Abstract
Riparian corridors and fencerows are hypothesized to increase the persistence of forest animals in fragmented landscapes by facilitating movement among suitable habitat patches. This function may be critically important for forest birds, which have declined dramatically in fragmented habitats. Unfortunately, direct evidence of corridor use has been difficult to collect at landscape scales and this limits support for corridors in conservation planning. Using telemetry and handheld GPS units, we examined the movement of forest birds by translocating territorial individuals of barred antshrikes (Thamnophilus doliatus; a forest specialist) and rufous-naped wrens (Campylorhynchus rufinucha; a forest generalist) 0.7–1.9 km from their territories in the highly fragmented tropical dry forest of Costa Rica. In each translocation, the directly intervening habitat comprised 1 of 3 treatments: forested riparian corridor, linear living fencerow, or open pasture. Antshrikes returned faster and with greater success in riparian corridors relative to pasture treatments. This species also traveled more directly in riparian corridor treatments, detoured to use forested routes in the other 2 treatments, and did not use fencerows even when they led directly to their home territories. By contrast, wrens were more likely to use fencerows when returning, and return time and success were equivalent among the 3 treatments. Both species crossed fewer gaps in tree cover during riparian corridor treatments than in fencerow or pasture treatments. We conclude that antshrikes, which may be representative of other forest specialists, use forested corridors for movement in this landscape and that fencerows are avoided as movement conduits.
Keywords: animal movement, Campylorhynchus rufinucha, habitat connectivity, hedgerows, Thamnophilus doliatus
Land-use change in tropical forests is expected to be the primary threat to global biodiversity for the remainder of this century (1). Because movement among remaining patches is important to population persistence (e.g., ref. 2), corridors have been widely advocated as a means to maintain biodiversity and ecological processes in fragmented landscapes (3, 4). Several studies have demonstrated that target organisms occur in corridors (5), providing indirect evidence that they facilitate movement. A few studies have measured movement directly to assess the functional connectivity (in the sense of ref. 6) provided by corridors (7–10), but this has been difficult to achieve for small species, like birds, that move at landscape scales (6). More specific movement information from free-ranging animals is especially important for forest specialists, particularly understory insectivores, because this group appears to be most sensitive to the isolation effects of fragmentation (4, 11, 12). Both forested corridors and fencerows of individual, living trees have been promoted as landscape elements to facilitate the movement of birds and other forest dependent animals (11, 13), but no studies of birds have directly measured movement in these habitats.
One tropical region where corridors and other landscape configurations appear to be important is the dry forest of Costa Rica. This area is part of the Mesoamerican biodiversity hotspot (14), but the contiguous tropical dry forest that once dominated the landscape is now defined within a matrix of pasture. Consequently, these dry forests are now highly fragmented and are one of the most endangered forest types in the tropics (15). The relatively low rates of forest cover that remain in the dry forest likely increase the importance of habitat configuration to biodiversity conservation (16). Indeed, much of the remaining dry forest exists as riparian corridors, which typically have a closed canopy and moderate understory. Another forest element is formed by the linear fencerows of individual living trees that demark pasture edges. There are also individual trees scattered within the pastures, which may function as stepping stones (in the sense of ref. 17) for forest-dependent animals. Because agricultural demands are expected to place large pressures on remaining forest over the next 50 years (18), demonstrations of the utility to forest animals of riparian corridors and fencerows could provide important information to landowners and land use planners in Mesoamerica and elsewhere.
Here, we test the efficacy of forested corridors and fencerows in facilitating the movement of forest birds in a highly fragmented tropical forest. Direct information about corridor use has come from experiments at small scales (19, 20). Studies at broader landscape scales have shown that birds make some use of corridors but have not followed moving individuals closely enough to collect detailed information about their route (e.g., refs. 21 and 22). We addressed this deficiency by following moving forest birds in real time at a landscape scale with unprecedented resolution. We translocated 30 territorial Barred Antshrikes (Thamnophilus doliatus, hereafter antshrikes) and 30 Rufous-naped Wrens (Campylorhynchus rufinucha, hereafter wrens). Both are common insectivores that hold territories year-round, but antshrikes are forest specialists, being found only in the understory of the most intact forest in this region, whereas wrens are forest generalists, being found in both intact and degraded forest (23). Birds were moved away from their territory in 1 of 3 treatments: along riparian corridors, along fencerows, and through pasture (see Methods). Using translocations allowed us to standardize the bird's motivation for moving, anticipate the direction it would predominantly travel, and choose the configuration of the intervening habitat (6). We predicted that birds would travel more quickly and successfully through the riparian corridors than through pasture, and that fencerows would provide intermediate travel speed and success. In addition, because the willingness of forest-dwelling species to cross open habitat or gaps in forest cover is often used as a measure of habitat permeability (19, 24), we predicted that birds would cross fewer gaps in riparian corridor than pasture treatments.
