Abstract
Previous studies have shown that a dominant negative form of c-Jun (TAM67) suppresses mouse skin carcinogenesis both in vitro and in vivo. The current study identifies Sulfiredoxin (Srx) as a unique target of activator protein-1 (AP-1) activation and TAM67 inhibition. Manipulation of Srx levels by ShRNA or over-expression demonstrates that Srx is critical for redox homeostasis through reducing hyperoxidized peroxiredoxins. In JB6 cells, knockdown of Srx abolishes tumor promoter-induced transformation and enhances cell sensitivity to oxidative stress. Knockdown of Srx also impairs c-Jun phosphorylation, implicating a role for Srx in the feedback regulation of AP-1 activity. Screening of patient tissues by tissue microarray reveals elevated Srx expression in several types of human skin cancers. Our study indicates that Srx is a functionally significant target of AP-1 blockade that may have value in cancer prevention or treatment.
Keywords: skin tumor, tumor promotion, peroxiredoxin
Tumorigenesis is a multistage process that includes initiation, promotion, and progression. Initiation involves mutagenesis while promotion occurs, following chronic exposure to growth factors or environmental agents that alter gene expression. Some of these alterations in gene expression drive the development of benign tumors and their progression to malignancy. Identification of critical gene-expression changes can elucidate mechanisms of carcinogenesis as well as implicate molecular targets for cancer prevention or intervention.
Activator protein 1 (AP-1) activation is required for tumor promoter-induced transformation in cell culture as well as carcinogenesis in mouse models. Upon tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment, AP-1-mediated gene transcription is rapidly stimulated and this effect is augmented through a positive-feedback loop (1). Inhibition of AP-1 activity, either by genetic inactivation or chemical inhibitors, significantly represses skin tumor development and malignant progression in mice (2, 3). TAM67 is an N-terminal deletion mutant of c-Jun, which lacks the transactivation domain but retains the DNA-binding and leucine zipper domains (4). Inhibition of AP-1 activation by TAM67 blocks tumor promoter-induced transformation in vitro (4, 5), skin papilloma formation and progression to carcinoma in vivo (6), and mammary gland tumorigenesis in tumor-prone transgenic mice (7). In mouse JB6 cells, lack of TPA-induced AP-1 activation contributes to the transformation resistant phenotype (8).
Blockade of tumorigenesis and subsequent malignant progression by TAM67 cannot be attributed to inhibition of cell proliferation or cell survival (6). Therefore, we hypothesize that TAM67 targets only a handful of AP-1-dependent genes that are critical for transformation and tumorigenesis. We previously identified a subset of genes whose expression was induced by TPA and inhibited by TAM67 using DNA microarray profiling of K14-TAM67 and wild-type mouse epidermis exposed to tumor promoter (9). Among these target genes are cyclooxygenase-2 (Cox-2), matrix metalloproteinase-10 (MMP-10), urokinase plasminogen activator (Plaur/uPAR), osteopontin (Spp1/Opn) and CD44 (9), which are known to play significant roles in driving tumor promotion and progression. Unique target genes have also been identified. Among them, Sulfiredoxin (Srx) emerges as a gene whose expression is activated by a tumor promoter and downregulated by TAM67.
Srx was first discovered as an enzyme that regulates reactive oxygen species (ROS) signaling by reducing the hyperoxidized peroxiredoxins (Prxs) (10, 11). However, the role of Srx in tumorigenesis is not clear. In this study, we demonstrate that Srx is a transcriptional target of tumor promoter-induced AP-1 activation and is required for tumor promoter-induced transformation in JB6 cells. Srx appears to contribute to a positive feedback regulation of c-Jun activity. Tissue microarray by immunohistochemistry staining reveals elevated Srx expression in several human tumors compared with normal adjacent tissues. Therefore, Srx emerges as a functionally significant target of AP-1 blockade that might be targeted for cancer prevention or treatment.
Results
Induction of Srx Expression by Tumor-Promoter TPA in Mouse Epidermis and JB6 Cells.
