Abstract
Rat saliva agglutinated Streptococcus mutans Ingbritt and NCTC 10449 and Streptococcus sanguis NCTC 7864 but not S. mutans NCTC 10921, GS 5, or LM 7, Streptococcus sobrinus 6715-13 or OMZ 65, or Streptococcus cricetus HS 6, as measured turbidometrically. The specificity of agglutination by rat saliva was the same as that by human saliva. Agglutination was associated with a mucin complex (rat salivary agglutinin complex [rS-A]) of sulfated sialoglycoproteins, with a trace of associated lipid and an apparent Mr of 1.6 X 10(6), isolated by gel-filtration Fast Protein Liquid Chromatography. The complex was dissociated in a high-ionic-strength buffer containing 6 M urea and then fractionated by gel filtration and anion-exchange Fast Protein Liquid Chromatography into four sulfated sialoglycoprotein components, designated rS-A-1Q1, rS-A-1Q2, rS-A-1Q3, and rS-A-2, with rS-A-1Q2 being polydisperse through differential glycosylation of the polypeptide backbone. The dissociation destroyed agglutination activity. The polypeptide backbones contained up to 42% serine plus threonine and up to 40% glycine plus alanine plus proline plus valine. The carbohydrate moiety of the rS-A sialoglycoproteins consisted of N-acetylgalactosamine, sialate, galactose, fucose, N-acetylglucosamine, and small amounts of mannose, with the predominant sugar being N-acetylgalactosamine. Agglutination was inhibited by 1 mM EDTA but was restored by 1.5 mM CaCl2. Agglutination was also inhibited by 5 mM CaCl2; nonimmune sera; cationic polymers; and wheat germ, lentil, soybean, and peanut lectins. However, agglutination was not affected by lipoteichoic acid, various anionic proteins, or various sugars. Neuraminidase treatment of rS-A did not affect activity, but tryptic digestion of S. mutans did prevent agglutination. The results are consistent with calcium bridging the negative groups within the rS-A complex and allowing the approach of rS-A to the bacterial cell surface to effect a specific conformational attachment.
Full text
PDF


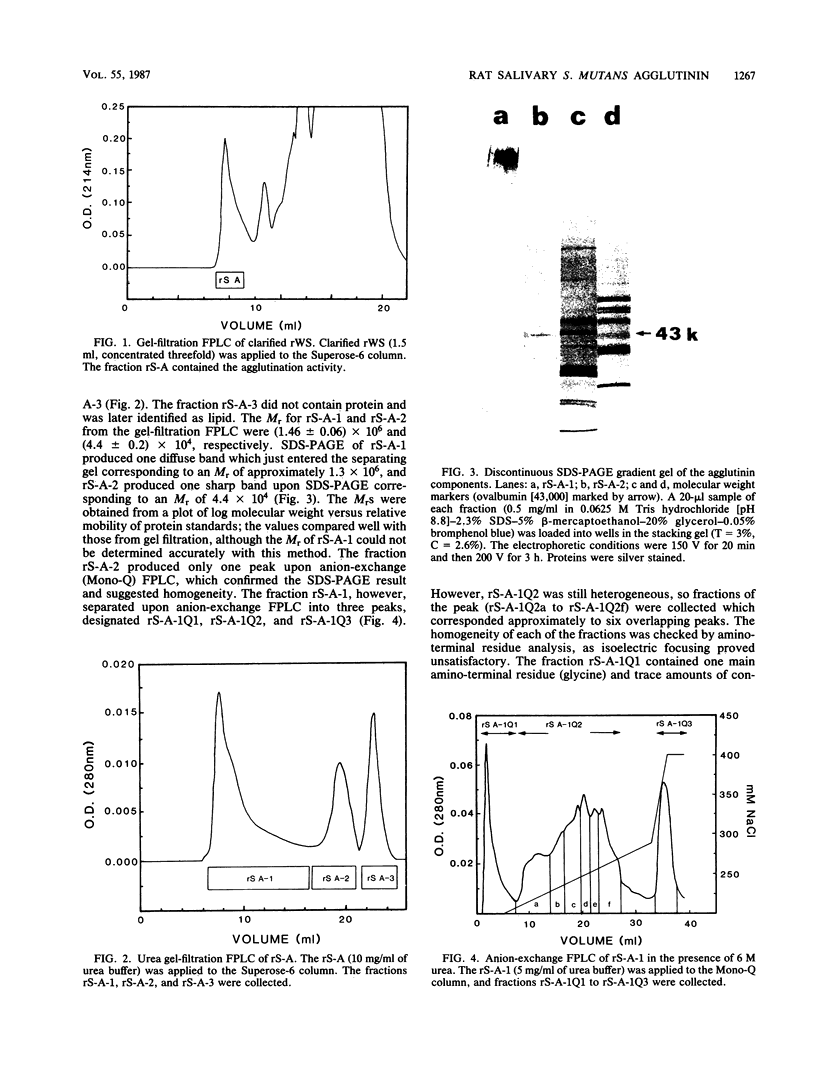
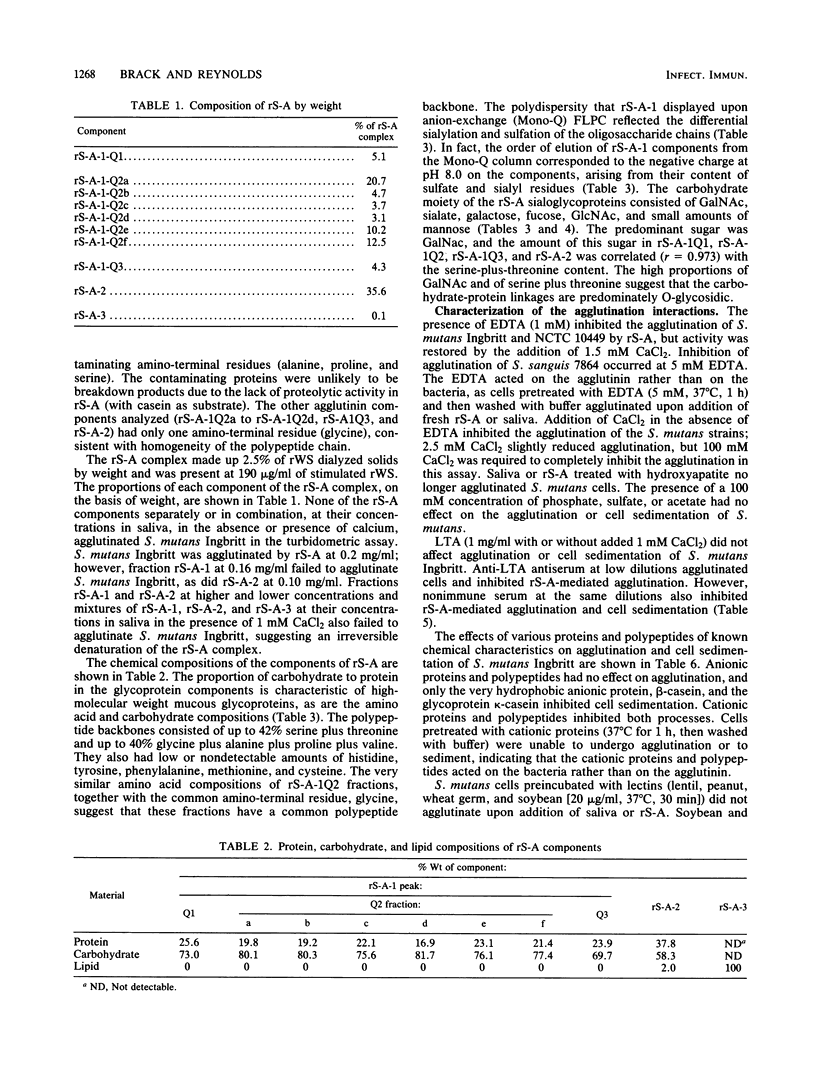