Results
Habitat Used to Return.
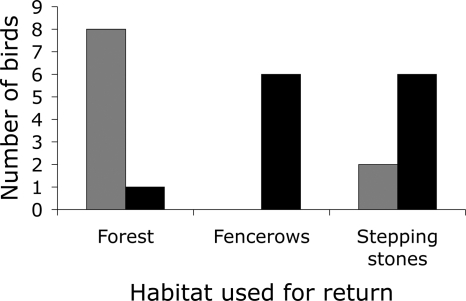
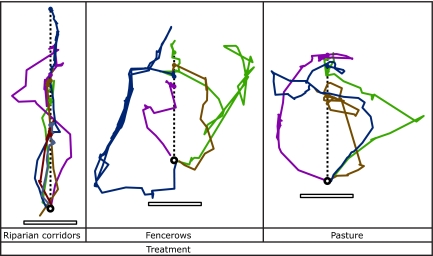
Although individuals from both species returned in fencerow and pasture treatments, there were large differences between the species in the habitat used to return within those treatments. Of the 10 antshrikes returning in fencerow and pasture treatments, 8 birds used an indirect forested route for the majority (>50%) of the distance during their return, and 2 crossed open pasture habitat by moving among stepping stones or small forest patches (Fig. 1). None of the antshrikes used fencerows for the majority of their return. In contrast, only 1 of 13 wrens returning in fencerow and pasture treatments used a forested route for the majority of its return. The remainder used fencerows or crossed gaps by moving among stepping stones and small forest patches. Returning antshrikes generally moved directly along the corridor in riparian corridor treatments, but traveled longer routes to circumvent the direct routes provided in both fencerow and pasture treatments (Fig. 2). Translocated birds typically made several initial forays out from the release point before moving in a more directed way to their territories (Fig. 2 and SI Appendix).
Fig. 1.
Habitat used by antshrikes (gray bars) and wrens (black bars) that returned after translocation in fencerow or pasture treatments.
Fig. 2.
Complete paths of returning antshrikes shifted to a common capture point (open circle) in riparian corridor (n = 6), fencerow (n = 4), and pasture (n = 4) treatments. Paths were rotated so release points (closed circles) occur on the same axis and each line represents 1 individual. Four individuals for which we only had partial path information are not included. The dashed line represents the direct route between the releases and the capture location and the approximate location of the riparian corridors or fencerows in these treatments. (Scale bars: 500 m.) Antshrikes demonstrated consistently direct paths in riparian corridor treatments, but traveled longer routes around fencerows and open pasture in the other 2 treatments. SI Appendix provides individual paths for these and the other 46 translocated individuals with each depicting habitat, capture and release points.
Return Success.
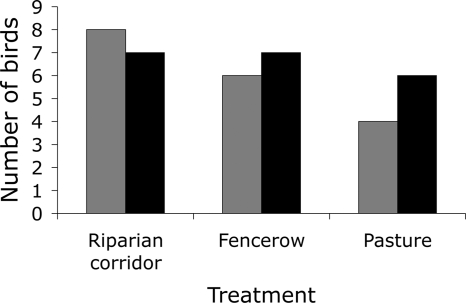
Of the 30 translocated individuals of each species, 18 antshrikes and 20 wrens returned (Fig. 3). Treatment was an important predictor of return success only for the antshrikes where success was half as likely in pasture translocations relative to riparian corridor translocations (Table 1; Fig. 3). Fencerow returns were intermediate for antshrikes, but not statistically different from either riparian corridor or pasture translocations. The proportion of tree cover (total area of forest, fencerow and stepping stone habitat) within an ellipse around the capture and release points (see Methods) was not a significant predictor of return success for either species. The return of both species was less likely as translocation distance increased (Table 1; overall models; antshrikes χ2 = 14.79, df = 3, P = 0.002, pseudo r2 = 0.37; wrens χ2 = 5.04, df = 1, P = 0.025, pseudo r2 = 0.13). Non-returning birds did not die but typically settled in a new territory after attempting to return home.
Fig. 3.
Return success by antshrikes (gray bars) and wrens (black bars) in the three treatments following translocation. Ten individuals of each species were translocated in each treatment.
Table 1.