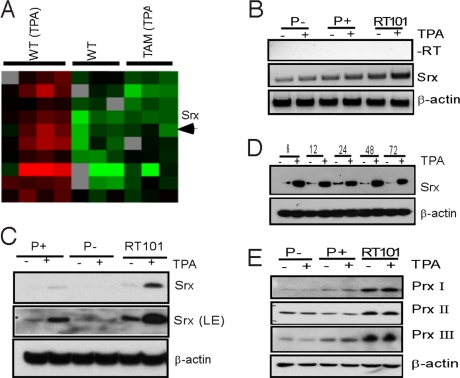
In an effort to identify genes that are stimulated by tumor promoter-induced AP-1 activation and inhibited by TAM67, comparative gene expression profiling of mouse epidermis from 12-dimethylbenz(a)anthracene (DMBA)-initiated wild-type or K14-TAM67 transgenic mice with or without exposure to TPA for 6 h was performed. As expected, only a small set of genes was shown to be significantly induced by a tumor promoter and blocked by TAM67 (9). Among them, Srx emerges as a unique target gene whose induction by TPA was blocked by TAM67. As shown in the heat map in Fig. 1A, TPA stimulated Srx expression in wild-type mice (WT TPA vs. WT no TPA), and this effect was blocked by the expression of TAM67 (TAM TPA vs. WT TPA) in transgenic mice.
Fig. 1.
Induction of Srx expression by TPA in mouse epidermis and JB6 cells. (A) DNA microarray expression profiling of mouse skin epidermis. (B) RT-PCR of Srx mRNA in JB6 cells. (C and D) Western blotting of Srx protein in JB6 cells. LE, longer exposure. (E) Western blotting of Prxs in JB6 cells.
To extend our findings from the mouse epidermal DNA microarrays, we examined the effect of TPA on Srx expression in transformation sensitive (P+) mouse JB6 cells. JB6 cells were originally derived from mouse epidermis and have been widely used in the study of tumor promotion and progression (12). After treatment with TPA for 6 h, a significant increase of Srx transcripts was demonstrated by RT-PCR (Fig. 1B). This stimulatory effect was also reflected at the endogenous protein level, as indicated by Western blotting (Fig. 1C). Interestingly, in transformation resistant (P−) cells, the amount of Srx at either the transcript or the protein level was lower than in transformation sensitive (P+) or transformed (RT101) cells. In contrast to P+ and RT101 cells, TPA produced no detectable effect on the expression of Srx in P− cells. These data suggest that Srx may play a role in TPA-induced transformation in JB6 cells.
A similar preferred expression of Srx in more transformed JB6 cell variants than in transformation-resistant cells was also noted by mRNA differential display in a previous study (13). To further characterize the induction of Srx in P+ cells by tumor promoter, cells were treated with TPA for an extended period. Srx-expression level was consistently induced by TPA treatment for as long as 72 h (Fig. 1D). Induction of Srx by TPA was also relatively specific, as the expression of other closely related proteins, such as Prxs, was not increased by TPA (Fig. 1E). Expression of Prxs in JB6 cells was also consistent with previous findings of Prx I to III expression in rat skin epidermis (14). Taken together, these observations suggest that Srx expression is activated by TPA in the mouse epidermis as well as in JB6 cells.
TPA-Induced Srx Expression Is AP-1-Dependent.
Previous study has found that AP-1 activity is strongly induced in response to TPA in P+ and transformed cells but not in P− cells (8). We then asked whether preferential activation of AP-1 by TPA contributed to the expression of Srx in P+ cells. Through computational analysis of transcription factor binding sites in the Srx promoter (TFSEARCH), we identified consensus NFκB, AP-1, and CREB binding sites in the proximal region of a mouse Srx promoter (Fig. 2A). These sites are also highly conserved in the same regions of rat and human Srx promoters. A series of promoter luciferase-reporter gene constructs was generated and transient transfection assays were performed. As shown in Fig. 2B, TPA strongly stimulated Srx-promoter activity. Deletion of the distal NFκB binding site had no significant effect on the promoter activity. Mutation of either AP-1 binding site decreased TPA-induced promoter activity, and mutation of both AP-1 sites led to a further decrease of both basal and TPA-induced Srx promoter activity (see Fig. 2B). The induction of promoter activity by TPA was also blocked by co-expression of TAM67 (Fig. 2C). Mutation of either binding site significantly compromised TAM67 inhibition, which indicates that both AP-1 binding sites are important for Srx promoter activity. This was also demonstrated by a further loss of TAM67 inhibition in the Srx promoter-reporter in which both AP-1 sites were mutated (Fig. 2C).
Fig. 2.
Srx promoter activity is stimulated by TPA-induced AP-1 activation and blocked by co-expression of TAM67. (A) Srx promoter-luciferase reporter constructs. The mutated AP-1-binding site is indicated by a star (*). (B) Luciferase reporter assay. Relative luciferase unit (RLU) is determined as the ratio of firefly luciferase value versus renila luciferase value. Assays were performed in triplicate and data are presented as x ± SD. *, P > 0.05; **, P < 0.05 (t test). (C) As in (B), but with cotransfection of a TAM67-expression plasmid. RLU is determined as the percentage of luciferase ratio to vector control. **, P < 0.05 (t test). (D) Chromatin immunoprecipitation assays were performed in HA-c-Jun transfected P+ cells using anti-HA antibody.