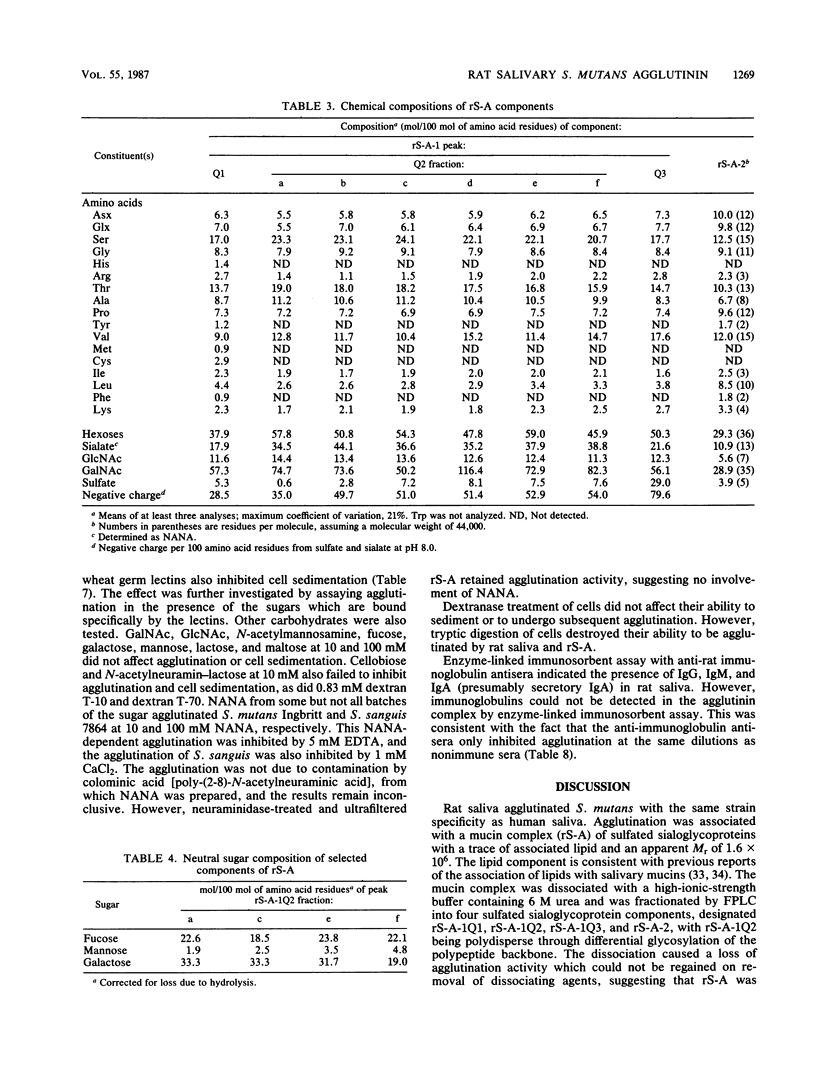



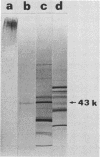
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Clarke A. E., Denborough M. A. The interaction of concanavalin A with blood-group-substance glycoproteins from human secretions. Biochem J. 1971 Mar;121(5):811–816. doi: 10.1042/bj1210811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson D., Bratthall D., Björck L., Myhre E., Kronvall G. Interactions between human serum proteins and oral streptococci reveal occurrence of receptors for aggregated beta 2-microglobulin. Infect Immun. 1979 Jul;25(1):279–283. doi: 10.1128/iai.25.1.279-283.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L. N., Knox K. W., Brown R. A., Wicken A. J., Fitzgerald R. J. Comparison of extracellular protein profiles of seven serotypes of mutans streptococci grown under controlled conditions. J Gen Microbiol. 1986 May;132(5):1389–1400. doi: 10.1099/00221287-132-5-1389. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Hogg S. D., Embery G. The isolation and partial characterization of a sulphated glycoprotein from human whole saliva which aggregates strains of Streptococcus sanguis but not Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):791–797. doi: 10.1016/0003-9969(79)90040-2. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Johnson G. S., White B., Scocca J. An accelerated system for analysis of neutral sugars in complex carbohydrates. Anal Biochem. 1971 Oct;43(2):640–643. doi: 10.1016/0003-2697(71)90301-0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- Malamud D., Brown C., Goldman R. Inhibition of bacterial aggregation by serum- and blood-derived proteins. Infect Immun. 1984 Jan;43(1):386–390. doi: 10.1128/iai.43.1.386-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H., Yoshii J., Nakamura R., Tsunemitsu A. The effects of human saliva and concanavalin A on the agglutination of oral Streptococci. Arch Oral Biol. 1981;26(2):127–130. doi: 10.1016/0003-9969(81)90082-0. [DOI] [PubMed] [Google Scholar]