Variables influencing the return success of translocated birds
| Species | Variable | Coefficient | SE | P |
|---|---|---|---|---|
| Antshrikes | Distance, km | −4.88 | 1.89 | 0.010 |
| Treatment-riparian corridor | 2.78A | 1.42 | 0.051 | |
| Treatment-fencerow | 1.26A | 1.17 | 0.282 | |
| Constant | 5.14 | 2.19 | 0.019 | |
| Wrens | Distance, km | −2.55 | 1.24 | 0.040 |
| Constant | 3.86 | 1.63 | 0.018 |
The reference category for Treatment is pasture. Superscripts on the treatment variables indicate group membership based on post-hoc comparisons (P ≥ 0.05 for membership).
Return Time.
Mean return times were 33.7 daylight hours for antshrikes and 26.2 daylight hours for wrens. Measured using Cox proportional hazards regression, the returns of antshrikes were significantly faster in riparian corridor than pasture treatments (Table 2). Fencerow treatments were intermediate and not significantly different from riparian corridor or pasture treatments. Similar to the results for return success, the return time of wrens was not affected by treatment and the return time of neither species was significantly affected by the amount of tree cover. Returns of both species were slower as translocation distance increased (Table 2; overall models; antshrikes χ2 = 15.80, df = 3, P = 0.001; wrens χ2 = 8.96, df = 1, P = 0.003).
Table 2.
Variables influencing the return time of translocated birds
| Species | Variable | Hazard ratio | SE | P |
|---|---|---|---|---|
| Antshrikes | Distance, km | 0.046 | 0.044 | 0.001 |
| Treatment-riparian corridor | 3.726A | 2.423 | 0.043 | |
| Treatment-fencerow | 2.422A | 1.628 | 0.188 | |
| Wrens | Distance, km | 0.121 | 0.092 | 0.005 |
Data are as in Table 1.
Gap Crossing.
Additional information about habitat used in return paths is provided by differences among treatments in the number and distance of gaps crossed. Antshrikes crossed an average of almost 3 fewer gaps/km in riparian corridor treatments than in fencerow or pasture treatments (Table 3; gamma regression, overall model χ2 = 14.6, df = 2, P < 0.001). In contrast, wrens crossed approximately 1 and 2 fewer gaps/km in fencerow and riparian corridor treatments than in pasture treatments, respectively (Table 3; gamma regression, overall model χ2 = 54.0, df = 4, P < 0.001). The mean width of gaps crossed was similar between species (54.6 m for wrens, 57.2 m for antshrikes, n = 45, gamma regression P = 0.787), but wrens crossed twice as many gaps/km of known path (2.77 vs. 1.37 gaps/km, n = 60, gamma regression P = 0.026). Mean path lengths in these analyses were 3.4 km for the antshrikes and 5.4 km for the wrens. For antshrikes, the proportion of tree cover was negatively correlated with mean gap size and returning individuals crossed larger gaps than non-returning ones (Table 3; gamma regression, overall model χ2 = 14.4, df = 2, P < 0.001). Returning wrens crossed more gaps/km than non-returning birds and female wrens crossed more gaps/km than males (Table 3). These results, based on the number of gaps/km were qualitatively unchanged when we based analyses (not presented here) on the total distance of gaps crossed/km in return trajectories.
Table 3.
Variables influencing the mean gap size crossed and number of gaps crossed / km for translocated antshrikes and wrens
| Species | Measure | Variable | Coefficient | SE | P |
|---|---|---|---|---|---|
| Antshrikes | Mean gap | Returned | 0.91 | 0.22 | <0.001 |
| Size | Proportion tree cover | −2.11 | 0.99 | 0.032 | |
| Constant | 4.13 | 0.33 | <0.001 | ||
| Gaps/km | Treatment-riparian corridor | −2.92A | 0.69 | <0.001 | |
| Treatment-fencerow | −0.02B | 0.69 | 0.976 | ||
| Constant | 0.70 | 0.48 | 0.148 | ||
| Wrens | Gap size | Constant | 4.00 | 0.12 | <0.001 |
| Gaps/km | Treatment-riparian corridor | −1.92A | 0.45 | <0.001 | |
| Treatment-fencerow | −0.89B | 0.45 | 0.048 | ||
| Constant | 1.67 | 0.32 | <0.001 |
Data are as in Table 1.
Discussion
Using translocations that standardized motivation, we provide detailed information about movement behavior collected from birds using corridors at a landscape scale. Perhaps the most striking result of our study is the dramatic difference between antshrikes and wrens, both forest insectivores, in the nature of their return paths. Antshrikes returned more quickly and successfully in riparian corridor treatments, circumvented direct routes in the other treatments to use riparian corridors, crossed half as many gaps in forest cover as wrens did, and never used fencerows as a majority habitat type in their return paths. In short, antshrikes expressed much more dependence on forested corridors for movement in this landscape than the wrens did.