C-jun is often a major component of the AP-1 transactivation complex and it's phosphorylation serves as an indicator of AP-1 activation (15). To test the role of c-Jun in the regulation of endogenous Srx expression, an HA-tagged c-jun was transiently expressed in P+ cells and chromatin immunoprecipitation (ChIP) assay was performed. In the presence of TPA, HA-c-Jun was rapidly recruited to the endogenous Srx-gene promoter (Fig. 2D), consistent with increased levels of Srx transcript (see Fig. 1B). These observations indicate that c-Jun/AP-1 activation is important for endogenous TPA-induced Srx transcription in P+ cells.
Srx Reduces Hyperoxidized 2-Cys Prxs.
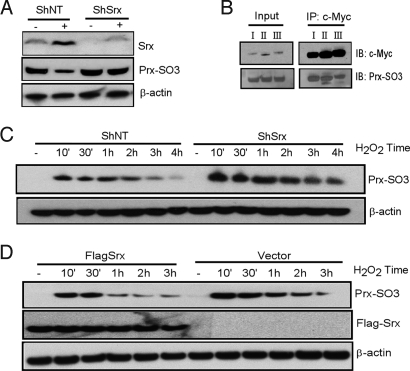
Srx functions as a peroxidase repair protein in yeast and mammalian cells, and plays a critical role in maintaining redox homeostasis through reducing hyperoxidized Prxs (10, 11). As shown in Fig. 1E, Prx I, II, and III were expressed in JB6 cells. However, the levels of Prxs in P+ cells remained unchanged in response to TPA treatment. We transfected an ShRNA construct containing a hairpin structure specific to Srx coding region (ShSrx) or a nontarget control RNA (ShNT) into P+ cells. Expression of ShSrx substantially knocked down basal and TPA-induced Srx expression in these cells (Fig. 3A). Using a specific antibody to recognize the hyperoxidized cysteine residue, we found that the endogenous hyperoxidized forms of Prxs in control cells decreased significantly upon TPA treatment, in accordance with the increase in Srx expression (see Fig. 3A), and this is not because of a change in Prx protein level (see Fig. 1E). In contrast, in Srx knockdown cells, the level of hyperoxidized Prxs did not change in response to TPA treatment (see Fig. 3A).
Fig. 3.
Srx reduces hyperoxidized 2-Cys Prxs in P+ cells. (A) P+ cells were stably transfected with a nontarget ShRNA (ShNT) or an ShRNA construct containing a hairpin structure specific to Srx coding region (ShSrx). Cells were treated with DMSO (−) or TPA (+) for 6 h and Western blotting was performed using whole-cell lysates. (B) Plasmid-expressing Myc-tagged Prx was transfected into P+ cells and immunoprecipitation (IP) was performed with anti-Myc antibody. Western blotting was performed using anti-Myc and anti-Prx-SO3 antibody. (C) Cells stably expressing ShNT or ShSrx were treated with H2O2 and then blotted for hyperoxidized Prxs. (D) As in (C), but cells stably expressing empty vector or Flag-tagged Srx.
The reduction of hyperoxidized Prxs by Srx is limited to 2-Cys Prxs, including I, II, III and IV, and involves Srx-Prx protein-protein interaction (16). Srx was found to interact with Prxs by co-immunoprecipitation in both HEK293T and JB6 cells. However, because of a similar molecular weight, PrxI, II, and III most likely comigrate on the gel. To clarify whether the Prx-SO3 signal arose from a particular isoform of Prxs, we transfected P+ cells with myc-tagged single isoforms of Prx and immunoprecipitated with myc antibody. In these cells, all three isoforms were recognized by anti-Prx-SO3 antibody. There were slight differences in the hyperoxidized protein levels in that the Prx III showed a lower level of oxidation than the others (Fig. 3B).
To investigate the biochemical function of Srx expression in P+ cells, we treated these cells with oxidative stress inducer H2O2. Cells stably expressing ShSrx showed a higher level of hyperoxidized Prxs and a slower rate of reduction of hyperoxidized Prxs than control cells expressing ShNT (Fig. 3C). Inversely, cells stably expressing Flag-Srx showed a lower level of hyperoxidized Prxs and a faster rate of reduction of hyperoxidized Prxs than cells stably expressing a vector control (Fig. 3D). Therefore, through loss-of-function and gain-of-function studies, our data suggest that Srx reduces hyperoxidized Prxs in JB6 cells.
Srx Is Required for Tumor Promoter-Induced Neoplastic Transformation.
