Abstract
The requirement for TRPV6 for vitamin D-dependent intestinal calcium absorption in vivo has been examined by using vitamin D-deficient TRPV6 null mice and littermate wild-type mice. Each of the vitamin D-deficient animals received each day for 4 days 50 ng of 1,25-dihydroyvitamin D3 in 0.1 ml of 95% propylene glycol:5% ethanol vehicle or vehicle only. Both the wild-type and TRPV6 null mice responded equally well to 1,25-dihydroxyvitamin D3 in increasing intestinal calcium absorption. These results, along with our microarray data, demonstrate that TRPV6 is not required for vitamin D-induced intestinal calcium absorption and may not carry out a significant role in this process. These and previous results using calbindin D9k null mutant mice illustrate that molecular events in the intestinal calcium absorption process in response to the active form of vitamin D remain to be defined.
Keywords: microarrays, TRPV6 KO mice, vitamin D, vitamin D mechanism, calcium channel
A primary function of vitamin D is to markedly increase intestinal absorption of calcium and phosphate (1). During the 1950s, this absorption was shown to be primarily an active calcium transport process and an independent active phosphate transport process (2, 3). With the discovery of the vitamin D endocrine system came the understanding that this process was directly stimulated by the hormonal form of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3) (4). Because this hormonal form of vitamin D is regulated in response to the need for calcium, it became clear that the endogenous factor discovered by Nicolaysen and Egg-Larsen is the vitamin D endocrine system producing the final vitamin D hormone, 1,25-(OH)2D3 (4, 5). There is still debate whether vitamin D further influences the diffusional component of calcium absorption, taking place at high intestinal levels of calcium (6, 7).
The molecular mechanism underlying active calcium transport in response to vitamin D began unraveling with the discovery of calbindin D9k by Wasserman and colleagues (8). Schachter and colleagues (9) also found a transporter responsive to vitamin D. Others have reported that vitamin D stimulates the basal lateral membrane calcium ATPase believed to be a calcium transporter (10, 11). These components have been put together in a diagrammatic fashion to present the current hypothesis of how 1,25-(OH)2D3 stimulates active intestinal calcium absorption. TRPV6 is a calcium channel protein clearly induced by 1,25-(OH)2D3 (12). Calcium entering through this channel is believed to associate with calbindin D9k, which serves as a shuttle for calcium, presenting it to the basal lateral membrane calcium ATPase. This step provides the energy input for the transfer of calcium against a concentration gradient. All three of these genes are clearly under the influence of the active form of vitamin D (13). Unfortunately not all evidence is currently in support of this hypothesis. Transgenic mice in which calbindin D9k has been eliminated have shown that calbindin D9k is not required for 1,25-(OH)2D3-stimulated intestinal calcium absorption (14, 15). Focus has now been placed on the TRPV6, the 1,25-(OH)2D3-induced calcium channel, at the apical membrane. A surprising result occurred when a double knockout of calbindin D9k and the TRPV6 reduced but did not eliminate active calcium transport (16). The question remained as to whether TRPV6 is the key factor in 1,25-(OH)2D3-induced intestinal calcium absorption.
In the present study, we have carried out an examination by using TRPV6 null mice to ask the question of whether this protein is required for the vitamin D-induced intestinal calcium absorption phenomenon. The results reported herein show that the TRPV6 is not required for the vitamin D-induced intestinal calcium absorption. Therefore, the key protein(s) induced by the vitamin D hormone responsible for intestinal calcium absorption has yet to be identified.
Results
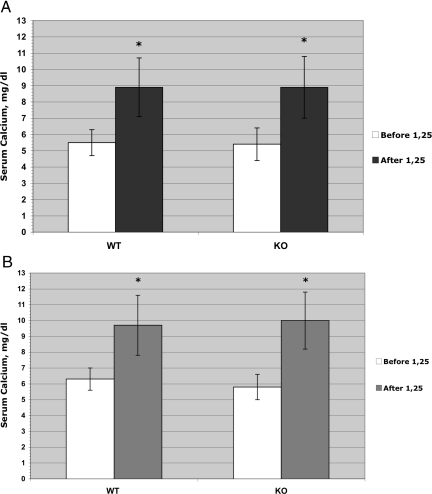
Vitamin D-deficient wild-type mice respond as expected to the administration of 1,25-(OH)2D3 with an elevation of serum calcium. This is true of both males and females, and because these animals are maintained on a 0.47% calcium diet, it is likely that the serum calcium response is largely because of intestinal absorption of calcium. Of considerable interest is that the TRPV6 knockout (KO) mice show at least an equal elevation of serum calcium in the case of males and a significant elevation of serum calcium in females (Fig. 1). These results alone argue that the ability of the intestine to respond to 1,25-(OH)2D3 in terms of calcium absorption is not impaired in the TRPV6 KO animals.