One proximate reason for the more conservative movement behavior by the antshrikes relative to the wrens may be their unwillingness to cross gaps in forest cover that are formed by the pasture matrix. Our results indicate that this matrix offered high resistance to the movement of the antshrikes and much more moderate resistance for the wrens. Other understory birds were also impeded by landscapes that contained fewer trees in both the Amazon (12) and Chile (22). In addition to crossing fewer gaps than wrens, antshrikes crossed fewer gaps in riparian corridor treatments than in fencerow or pasture treatments. Gaps in forest cover may generally be perceived as inhospitable by forest birds (19, 24, 25), because they are more detectable by predators there (26). Other studies have suggested that forest birds avoid gaps over a certain threshold of size (19, 27), but no previous corridor study has provided enough spatial resolution to assess cumulative gap crossing behavior at a landscape scale.
A second proximate reason for greater reliance on riparian corridors by antshrikes may be the quality of habitat contained in the fencerows, which were composed of large trees with little or no understory. Whereas we found wrens in both degraded and more intact forest habitat, we never detected antshrikes in degraded forest with little understory. Research on 5 species of understory birds in Chile found that the availability of dense understory was the primary predictor of whether they would travel along narrow corridors (20). Both studies suggest that understory specialists are particularly sensitive to the habitat characteristics of movement corridors.
Separating the limitation to movement caused by gap crossing from the limitation caused by habitat quality will be difficult because the two likely covary. Indeed, others have reported that habitat specialist species are less likely to cross gaps than generalists (24, 25, 28, 29). Terrestrial insectivores in particular seem to be sensitive to fragmentation (12, 30) partly because they are unwilling to cross forest gaps (12, 31–33). This covariance would be expected if animals select travel routes with the same criteria they use to select habitat for foraging and other activities (34). In our study, the reluctance of antshrikes to cross gaps and avoidance of fencerows could both result from habitat selection for dense forest understory. Viewing movement behavior in fragmented habitats more holistically as a process of habitat selection may advance our understanding of corridors in this and other fragmented landscapes.
Regardless of its proximate mechanisms, the more conservative route choices of antshrikes have some important conservation implications for this and perhaps other forest specialist species in both temperate and tropical forests. Specialist species are generally more susceptible to extinction (e.g., ref. 35), and there are many species in other tropical forests that are more dependent on forest than barred antshrikes (23). Retaining these specialist species will require further work on landscape-level movement for the benefit of conservation planning. Beyond the variation among species, there may be heartening news in the variation in movement responses among individuals within species. Antshrikes that returned crossed larger gaps than antshrikes that did not return, and returning wrens crossed more gaps/km than non-returning birds did. The importance of this individual variation is an unexplored and potentially profitable area for future research. It would be particularly interesting to know what role inherent variation in traits like boldness played in return success. The presence of bold individuals in a population (in the sense of ref. 36) could be important if even rare dispersal events are sufficient to provide rescue effects for isolated populations (37).
In contrast to the differences between species our results emphasized, 2 aspects of our results might be generalized among species. First, our translocation protocol provided substantial information about homing behavior after release (SI Appendix). Like a boy scout is trained to do, individuals of both species responded to being “lost” by exploring the immediate vicinity of the release point and making increasingly large forays from it. This pattern of movement appears to be similar to the way squirrels (38, 39) and butterflies (40) move before dispersal. In our study, these forays continued to increase in length until birds appeared to determine the correct direction of travel and found a suitable route to their territory. Homing pigeons (Columba livia) exhibit similar behavior and their movement becomes more directed as they recognize landmarks closer to their home lofts (41). Our nonreturning birds made the same forays but eventually abandoned their search and settled in a new territory or wandered to a new area. The ubiquity of these preliminary forays, and the failure of some birds to return both suggest that even birds do not possess a “bird's eye view” of the landscape as conservation planners, benefitting from maps of land cover, might assume they do. Dispersing animals too may fail to recognize corridors as movement conduits and instead assess habitat with proxies of suitability in an iterative and incomplete way (42).
A second general implication of our results is to address the ongoing debate about the relative importance of habitat configuration and composition for conservation (16). Although much of this literature has focused on predicting occurrence or population size (43–45), movement is the domain most pertinent to configuration. Our measure of configuration (treatment) was included in models much more often than our composition variable (proportion tree cover). Configuration of the habitat influenced all of our measures of movement behavior for the antshrikes and the gap crossing of the wrens. By contrast, the amount of forest cover affected only the size of gaps crossed by antshrikes. This result contrasts with other studies that have found that the amount of forest cover predicts return time and success in translocated birds (46, 47), but they did not have an analogous measure of configuration. Our results suggest that configuration is an important component influencing movement of at least 1 forest specialist species, perhaps predictably so in this largely deforested region (16).