The above experiments indicate that Srx is an AP-1 target gene that is critical for maintaining cellular redox homeostasis through reducing hyperoxidized Prxs. We then asked whether Srx was required for TPA-induced transformation in JB6 cells. Anchorage-independent colony formation is a hallmark of transformation and an in vitro correlate of tumorigenicity in vivo (12, 17). Therefore, cells stably expressing control vectors or ShSrx were cultured in soft agar in the presence or absence of TPA, and colony induction was evaluated. TPA strongly induced anchorage-independent colony formation in control P+ cells. Under the same conditions, depletion of Srx by ShSrx completely abolished the transformation response, as shown by lack of colony formation (Fig. 4 A–C).
Fig. 4.
Knockdown of Srx abolishes TPA-induced transformation in P+ cells. (A and B) Microscopic images (×4) of anchorage independent growth of P+ cells stably expressing ShNT (A) or a ShSrx (B) in the presence of TPA. (C) Colonies from random fields under the microscope were counted and number of colonies per field was presented as x ± SD. *, P > 0.05; **, P < 0.05 (t test). (D) Cell proliferation under adherent conditions measured by XTT assay. Data at each time point are from six replicates and are presented as x ± SD.
Knockdown of Srx Does Not Inhibit Cell Proliferation Under Adherent Conditions.
The incapability of Srx knockdown cells to form colonies in soft agar might result from inhibition of cell proliferation. To test whether knockdown of Srx affects cell proliferation, wild-type cells and cells stably expressing vector (ShV), ShNT, or ShSrx were cultured at low density and cell proliferation until confluence was measured by a modified XTT assay. The doubling rate of parental P+ cells was similar to previous findings and did not change with the expression of an empty vector or nontarget shRNA. Compared with control cells, knockdown of Srx did not inhibit cell proliferation under adherent conditions (Fig. 4D).
Knockdown of Srx Impairs c-Jun Activation and Increases Cell Death Under Oxidative Stress Conditions.
We hypothesized that the transformation resistant phenotype of Srx knockdown cells might result from a change in kinase signaling. Therefore, we treated these cells with EGF to investigate the MAPK signaling. Upon EGF treatment, Srx expression in control cells was increased but not in knockdown cells [supporting information (SI) Fig. S1]. Srx knockdown cells also showed slightly increased MAPK signaling, as indicated by increases in EGF receptor phosphorylation and activation of downstream kinases (Fig. 5A). Surprisingly, C-Jun was less activated in Srx knockdown cells than in control cells, as indicated by the levels of Ser-73 phosphorylation upon EGF treatment (see Fig. 5A). JNK 1 and 2, the major upstream kinases that activate c-Jun (18), showed impaired activation in Srx knockdown cells in response to EGF treatment (see Fig. 5A). It is noteworthy that the total protein levels of JNK1/2 in Srx knockdown cells were lower than in control cells (see Fig. 5A).
Fig. 5.
Knockdown of Srx impairs AP-1 activation. (A and B). Cells after serum starvation were treated with DMEM containing 50 ng/ml EGF (A) or 5% FBS (B) for indicated time and Western blotting was performed. (C and D). Assay of 4XAP-1 luciferase reporter activity in Srx knockdown cells (C) or in cells co-expressed with Srx (D). Assays were performed in triplicate and data are presented as x ± SD. **, P < 0.05 (t test).
To ask whether the impairment of EGF-induced c-Jun activation in Srx deficient cells extends to other inducers, we investigated c-Jun activation by serum addition after starvation. Serum addition increased Srx expression in control cells but not in Srx knockdown cells (see Fig. S1A). As shown in Fig. 5B, addition of serum rapidly induced Ser-73 phosphorylation of C-Jun. However, in Srx knockdown cells, addition of serum showed a much weaker induction of Ser-73 phosphorylation within the time frame examined. The c-Jun phosphorylation correlated with the time course of JNK1/2 activation in these cells, and the lack of activation in Srx knockdown cells (Fig. 5B). The basal protein levels of JNK1/2 were also lower in Srx knockdown cells, contributing to the lack of activated JNK1/2 (see Fig. 5 A and B). TPA is known to activate c-Jun by inducing phosphorylation (8). Knockdown of Srx also impaired TPA-induced Ser-73 phosphorylation (see Fig. S1B). Again, this appears to be because of reduced basal JNK1/2 protein levels and impaired JNK1/2 activation in these cells.
