On any given day you turn over your body weight equivalent in ATP, the principal energy currency of the cell. Mitochondria, which are believed to arise from the capture of a bacterium by an ancestral eukaryotic host cell (1), regenerate ATP from ADP and inorganic phosphate through oxidative phosphorylation. These organelles, termed the powerhouse of eukaryotic cells, contain both an inner and outer membrane, where ATP synthesis in the mitochondrial matrix is coupled to a transmembrane proton-motive force by ATP-synthase. Regenerated ATP is then actively exported across the inner mitochondrial membrane by the ADP/ATP antiporter (2). In contrast, the transport of ATP, ADP, and other metabolites across the outer mitochondrial membrane is passive, with the major pathway being through a voltage-dependent anion channel (VDAC) (3).
VDAC is the most abundant protein of the mitochondrial outer membrane. This eukaryotic porin not only links the aqueous domains of the cytoplasm to the intermembrane space of the mitochondria, but it also regulates the energy-dependent metabolism of the cell by fine-tuning the diffusion barrier to ions and metabolites. When inserted into lipid bilayers, VDAC adopts an open conformation that tends to be weakly selective for anionic solutes of low molecular mass (<3 kDa). Remarkably, the application of a relatively small transmembrane voltage (≈30 mV) significantly lowers the conductance (3, 4) and the pore becomes weakly cationic selective. The puzzling biological implications of this complex voltage-gating mechanism have been a matter of debate, but endogenous potentials due to chemical gradients across the outer membrane may be sufficient to regulate this channel (5). Thus, although VDAC's role as the mitochondrial gatekeeper in vivo is firmly established, relatively little is known regarding the structural mechanism of voltage-induced gating.
Three recent publications present, with increasing detail, the architecture of this remarkable pore and provide enticing clues as to the mechanism of voltage-sensitive gating (6–8). Hiller et al. (6) applied NMR spectroscopy to recover the solution structure of detergent-solubilized human VDAC; Bayrhuber et al. (7) exploited constraints recovered from NMR spectroscopy to assist in solving the X-ray structure of detergent-solubilized human VDAC to medium resolution; and, in a recent issue of PNAS, Ujwal et al. (8) applied the technique of lipidic bicelle crystallization (9) to recover a high-resolution X-ray structure of mouse VDAC within a lipid environment. All 3 structures reveal a novel 19-strand β-barrel fold for VDAC, with all β-strands arranged antiparallel except for strands β1 and β19, which combine to close the barrel with a parallel interface. This fold is unique when compared with the 32 β-barrel structures of prokaryotic membrane proteins solved to date, which all contain an even number of β-strands. Thus, earlier fears that the structure of VDAC in detergent (6, 7) and lipidic (8) environments would be markedly different (10) appear to be misplaced.
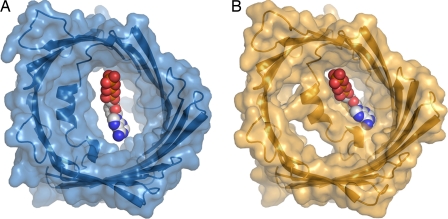
As with other outer membrane porins, VDAC presents a hydrophobic surface to the membrane while creating a polar channel interior, establishing an obvious pore through which charged metabolites may pass through the membrane. Its channel entrance appears almost circular on both sides of the membrane, but the pore diameter decreases to ≈14 Å by 27 Å at its narrowest point (8), which is nevertheless sufficient to allow the passage of ATP (Fig. 1A). Asymmetry in the pore profile arises because of the presence of an N-terminal α-helix approximately half-way through the pore which aligns at an angle almost parallel to the plane of the membrane. Mutational studies have established that this N-terminal α-helix forms a key component in regulating the flux of metabolites through the channel (11), and thus, its placement within the pore is highly suggestive of a structural mechanism for channel gating. Moreover, the conformation of this helix appears to be flexible because only those N-terminal residues which were located near the pore walls could be assigned using NMR spectroscopy (6) and the helix is rotated almost 180° about its axis in the 4-Å resolution X-ray structure (7) relative to that observed at 2.3-Å resolution (8).
Fig. 1.
Conformational flexibility within the N-terminal region of VDAC and its putative role in gating. (A) Diagram and surface representation of the X-ray structure of mVDAC1 to 2.3-Å resolution (8). (B) Proposed model for the closed conformation (8). A space-filling model for ATP indicates its size relative to the pore diameter. A path through VDAC that is sufficiently large for ATP to pass becomes partially occluded in the putative closed conformation.
