurosci Methods. 2003;130:65–73. doi: 10.1016/s0165-0270(03)00202-4. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs G, et al. NR2B containing NMDA receptor-dependent windup of single spinal neurons. Neuropharmacology. 2004;46:23–30. doi: 10.1016/s0028-3908(03)00339-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.