The above observations raise the possibility that Srx functions as a positive regulator of c-Jun activation in addition to being a target of AP-1 activation. Indeed, knockdown of Srx significantly repressed AP-1-mediated luciferase activity (Fig. 5C). This inhibitory effect was relatively specific, as other signaling pathways, such as NFκB signaling, were not affected (Fig. S2A). In contrast, when Srx was over-expressed in P+ cells, it strongly stimulated basal as well as TPA-induced AP-1 activity (Fig. 5D). Under the same conditions, over-expression of Srx in these cells did not affect NFκB signaling (Fig. S2B). Therefore, Srx appears to be required for sustained c-Jun/AP-1 activation through a positive feedback loop.
As c-Jun activity is important for cell growth and survival (19, 20), we further examined the tolerance threshold of Srx to oxidative stress by XTT assay. As shown in Fig. S3, Srx knockdown cells were much more sensitive than control cells to hydrogen peroxide-induced cell death. The IC50 of Srx knockdown cells was around 20 μM, while the control cells had an IC50 of around 100 μM (see Fig. S3B). This effect was most likely a result of the knockdown of Srx in these cells, because expression of ShV or a ShNT did not have a similar effect (see Fig. S3A). Therefore, the impaired AP-1 activation and increased susceptibility of Srx knockdown cells to oxidative stress may contribute to the lack of transformation response in these cells, consistent with an earlier observation of decreased antioxidant response and excessive toxicity by oxidant promoters in transformation resistant JB6 cells (21).
Srx Is Highly Expressed in Certain Types of Human Tumors.
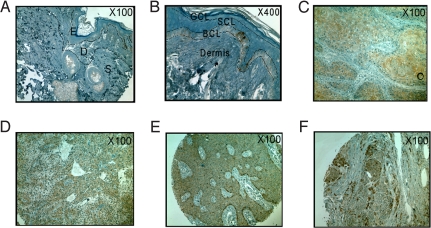
To evaluate the expression of Srx in human skin tissues, tissue microarrays were analyzed by anti-Srx immunohistochemistry. Strong punctuate staining of Srx was found to be limited to the basal cell layer of the epidermis in normal skin (Fig. 6 A and B). Positive staining (brown) was mainly found in tumor tissues from patients with squamous cell carcinoma (SCC, 88.1%), sweat gland carcinoma (SGC, 81.8%), basal cell carcinoma (BCC, 74.4%) and melanoma (65.2%) (Fig. 6 C–F and Table S1). A significant portion of tissues from BCC or melanoma patients were very strongly positive (+++), with a percentage of 27.9% and 34.8%, respectively. In contrast, with the exception of the basal cell layer, an overall negative staining was found in skin tissues from patients with hyperplasia or chronic inflammation (see Table S1). Tissues from patients with condyloma, papilloma, or dermatofibrosarcoma were negative or weakly positive. Therefore, Srx was found to be highly expressed in certain human skin malignancies and might have potential value in diagnosis, prevention, or treatment of these tumors.
Fig. 6.
Representative immunohistochemistry staining of Srx in normal human skin (A and B), squamous cell carcinoma (C), sweat gland carcinoma (D), basal cell carcinoma (E), and melanoma (F). Note the positive (+) staining of basal cell layer in (A) and (B); (C) and (D), strong positive (++); (E) and (F), very strong positive (+++). BCL, basal cell layer; D, dermis; E, epidermis; GCL, granular cell layer; S, subcutis; SCL, squamous cell layer.
To extend our findings to other cancer sites, we examined Srx expression in cancer tissues from a variety of organ and tissue origins. A total number of 42 patient tissues (including tumors from esophagus, stomach, colon, rectum, liver, lung, kidney, breast, cervix, ovary, bladder, lymph node, brain, and prostate) and 42 paired, adjacent normal control tissues have been examined. Compared with adjacent normal tissue, Srx expression was increased in several cases. In particular, Srx was highly expressed in rectal carcinoma and lung adenocarcinoma but not in paired normal tissues (Fig. S4). Therefore, it is possible that Srx may also play a more general role in other cancer sites.
Discussion
In this study we identified sulfiredoxin as a functionally significant target of AP-1 blocker TAM67 when it specifically inhibited tumorigenesis in mice. AP-1 activation was found to be required for TPA-induced Srx gene expression. In JB6 cells, Srx interacts with peroxiredoxins and regulates the levels of hyperoxidized peroxiredoxins. Deficiency of Srx renders JB6 P+ cells resistant to tumor promoter-induced transformation. This loss of transformation response is not attributable to a general inhibition of cell proliferation. Srx is also found to be highly expressed in several human cancer tissues, suggesting that Srx might be targeted for cancer prevention or treatment.
The Prx/Srx Axis and Tumorigenesis.