In sum, our study tracked closely the movement of forest birds, using corridors at a landscape scale. It showed that movement by barred antshrikes after translocation depended highly on the riparian corridors that provide much of the remaining forest cover in the highly fragmented dry forest of Costa Rica. Compared with rufous-naped wrens, barred antshrikes avoided pasture habitat, crossed fewer gaps in forest cover, and made little use of fencerows. These results provide mechanistic detail in support of the studies that have inferred the benefits of corridors from higher rates of interpatch movement (5, 9), pollination (8) and seed dispersal (8, 10). Ultimately, corridors appear to promote higher species richness (48, 49), support greater population persistence (2) and reduce changes to community structure (50) in fragmented landscapes. Although these inferential studies are important, we amplify caution that detailed information about movement behavior is critical in the study of corridors (5, 34). Our conclusions would have been quite different if we had tested only the forest generalist wren or lacked the detailed route information. Both scenarios would have suggested that living fencerows are adequate to facilitate movement, whereas the detailed results from antshrikes revealed fencerows to be almost useless as movement corridors. Retaining or creating corridors with the habitat characteristics needed by their target species is likely to be important for conserving birds and other forest-dwelling species in tropical agricultural landscapes where little natural habitat remains (51, 52).
Methods
To collect information about forest bird movement in fragmented landscapes, we captured territorial individuals of 2 species in an agricultural landscape of northwestern Costa Rica near the town of Liberia. This landscape was once contiguous tropical dry forest, but is now dominated by cattle pasture. Remaining forest covers ≈25% of the landscape and is often confined to riparian areas. Captured birds were translocated from their home territory to another location after which we followed their return with radio-telemetry. All protocols were approved by the Biosciences Animal Service at the University of Alberta.
We conducted translocations from June to August 2000 and January to June 2002. All individuals were caught by 0940 local time (mean capture time = 0659 h ± 65 min) by attracting them into a mistnet with a playback of a conspecific song. Antshrikes typically hold territories as a pair and the wrens, which breed cooperatively, hold territories as a family group with 2 to 5 individuals (23). We moved male antshrikes and both male and female adults of the monomorphic wrens. We attached a radio transmitter using eyelash adhesive to trimmed feathers on the backs of translocated individuals. A plastic colored leg band was also attached to facilitate identification if the transmitter fell off prematurely. Individuals were moved from unique forested territories to unique release locations (> 100 m from the nearest release for the same species) in 1 of 3 treatments: along a riparian corridor, along a fencerow, or across pasture. Translocation treatments for each distance were chosen a priori from aerial photos. Treatments were not assigned randomly because not all treatments were available from a single territory, but we endeavored to move all of the birds through similar landscapes so that the directly intervening habitat was the primary difference among treatments. Birds were released in fencerow or forest habitat. Forest habitat was diverse, but common tree species included Enterolobium cyclocarpum, Tabebuia rosea, T. ochracea, and Samanea saman. Fencerows were typically Gliricidia sepium, Caesalpinia eriostachys, or Guazuma ulmifolia. Due to the rarity of fencerows in the study area, the same fencerow was used for t wo treatments (one of each species) on 3 occasions. Thus, 17 fencerows were used for 20 translocations. In these cases, we moved an individual of each species differing distances, which resulted in 10 unique fencerows for each species. Most wrens (23 of 30) were sexed by extracting DNA from a whole tail feather (53). The remaining individuals were sexed by comparing their weight, tarsus length, and exposed culmen length to measurements of individuals of known sex using a discriminant function analysis. We translocated 14 female and 16 male wrens. Individuals of both species responded to species-specific songs throughout the 2 field seasons and we found no evidence that season (wet vs. dry) affected territoriality or homing success.
After release, we recorded with radio-telemetry and hand-held GPS units the location of each translocated bird approximately every 15 min (mean = 14.8 min ± 8.2 min standard deviation) for up to 4 days and daily thereafter for 10 days or until they returned, whichever was earlier. Two observers closely followed individuals by simultaneously triangulating their location from a mean distance of 27 m ± 13 m. These positions provided trajectories of moving birds from which we assessed the habitat used for movement and their return time and success. The return of 9 birds that lost their transmitters was checked daily by playing the song of a conspecific at the capture site. We had path information of 1 or more days for each of these 9 birds. Two wrens lost their transmitters, but were still included in the summary of habitat used to return because they lost their transmitters after traveling the majority of the distance back to their territories. Sixty individuals were translocated; 1 bird from each species was translocated in each treatment at each of 10 distances (0.7–1.3 km in 0.1-km intervals, then 1.45, 1.6, and 1.9 km). Even the shortest translocations were well outside the home range of these birds. Although empirical information for the home range size of these species is not available, home range radius was ≈60 m for a congener to the antshrike in Brazil (T. caerulescens; 54) and ≈75 m for a cogener to the wren in Venezuela (C. nuchalis; 55). Riparian corridors were forested and typically between 50 m and 150 m wide. Fencerows were typically 15 m to 30 m wide with little understory.