- Oemrawsingh I., Roukema P. A. Isolation, purification and chemical characterization of mucins from human submandibular glands. Arch Oral Biol. 1974 Aug;19(8):615–626. doi: 10.1016/0003-9969(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Reynolds E. C., Wong A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect Immun. 1983 Mar;39(3):1285–1290. doi: 10.1128/iai.39.3.1285-1290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. C., del Rio A. Effect of casein and whey-protein solutions on caries experience and feeding patterns of the rat. Arch Oral Biol. 1984;29(11):927–933. doi: 10.1016/0003-9969(84)90093-1. [DOI] [PubMed] [Google Scholar]
- Rundegren J., Ericson T. An evaluation of the specificity of salivary agglutinins. J Oral Pathol. 1981 Aug;10(4):261–268. doi: 10.1111/j.1600-0714.1981.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Simonson L. G., Reiher D. A. Effect of human saliva and various compounds on the adsorption of the bacterium Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1981;26(2):143–146. doi: 10.1016/0003-9969(81)90085-6. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Takagi A., Tsukada H., Kosmala M., Slomiany A. Fatty acid acylation of salivary mucin in rat submandibular glands. Arch Biochem Biophys. 1985 Nov 1;242(2):402–410. doi: 10.1016/0003-9861(85)90224-3. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Witas H., Murty V. L., Slomiany A., Mandel I. D. Association of lipids with proteins and glycoproteins in human saliva. J Dent Res. 1983 Jan;62(1):24–27. doi: 10.1177/00220345830620010601. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE W. W., VANDER A. J., SKRENTNY B. A., DEJONG R. N. A study of lipids in the cerebrospinal fluid. II. The determination of total lipids. J Lab Clin Med. 1958 Sep;52(3):481–490. [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Mirels L., Monte L. D., Ridall A. L., Levine M. J., Loomis R. E., Lindauer F., Reddy M. S., Baum B. J. Isolation and characterization of a mucin-glycoprotein from rat submandibular glands. Arch Biochem Biophys. 1985 Nov 1;242(2):383–392. doi: 10.1016/0003-9861(85)90222-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]