There are six isoforms of Prxs in mammals, with distinct cellular locations, including the cytosol (Prx I, II, and VI), nucleus (Prx I), mitochondria (Prx III, VI), peroxisomes (Prx V), and endoplasmic reticulum (IV) (22). It is possible that different subcellular distributions of Prx isoforms may have distinct consequences for cellular function. The typical 2-Cys Prxs, including I, II, III, and IV, are the best-characterized members of the Prx family. In addition to scavenging free radicals and ROS through the conserved cysteine residues, these Prxs also participate in multiple cell-signaling pathways, such as PDGF receptor signaling (23).
Considerable evidence implicates Prx I as a putative tumor suppressor. Prx I was first identified as a gene activated during cell proliferation in human mammary epithelial cells (24), and was later found to interact with the oncoprotein c-Abl and to inhibit its tyrosine kinase activity (25). Loss of Prx I in mice leads to premature death from spontaneous cancer formation in multiple organs (26), supporting the notion that Prx I functions as a tumor suppressor. This activity is mediated, at least in part, through interaction with c-Myc and selective alterations of its target gene expression (27, 28). Prx I over-expression significantly reduces colony formation in vitro and tumorigenicity of Myc-transformed fibroblasts in vivo (28), whereas Prx I knockout cells are much more susceptible to ras transformation alone (27). Prxs I and II also function as molecular chaperones to enhance resistance to heat shock and oxidative stress in yeast and mammals (29, 30)
Prxs are rapidly inactivated through hyperoxidation of the catalytic cysteine residue during hydrogen peroxide-mediated cell signaling. Hyperoxidation of Prxs results in inactivation of peroxidase function and facilitates proteasome-mediated protein degradation (31, 32). In the presence of ATP and magnesium, the hyperoxidized form of Prxs can be reduced back to the active form through interaction with Srx (10, 11, 32), and this function of Srx is specific to 2-Cys Prxs including I, II, III, and IV. Expression of Srx increases cells' ability to scavenge hydrogen peroxide and protects them from ROS-induced cell senescence and apoptosis (33, 34).
Given the importance of Prxs in tumorigenesis, it is noteworthy that Srx is also required for tumor promoter-induced transformation in JB6 cells. Because several Prxs are expressed in these cells and in mouse epidermis, a combinatorial effect, rather than a particular Prx-Srx interaction, may contribute to this process. It is of future interest to investigate if other functions of Srx may also be required in addition to its peroxidase reductase activity.
In this study, Srx emerges as a target of AP-1 blockade in vivo when it specifically inhibits tumorigenesis, and is functionally significant in driving tumor promoter-induced transformation in the mouse JB6 model, a model shown to be predictive for in vivo outcomes (5, 6). However, whether Srx deficiency will render mice resistant to carcinogenesis in vivo needs to be further investigated.
Srx Acts Upstream and Downstream of AP-1.
Our study reveals that loss of tumor promoter-sensitive phenotype in P+ cells upon Srx depletion involves an impaired AP-1 activation and increased cell death under oxidative stress conditions. Therefore, Srx is not only an AP-1 target gene but also contributes to the activation of c-Jun. This suggests that Srx is a unique component of a positive feedback loop regulating AP-1 activity. This is achieved at least in part through the regulation of JNK1/2 kinase activity and protein levels.
The regulation of JNK by Srx may occur through a secondary mechanism, as we were unable to detect direct association of Srx with JNK1/2 or c-Jun by co-immunoprecipitation. Hyperoxidized Prxs have been reported to inhibit JNK activities either by removal of hydrogen peroxide (35), or by directly interacting with GSTpi-JNK complex (36). Therefore, Srx may positively regulate JNK activity by maintaining a constant level of reduced Prxs, facilitating the release and activation of JNK from the GSTpi-JNK complex (36).
The regulation of c-Jun activity by Srx appears to occur mainly through JNK1/2-mediated phosphorylation. However, this does not exclude the regulation of c-Jun activity through other mechanisms involving antioxidant proteins. For example, a recent study demonstrated that depletion of another redox protein, Ref-1, led to loss of transformation and impaired AP-1 activation in JB6 cells (37). In yeast, activation of Tpx1 (yeast homologue of Prx I) by Srx facilitated reduction of oxidized cysteine in PAP1 (yeast homologue of c-Jun) and activated PAP1-dependent gene transcription (33, 38). Furthermore, Srx also plays a positive role in the deglutathionylation of multiple proteins, including PTP 1B, and thus promotes cell proliferation (39, 40), which may also contribute to tumorigenesis.
Srx Expression in Human Tumors.