Fig. 1.
Serum calcium concentration in wild-type and TRPV6 mutant male (A) and female (B) mice after 1,25-(OH)2D3 administration. Five- to six-month-old vitamin D3-deficient wild-type and KO mice were injected i.p. daily with 50 ng 1,25-(OH)2D3 in 95% propylene glycol/5% ethanol or vehicle (95% propylene glycol/5% ethanol) for 3 consecutive days. Mice were bled on day 4 via their maxillary veins to determine serum calcium level. Data are expressed as mean ± SD (n = 8–12). Blood samples were processed according to the method described in Material and Methods. *, Difference with control (vehicle) is statistically significant (P < 0.005).
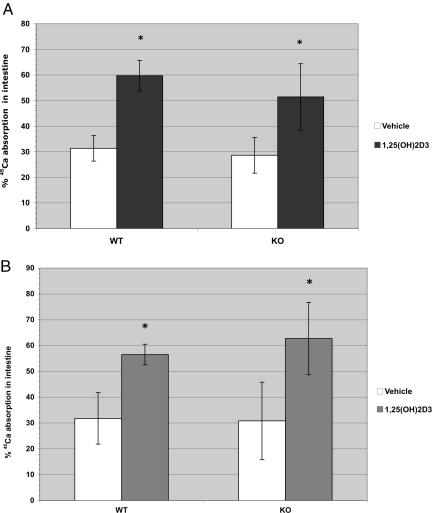
Nevertheless, a direct measurement of intestinal calcium absorption in response to 1,25-(OH)2D3 seemed to be in order. The absorption of calcium from the intestine as influenced by 1,25-(OH)2D3 is shown (Fig. 2). As expected, the wild-type mice responded to 1,25-(OH)2D3 with a marked elevation in calcium absorption both in the males and females. Furthermore, the TRPV6 KO mice showed exactly the same response to 1,25-(OH)2D3 in both the male and female mice. These results demonstrate clearly that TRPV6 protein is not required for the action of vitamin D on intestinal calcium absorption.
Fig. 2.
1α,25-Dihydroxyvitamin D3-stimulated 45Ca absorption in small intestine of wild-type and TRPV6 KO male (A) and female (B) vitamin D-deficient mice. TRPV6 wild-type and TRPV6 KO mice were injected i.p. with 50 ng 1,25-(OH)2D3 or vehicle (95% propylene glycol/5% ethanol) for 4 consecutive days. Twenty-four hours after the fourth injection, mice were dosed with 0.5 μCi 45CaCl2 via gastric gavage and killed 45 min later. Intestines were harvested, and the radioactivity remaining in intestines was measured by scintillation counting. The percentage of 45CaCl2 absorbed was calculated as described in the Methods section. All values are reported as mean ± SD (n = 8–9 for male mice and n = 9–12 for female mice). *, Significantly different from vehicle control (P < 0.001).
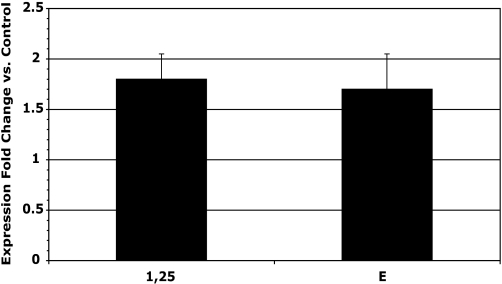
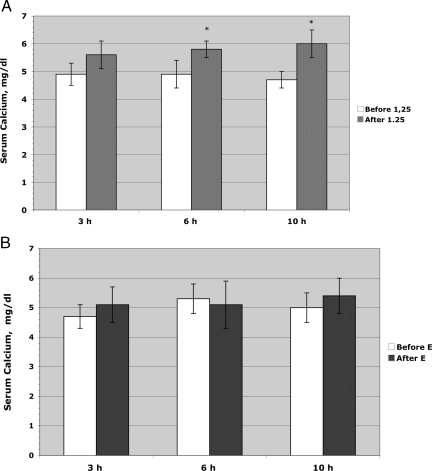
As a continuing program to discover the various genes that are either enhanced or suppressed by 1,25-(OH)2D3 in the small intestine, a microarray study was carried out. Among those genes is TRPV6, which is known to clearly be responsive to 1,25-(OH)2D3. As expected, the TRPV6 mRNA rose in response to 1,25-(OH)2D3 (Fig. 3). A similar response of TRPV6 was found with a vitamin D analog synthesized in our laboratory that did not raise serum calcium at these doses, that is, 17(E)-1α,25-dihydroxy-17 (20)-dehydro-2-methylene-19-nor-dihydroxyvitamin D3 (analog E) (Fig. 3). However, in the same animals, the serum calcium response to these two analogs revealed that 1,25-(OH)2D3 does increase serum calcium as a result of intestinal calcium absorption, whereas serum calcium does not respond to the analog E despite the elevation of the TRPV6 mRNA (Fig. 4). These results add a confirmatory note that the TRPV6 gene response is not predictive of an intestinal calcium transport response of 1,25-(OH)2D3 in the rat.