While following birds, we recorded the distance of all of the gaps crossed that were >15 m. We report this information for each bird as the mean gap width and the number of gaps/km of path where the path of the bird was known. Because the birds only traveled during the day, we calculated total monitoring time by summing the total daylight between a bird's release and its return or end of monitoring. We defined day length as the time between the beginning and end of local civil twilight (56, 57), because observed waking times of these birds most closely matched the beginning of local civil twilight.
Land cover information for the study area was developed from a series of high-resolution (≈1 m pixel size) infrared images taken by the Airborne Sensor Facility at the National Aeronautics and Space Administration as part of the CARTA program during March 2003 (http://asapdata.arc.nasa.gov). Images were orthorectified using a digital elevation model and the coordinates of known locations in the field with the OrthoBASE package in ERDAS IMAGINE 8 (58). Land cover was delineated on these images using ArcGIS (59). The calculation of total tree cover (habitat composition) for each individual was measured inside an ellipse with foci on the release and capture points and an eccentricity of 1.4. This ellipse approximated the region or landscape in which these birds typically moved while returning. The amount of tree cover in riparian corridor treatments, fencerow treatments and pasture treatments was 40%, 31%, and 24%, respectively.
Unless otherwise noted, candidate variables for inclusion in our statistical models were treatment, distance, proportion of tree cover in the ellipse, whether the bird returned, and sex (wrens only). Because n = 30 for most of the analyses, we felt it was inappropriate to include all of the covariates in a single model. Statistical models were built using forward stepwise entry of variables (P < 0.1 for the coefficient for addition) (Tables 1–3). We used P < 0.1 as the threshold for addition to models and considered variables in combined models to be statistically significant at P < 0.05. Analyses were performed using Stata 8.2 (60). Return success and return time analyses used logistic and Cox regression, respectively. Analysis of the mean gap size and gaps/km used gamma regression with a log link function. Posthoc tests for group membership used the test procedure in Stata (60).
Supplementary Material
Acknowledgments.
We thank E. Carman, M. Gamboa-Poveda, and S. Perez-Brenes for superb help in the field; J. Zook for critical assistance and advice at the start of the project; R. Blanco and the Guanacaste Conservation Area for support throughout the project; G. A. Sanchez-Azofeifa for providing imagery used to develop the land cover information; E. Bayne, E. Crone, S. Hannon, M. Lewis and 2 anonymous reviewers whose comments improved the manuscript; and especially the landowners and managers that gave us permission to conduct this work on their land. This work was supported by grants from the Animal Behavior Society, American Ornithologists Union, American Wildlife Research Foundation, Association of Field Ornithologists, Canada Foundation for Innovation, Fund in Support of International Development Activities at the University of Alberta, the International Development Research Centre, the Natural Science and Engineering Research Council (NSERC) and National Geographic Society Committee for Research and Exploration. C.S.G. was supported by scholarships from the NSERC and the Province of Alberta.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19569.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803530105/DCSupplemental.