Elevated expression of major regulators of ROS signaling, such as Prxs and Trxs, has been identified in a wide range of human cancers (41). Using a tissue microarray, we showed that Srx was expressed in some basal cells of normal skin epidermis. The identity of these Srx-expressing cells is still unclear. Interestingly, we also showed that Srx was highly expressed in skin tumors, including SCC, SGC, BCC, and melanoma, representing several histological origins. This finding also extends to other human tumors, as elevated expression of Srx is evident in rectal carcinoma and lung adenocarcinoma but not in their paired normal tissues. Therefore, Srx may be a target gene of potential value for cancer prevention or treatment. In the future, it will be of interest to investigate whether Srx expression correlates with the progression of malignancy in tumors with clinically defined stages.
Materials and Methods
DNA Microarray and Cell Culture.
JB6 cells were cultured in standard conditions with 4% FBS. DNA microarrays, transient transfections, and luciferase reporter assays were performed as previously reported (9). For cell growth and proliferation assay, 103 cells were plated in each well of 96-well plate. For cell viability assay, 2 × 104 cells were plated in each well of 96-well plate. Modified XTT assays for cell proliferation and cell survival were performed following commercial kit protocols (Roche).
Cloning, RT-PCR and Chromatin Immunoprecipitation.
Mouse Srx promoter was amplified from genomic DNA and cloned into upstream of the luciferase coding region in the pGL3 vector (Promega). Mutation of the AP-1 site in the Srx promoter was achieved by site-directed mutagenesis (Stratagene) and the mutation was confirmed by DNA sequencing. Total mRNA from JB6 cells was extracted and purified following commercial kit protocols (Qiagen). Reverse transcriptase PCR (RT-PCR) was performed using a gene-specific primer and SuperScript reverse transcriptase (Invitrogen). Chromatin immunoprecipitation assay of P+ cells transfected with HA-c-Jun was performed using anti-HA antibody (Roche) and commercial kit (Upstate). To construct C-terminal Myc-tagged peroxiredoxin expression plasmids, human peroxiredoxins I, II, and III coding regions were amplified from HEK293T total mRNA by RT-PCR and cloned into BamH I/XhoI sites of pcDNA3.1-MycHis expression vector. The inserted sequences were confirmed by DNA sequencing. Plasmid-expressing Flag-Srx and mouse anti-Srx mAb were kindly provided by Dr. Kenneth Tew (Medical University of South Carolina).
Western Blotting and Immunoprecipitation.
Western blotting and immunoprecipitation were performed using standard procedures. Antibodies used were, Sulfiredoxin, c-Jun, and c-Myc (Santa Cruz), β-actin, and Flag (Sigma-Aldrich), Prx I, II, III, and PrxSO3 (Abcam). Other antibodies, including EGFR, phosphor-EGFR, phosphor-c-Jun, phosphor-MEK1/2, phosphor-ERK1/2, phosphor-JNK1/2, and JNK were from cell signaling.
Knockdown of Srx in JB6 Cells.
JB6 cells were transfected with mission ShRNA (Sigma-Aldrich) and selected in medium containing 1-μg/ml Puromycin. MISSION shRNA lentiviral transduction particles were also used to infect JB6 cells to produce pools of Srx knockdown cells. Control vectors and virus particles used were MISSIONpLKO.1-puro control vector, MISSION Non-Target shRNA control vector, MISSION pLKO.1-puro control transduction particles, and MISSION Non-Target shRNA control transduction particles. Knockdown experiments were designed according to previous suggestions (42).
Transformation in Soft Agar.
Anchorage-independent transformation assays were performed using a modified CytoSelect 96-well cell transformation assay kit (Cell Biolabs). Briefly, cells were plated in soft agar in a 96-well plate at 2,000 cells per well. Culture medium containing TPA or solvent was changed every 3 days and cells were in culture for 2 weeks. Colonies were counted and images were taken under microscope (×4) and analyzed using the Image-Pro software (MediaCybernetics).
Tissue Microarray Immunohistochemistry.
Tissue slides containing various human tumor samples, skin diseases, or paired adjacent normal skin tissues were commercially obtained (Biomax). After deparaffinization and antigen retrieval according to the manufacturer's suggestions, anti-Srx immunohistochemistry staining was performed using anti-Srx antibody with a commercial kit (R & D Systems). Slides were counterstained with Hematoxylin (Sigma) and mounted after dehydration. Staining images were taken under microscope (×100, or otherwise specified) and were evaluated and scored by using the Image-Pro software.
Supplementary Material
Acknowledgments.