Fig. 3.
TRPV6 expression fold increase over control in rat duodenum by microarray analysis (Materials and Methods) 3 h after IV injection of 90 ng 1,25-(OH)2D3 or 700 ng E analog per kg of body weight. The bars represent SEM for 6 observations. The fold increase is significant for both compounds (P < 0.01).
Fig. 4.
Serum Ca2+ level in rats (n = 6) after IV injection of (A) 90 ng 1,25-(OH)2D3 or (B) 700 ng E analog per kg of body weight. *, Significantly different from value measured before administration of 1,25-(OH)2D3 (P < 0.01). There were no changes observed in the E analog-treated group.
Discussion
The exciting development of the vitamin D-dependence of a calcium channel protein, TRPV6, might be the key actor in the vitamin D-induced calcium absorption phenomenon appeared to provide the missing piece in the molecular mechanism of action of vitamin D in the small intestine (17). A large number of laboratories have clearly confirmed that the TRPV6 gene is responsive to 1,25-(OH)2D3. Clearly, the TRPV6 protein is a calcium channel protein, and thus, it is naturally expected that TRPV6 is a key factor in the intestinal absorption of calcium. We have now tested directly in vivo the ability TRPV6 null mice to absorb calcium in response to the vitamin D hormone. As expected, wild-type mice responded to 1,25-(OH)2D3 treatment by raising serum calcium and by increasing intestinal calcium absorption. Mice lacking the TRPV6 were fully able to absorb calcium from the intestine in response to 1,25-(OH)2D3 treatment. Thus, the lack of TRPV6 channel, considered to be crucial for vitamin D-mediated Ca2+ entry into the cell, did not eliminate or diminish intestinal calcium absorption. Further, the increased expression of TRPV6 in rat intestine caused by the vitamin D analog E failed to induce the intestinal Ca2+ absorption in rats.
Previous results from our research group clearly showed that elimination of the calbindin D9k gene failed to impair the 1,25-(OH)2D3-induced intestinal calcium absorption in the mouse (14, 15). Benn et al. generated a double KO mouse by back-crossing TRPV6 KO mice with calbindin D9k KO mice (16). Using ex vivo the everted intestinal sac method, Benn et al. found in these mice a significant induction in intestinal calcium transport after 1,25-(OH)2D3 administration, suggesting that the vitamin D-dependent transport process was occurring without the presence of this calcium channel protein.
These results taken altogether illustrate very clearly that the TRPV6 is not of critical importance to vitamin D-induced intestinal calcium absorption. Therefore, having eliminated two of the well known calcium-related protein responses to 1,25-(OH)2D3 as key factors, it is clear that we must reexamine our current model of the vitamin D-induced intestinal calcium absorption. The one known remaining protein with activity that has not yet been examined in terms of playing a key role in intestinal calcium transport is the calcium-dependent ATPase (ATP2B1) or the PMCA1. Unfortunately, a knockout of this gene is lethal and, therefore, its role in intestinal calcium transport in response to vitamin D has not been examined directly (18).
Other mechanisms have been proposed. We and others suggested the involvement of vitamin D in the regulation of paracellular calcium transport in the intestine (6, 7, 19, 20). From a microarray study in rats, it seemed likely that vitamin D regulates tight junction permeability in intestine by regulating expression of several tight junction proteins including claudin-3 (6). Recently, tight junction protein claudin-12 was also suggested as a component in vitamin D-dependent Ca2+ absorption (21).
It is very clear that we are missing some important information on intestinal calcium absorption, and we must recognize that our current hypothesis of the molecular mechanism of vitamin D-induced calcium absorption is inadequate and must be further explored.
Materials and Methods
Materials.