References
- 1.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 2.González A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science. 1998;281:2045–2047. doi: 10.1126/science.281.5385.2045. [DOI] [PubMed] [Google Scholar]
- 3.da Silva JMC, Tabarelli M. Tree species impoverishment and the future of the Atlantic forest of northeast Brazil. Nature. 2000;404:72–74. doi: 10.1038/35003563. [DOI] [PubMed] [Google Scholar]
- 4.Lens L, Dongen SV, Norris K, Githiru M, Matthysen E. Avian persistence in fragmented rainforest. Science. 2002;298:1236–1238. doi: 10.1126/science.1075664. [DOI] [PubMed] [Google Scholar]
- 5.Beier P, Noss RF. Do habitat corridors provide connectivity? Conserv Biol. 1998;12:1241–1252. [Google Scholar]
- 6.Bélisle M. Measuring landscape connectivity: The challenge of behavioral landscape ecology. Ecology. 2005;86:1988–1995. [Google Scholar]
- 7.Beier P. Dispersal of juvenile cougars in fragmented habitat. J Wildl Manage. 1995;59:228–237. [Google Scholar]
- 8.Tewksbury JJ, et al. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci USA. 2002;99:12923–12926. doi: 10.1073/pnas.202242699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddad NM, et al. Corridor use by diverse taxa. Ecology. 2003;84:609–615. [Google Scholar]
- 10.Levey DJ, Bolker BM, Tewksbury JJ, Sargent S, Haddad NM. Effects of landscape corridors on seed dispersal by birds. Science. 2005;309:146–148. doi: 10.1126/science.1111479. [DOI] [PubMed] [Google Scholar]
- 11.Şekercioğlu ÇH, et al. Disappearance of insectivorous birds from tropical forest fragments. Proc Natl Acad Sci USA. 2002;99:263–267. doi: 10.1073/pnas.012616199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stouffer PC, Bierregaard RO, Jr, Strong C, Lovejoy TE. Long-term landscape change and bird abundance in Amazonian rainforest fragments. Conserv Biol. 2006;20:1212–1223. doi: 10.1111/j.1523-1739.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg DK, Noon BR, Meslow EC. Biological corridors: Form, function, and efficacy. BioScience. 1997;47:677–687. [Google Scholar]
- 14.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca EAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 15.Laurance WF. Reflections on the tropical deforestation crisis. Biol Conserv. 1999;91:109–117. [Google Scholar]
- 16.Fahrig L. When does fragmentation of breeding habitat affect population survival? Ecol Model. 1998;105:273–292. [Google Scholar]
- 17.Diamond J. The Island Dilemma: Lessons of modern Biogeographic studies for the design of nature reserves. Biol Conserv. 1975;7:29–146. [Google Scholar]
- 18.Tilman D, et al. Forcasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 19.St. Clair CC, Bélisle M, Desrochers A, Hannon S. Winter responses of forest birds to habitat corridors and gaps. Conserv Ecol. 1998;2:13. [Google Scholar]
- 20.Sieving KE, Willson MF, de Santo TL. Defining corridor functions for endemic birds in fragmented south-temperate rainforest. Conserv Biol. 2000;14:1120–1132. [Google Scholar]
- 21.Haas CA. Dispersal and use of corridors by birds in wooded patches on an agricultural landscape. Conserv Biol. 1995;9:845–854. [Google Scholar]
- 22.Castellón TD, Sieving KE. An experimental test of matrix permeability and corridor use by an endemic understory bird. Conserv Biol. 2006;20:135–145. doi: 10.1111/j.1523-1739.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 23.Stiles FG, Skutch AF. A guide to the birds of Costa Rica. New York: Cornell Univ Press; 1989. [Google Scholar]
- 24.Desrochers A, Hannon SJ. Gap crossing decisions by forest songbirds during the post-fledging period. Conserv Biol. 1997;11:1204–1210. [Google Scholar]
- 25.Harris RJ, Reed JM. Behavioral barriers to non-migratory movements of birds. Ann Zool Fennici. 2002;39:275–290. [Google Scholar]
- 26.Rodríguez A, Andrén H, Jansson G. Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos. 2001;95:383–396. [Google Scholar]
- 27.Machtans CS, Villard MA, Hannon SJ. Use of riparian buffer strips as movement corridors by forest birds. Conserv Biol. 1996;10:1366–1379. [Google Scholar]
- 28.St. Clair CC. Comparative permeability of roads, rivers, and meadows to songbirds in Banff National Park. Conserv Biol. 2003;17:1151–1160. [Google Scholar]
- 29.Hannon SJ, Schmiegelow FKA. Corridors may not improve the conservation value of small reserves for most boreal birds. Ecol Appl. 2002;12:1457–1468. [Google Scholar]
- 30.Renjifo LM. Composition changes in a Subandean avifauna after long-term forest fragmentation. Conserv Biol. 1999;13:1124–1139. [Google Scholar]
- 31.Sieving KE, Willson MF, de Santo TL. Habitat barriers to movement of understory birds in fragmented south-temperate rainforest. Auk. 1996;113:944–949. [Google Scholar]