We thank Dr. Kenneth Tew for kindly providing reagents. We also thank Dr. Matthew Young and Ms. Alyson Baker for help with the mouse epidermis and JB6 models, and all other members of the Colburn laboratory for thoughtful discussions and suggestions. Q.W. is a recipient of the Cancer Research Training Award from the National Cancer Institute. This work was supported in part by the Intramural Research Program of the National Cancer Institute (N.H.C).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810676105/DCSupplemental.
References
- 1.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product. Jun/AP-1 Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 2.Saez E, et al. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 3.Zenz R, et al . c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd A, Yancheva N, Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991;352:635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young MR, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Q, et al. Targeting the activator protein 1 transcription factor for the prevention of estrogen receptor–negative mammary tumors. Cancer Prev Res 1. 2008 doi: 10.1158/1940-6207.CAPR-08-0034. first published on line March 31, 2008, 2010.1158/1940–6207.CAPR-2008–0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- 9.Matthews CP, et al. Dominant-negative activator protein 1 (TAM67) targets cyclooxygenase-2 and osteopontin under conditions in which it specifically inhibits tumorigenesis. Cancer Res. 2007;67:2430–2438. doi: 10.1158/0008-5472.CAN-06-0522. [DOI] [PubMed] [Google Scholar]
- 10.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 11.Chang TS, et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 12.Colburn NH, et al. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978;38:624–634. [PubMed] [Google Scholar]
- 13.Sun Y, Hegamyer G, Colburn NH. Molecular cloning of five messenger RNAs differentially expressed in preneoplastic or neoplastic JB6 mouse epidermal cells: one is homologous to human tissue inhibitor of metalloproteinases-3. Cancer Res. 1994;54:1139–1144. [PubMed] [Google Scholar]
- 14.Lee SC, et al. Peroxiredoxin is ubiquitously expressed in rat skin: isotype-specific expression in the epidermis and hair follicle. J Invest Dermatol. 2000;115:1108–1114. doi: 10.1046/j.1523-1747.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 16.Woo HA, et al. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 17.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 18.Minden A, et al . Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 19.Hilberg F, Aguzzi A, Howells N, Wagner EF. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 21.Jain PT, et al. Differential cytotoxicity in mouse epidermal JB6 cells: a potential mechanism for oxidant tumor promotion. Mol Carcinog. 1994;11:164–169. doi: 10.1002/mc.2940110307. [DOI] [PubMed] [Google Scholar]
- 22.Tavender TJ, Sheppard AM, Bulleid NJ. Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem J. 2008;411:191–199. doi: 10.1042/BJ20071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi MH, et al . Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 24.Prosperi MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993;268:11050–11056. [PubMed] [Google Scholar]
- 25.Prosperi MT, Ferbus D, Rouillard D, Goubin G. The pag gene product, a physiological inhibitor of c-abl tyrosine kinase, is overexpressed in cells entering S phase and by contact with agents inducing oxidative stress. FEBS Lett. 1998;423:39–44. doi: 10.1016/s0014-5793(98)00057-x. [DOI] [PubMed] [Google Scholar]
- 26.Neumann CA, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 27.Egler RA, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 28.Mu ZM, Yin XY, Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002;277:43175–43184. doi: 10.1074/jbc.M206066200. [DOI] [PubMed] [Google Scholar]
- 29.Jang HH, et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Moon JC, et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J Biol Chem. 2005;280:28775–28784. doi: 10.1074/jbc.M505362200. [DOI] [PubMed] [Google Scholar]
- 31.Jeong W, Park SJ, Chang TS, Lee DY, Rhee SG. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J Biol Chem. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- 32.Jonsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozonet SM, et al. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 34.Rey P, et al. The Arabidopsis thaliana sulfiredoxin is a plastidic cysteine-sulfinic acid reductase involved in the photooxidative stress response. Plant J. 2007;49:505–514. doi: 10.1111/j.1365-313X.2006.02969.x. [DOI] [PubMed] [Google Scholar]
- 35.Kang SW, et al. Cytosolic peroxiredoxin attenuates the activation of Jnk and p38 but potentiates that of Erk in Hela cells stimulated with tumor necrosis factor-alpha. J Biol Chem. 2004;279:2535–2543. doi: 10.1074/jbc.M307698200. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, et al. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66:7136–7142. doi: 10.1158/0008-5472.CAN-05-4446. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Misner BJ, Chiu RJ, Meyskens FL., Jr. Redox effector factor-1, combined with reactive oxygen species, plays an important role in the transformation of JB6 cells. Carcinogenesis. 2007;28:2382–2390. doi: 10.1093/carcin/bgm128. [DOI] [PubMed] [Google Scholar]
- 38.Vivancos AP, et al. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Findlay VJ, et al. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei K, Townsend DM, Tew KD. Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds A, et al. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.