What do these findings imply regarding the mechanism of VDAC regulation in vivo? Several factors serve to shift the dynamical equilibrium and favor the closed conformation of VDAC in addition to the application of transmembrane potentials. For example, protein–protein interactions (12) and the binding of medium-sized molecules such as NADH (13) have also been suggested to induce pore closure. Ujwal et al. (8) observe that the site of NADH binding identified from the NMR spectroscopy study (6) flanks a short Gly-X-Gly-X-Gly sequence that links the N terminus to strand β1, which appears to be smoking-gun evidence for a protein hinge region. Moreover, in the X-ray structure this affinity site lacks the space needed for NADH to bind, yet a modest 10° rotation about this hinge would simultaneously create the required space and partially occlude the channel (Fig. 1B). Should this plausible structural mechanism for metabolite gating by VDAC survive further scrutiny, then changes in the diameter and distribution of charges within the modified pore should also explain the shift from weakly anionic to weakly cationic selectivity. Curiously, structures of both the open and closed conformations of the bacterial pore OmpG reveal a very different mechanism for outer membrane porin gating (14). In that case it is an extended loop of OmpG that folds across the channel and occludes the pore.
A protein structure has value not only because of the questions that it answers, but also because of those it asks. A slight asymmetry in the polarity and lengths of the loop regions has been used by Bayrhuber et al. (7) to argue that VDAC inserts with its C terminus extending into the intermembrane space, but this may not be the final word because there have been conflicting earlier assignments of the topology (15, 16). VDAC's ability to regulate the exchange of metabolites between the mitochondria and the cytosol is also tightly coupled to cell survival. Indeed, apoptotic factors such as members of the Bcl-2 protein family have been shown to interact with VDAC (17) and either promote or prevent apoptosis by stimulating VDAC closure or opening, respectively (18). Hiller et al. (6) mapped a potential binding site for Bcl-xL to strands β18 and β19, yet more detailed characterization is needed before the mechanism by which these interactions influence conductance can be understood. VDAC has also been shown to interact with hexokinase (19), a glycolytic enzyme that is expressed to high levels in cancer cells. Hexokinase binding is suggested to block metabolite conductance through VDAC and thereby suppress aerobic respiration and promote aerobic glycolytic activity (18), a change that is frequently encountered in cancer cells. Again, structural insights into the mechanism by which VDAC is gated by the binding of an external mediator would be of tremendous value.
VDAC is the most abundant protein of the mitochondrial outer membrane.
VDAC is a versatile pore that primarily serves to regulate cellular metabolism, yet which may also be seconded into critical roles in apoptosis and the development of cancer. Recent structures of this gated channel have fundamental implications for the exchange of ADP and ATP across the outer membrane of mitochondria, a process essential for life in all eukaryotes. Thus, although a camel cannot pass through the eye of a needle, it is extraordinary to imagine that every day its body weight's equivalent in metabolites tunnel backward and forward through an integral membrane aperture ≈6 orders of magnitude smaller in diameter.
Acknowledgments.
This work was supported by the Swedish Science Research Council (VR).
Footnotes
The authors declare no conflict of interest.
See companion article on page 17742 of issue 46 of volume 105.
References
- 1.Lang BF, Gray MW, Burger G. Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet. 1999;33:351–397. doi: 10.1146/annurev.genet.33.1.351. [DOI] [PubMed] [Google Scholar]
- 2.Pebay-Peyroula E, et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 3.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 4.Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979;279:643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- 5.Porcelli AM, et al. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 6.Hiller S, et al. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayrhuber M, et al. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ujwal R, et al. The crystal structure of mouse VDAC1 at 2.3 Å resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faham S, Bowie JU. Bicelle crystallization: A new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J Mol Biol. 2002;316:1–6. doi: 10.1006/jmbi.2001.5295. [DOI] [PubMed] [Google Scholar]
- 10.Shanmugavadivu B, Apell HJ, Meins T, Zeth K, Kleinschmidt JH. Correct folding of the beta-barrel of the human membrane protein VDAC requires a lipid bilayer. J Mol Biol. 2007;368:66–78. doi: 10.1016/j.jmb.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 11.Thomas L, Blachly-Dyson E, Colombini M, Forte M. Mapping of residues forming the voltage sensor of the voltage-depenent anion-selective channel. Proc Natl Acad Sci USA. 1993;90:5446–5449. doi: 10.1073/pnas.90.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 13.Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz O, Vinothkumar KR, Goswami P, Kuhlbrandt W. Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation. EMBO J. 2006;25:3702–3713. doi: 10.1038/sj.emboj.7601237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley S, Dias JA, D'Arcangelis D, Mannella CA. Peptide-specific antibodies as probes of the topography of the voltage-gated channel in the mitochondrial outer membrane of Neurospora crassa. J Biol Chem. 1995;270:16694–16700. doi: 10.1074/jbc.270.28.16694. [DOI] [PubMed] [Google Scholar]
- 16.De Pinto V, Prezioso G, Thinnes F, Link TA, Palmieri F. Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry. 1991;30:10191–10200. doi: 10.1021/bi00106a017. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 18.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator: Thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Hexokinase receptor complex in hepatoma mitochondria: Eevidence from N,N′-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986;25:1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]