- 32.Laurance SG, Stouffer PC, Laurance WF. Effects of road clearings on movement patterns of understory rainforest birds in Central America. Conserv Biol. 2004;18:1099–1109. [Google Scholar]
- 33.Moore RP, Robinson WD, Lovette IJ, Robinson TR. Experimental evidence for extreme dispersal limitation in tropical forest birds. Ecol Lett. 2008;11 doi: 10.1111/j.1461-0248.2008.01196.x. in press. [DOI] [PubMed] [Google Scholar]
- 34.Chetkiewicz CLB, St. Clair CC, Boyce MS. Corridors for conservation: Integrating pattern and process. Annu Rev Ecol Evol Syst. 2006;37:317–342. [Google Scholar]
- 35.Şekercioğlu ÇH, Daily GC, Ehrlich PR. Ecosystem consequences of bird declines. Proc Natl Acad Sci USA. 2004;101:18042–18047. doi: 10.1073/pnas.0408049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sih A, Bell A, Johnson JC. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Brown JH, Kodric-Brown A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology. 1977;58:445–449. [Google Scholar]
- 38.Haugland DL, Larsen KW. Exploration correlates with settlement: Red squirrel dispersal in contrasting habitats. J Anim Ecol. 2004;73:1024–1034. [Google Scholar]
- 39.Selonen V, Hanski I. Habitat exploration and use in dispersing juvenile flying squirrels. J Anim Ecol. 2006;75:1440–1449. doi: 10.1111/j.1365-2656.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 40.Conradt L, Roper TJ. Nonrandom movement behavior at habitat boundaries in two butterfly species: Implications for dispersal. Ecology. 2006;87:125–132. doi: 10.1890/05-0413. [DOI] [PubMed] [Google Scholar]
- 41.Guilford T, Roberts S, Biso D, Rezek I. Positional entropy during pigeon homing II: Navigational interpretation of Bayesian latent state models. J Theor Biol. 2004;227:25–38. doi: 10.1016/j.jtbi.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Stamps JA. In: Dispersal. Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. New York: Oxford Univ Press; 2001. pp. 230–242. [Google Scholar]
- 43.Andrén H. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos. 1994;71:355–366. [Google Scholar]
- 44.Villard MA, Trzcinski KM, Merriam G. Fragmentation effects on forest birds: Relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol. 1999;13:774–783. [Google Scholar]
- 45.Betts MG, Graham JF, Diamond AW, Taylor PD. Independent effects of fragmentation on forest songbirds: An organism-based approach. Ecol Appl. 2006;16:1076–1089. doi: 10.1890/1051-0761(2006)016[1076:ieofof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Bélisle M, Desrochers A, Fortin M-J. Influence of forest cover on the movements of forest birds: A homing experiment. Ecology. 2001;82:1893–1904. [Google Scholar]
- 47.Gobeil JF, Villard MA. Permeability of three boreal forest landscape types to bird movements as determined from experimental translocations. Oikos. 2002;98:447–458. [Google Scholar]
- 48.Gilbert F, González A, Evans-Freke I. Corridors maintain species richness in the fragmented landscapes of a microecosystem. Proc R Soc London Ser B. 1998;265:577–581. [Google Scholar]
- 49.Damschen EI, Haddad NM, Orrock JL, Tewksbury JJ, Levey DJ. Corridors increase plant species richness at large scales. Science. 2006;313:1284–1286. doi: 10.1126/science.1130098. [DOI] [PubMed] [Google Scholar]
- 50.Schmiegelow FKA, Machtans CS, Hannon SJ. Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology. 1997;78:1914–1932. [Google Scholar]
- 51.Stratford JA, Robinson WD. Gulliver travels to the fragmented tropics: Geographic variation in mechanisms of avian extinction. Front Ecol Environ. 2005;3:85–92. [Google Scholar]
- 52.Fischer J, Lindenmayer DB, Manning AD. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front Ecol Environ. 2006;4:80–86. [Google Scholar]
- 53.Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 54.Duca C, Guerra TJ, Marini MA. Territory size of three Antbirds (Aves, Passeriformes) in an Atlantic forest fragment in southeastern Brazil. Revista Brasilleira de Zoologica. 2006;23:692–698. [Google Scholar]
- 55.Yaber MC, Rabenold KN. Effects of sociality on short-distance, female-biased dispersal in tropical wrens. J Anim Ecol. 2002;71:1042–1055. [Google Scholar]
- 56.Nautical Almanac Office. The Astronomical Almanac for the Year 2000. Washington, DC: US GPO; 2000. [Google Scholar]
- 57.Nautical Almanac Office. The Astronomical Almanac for the Year 2002. Washington, DC: US GPO; 2002. [Google Scholar]
- 58.ERDAS, Inc. IMAGINE OrthoBase Pro User's Guide ERDAS IMAGINE Version 8.6. Atlanta, GA: Erdas, Inc.; 2002. [Google Scholar]
- 59.ESRI. ArcGIS Release 9.1. Redlands, CA: Environmental Systems Research Institute; 2005. Environmental Systems Research Institute. [Google Scholar]
- 60.Statacorp. Stata Statistical Software: Release 8.0. College Station, TX: Stata Corporation; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.