Animals were maintained and research was conducted in accordance with guidelines set forth by the Animal Care and Research Committee (University of Wisconsin, Madison, WI). 1,25-(OH)2D3 was purchased from Sigma Aldrich Fine Chemicals. Analog E (17(E)-1α,25-dihydroxy-17 (20)-dehydro-2-methylene-19-nor-vitamin D3) was synthesized in this laboratory by Dr. Bulli Padmaja Tadi (22). 1,25-(OH)2D3 was quantitated by measurement of UV absorption at 265 nm by using an extinction coefficient of 18,200 M−1cm−1. The E analog was also quantitated by UV absorption at 253 nm by using an extinction coefficient of 42,000 M−1cm−1.
Mice.
The strain of TRPV6 +/− heterozygote mice was received from the Laboratory of Professor M. Hediger (23). The TRPV6 KO mice were generated as described by Bianco et al. and had B6/129 background (23). The TRPV6 KO mice were backcrossed with the C57/B6 mice for three generations and were still carrying a mixed background of B6/129. Thus, as described, heterozygous pairs were used as breeders and littermates carrying the TRPV6 +/+ genotype were used as controls for all experiments. Breeders for colony maintenance were set up and maintained on chow diet (LabDiet 5015 Mouse Diet, PMI Nutrition International). Mice were maintained in a virus- and parasite-free facility and exposed to a 12/h/12 h light/dark cycle. Food and water were given ad libitum.
Genotyping of TRPV6 KO Mice.
Genotyping of TRPV6 KO mice was performed as described in ref. 23.
Preparation of Vitamin D-Deficient Mice.
To obtain vitamin D-deficient mice, a set of TRPV6 KO heterozygous mice were set up in a special room with incandescent lighting, where all potential sources of UV light and vitamin D were excluded. The animals were maintained on a highly purified vitamin D-deficient diet containing 0.47% Ca and 0.3% phosphorus supplemented three times per week with 500 mg of DL-α-tocopherol, 60 μg of menadione, and 40 mg of β-carotene in 0.1 ml of soybean oil (AEK) (24). After birth and weaning, the pups were kept in the same room and maintained on the purified diet until 5 weeks of age. At 5 weeks, mice were provided the same diet but containing 0.02% calcium and 0.3% phosphorus for one week. They were then returned to the 0.47% Ca, 0.3% phosphorus diet for 1 week. Mice were cycled between these two diets (0.47% or 0.02% calcium) for a total of three cycles. After these cycles, mice were maintained on a 0.47% calcium diet for 2 weeks before use in the 45Ca absorption assay. Vitamin D deficiency was achieved after three diet cycles as revealed by low serum calcium levels (5.9 ± 0.7 mg/dl).
Preparation of Vitamin D-Deficient Rats.
Sprague–Dawley male weanling rats (Harlan) were maintained on a highly purified vitamin D-deficient diet (24). The rats were housed in hanging wire cages and maintained on a 12 h light/12 h dark cycle. Rats fed the vitamin D-deficient diet were maintained in a room with incandescent lighting, and all potential sources of UV light and vitamin D were excluded. Severe hypocalcemia was used to confirm vitamin D depletion.
Serum Calcium Analysis.
Mice were bled via maxillary veins every other week while on a 0.47% calcium diet starting from week 7. Whole blood was centrifuged at 1100 × g for 15 min at 25°C to yield serum. Serum calcium concentration was determined by using an atomic absorption spectrometer (Model 3110, Perkin–Elmer) on samples diluted 1:40 with 1 g/L LaCl3 (25).
Intestinal 45Ca Absorption Assay.
Mice that became vitamin D-deficient (serum calcium <6 mg/dl) were used. Each mouse was injected i.p. (i.p) with either 50 ng of 1,25-(OH)2D3 (SAFC) in 95% propylene glycol/5% ethanol or with 95% propylene glycol/5% ethanol (vehicle) for 4 consecutive days. During this period, mice were maintained on 0.47% Ca and 0.3% P diet. Mice were bled before their fourth injection to determine serum calcium. On day 5, mice were weighed and fasted for 5–6 h and then given 0.3 ml of a solution containing 0.5 μCi of 45CaCl2, 10 mM Tris-acetate pH 7.5, and 0.5 mM of CaCl2 via gastric gavage. Mice were killed 45 min after administration of the 45Ca, and the entire gastrointestinal tract (esophagus to terminal colon) was harvested and blood was collected by heart puncture. Tissue and serum samples were stored at –20°C until analysis. A group of vehicle- and 1,25-(OH)2D3-treated mice (6 per group) were killed immediately after gavage of 45CaCl2 to determine efficiency of radioactivity recovery (see Methods below), which was consistently 85% or greater.
Processing Tissues for Counting Radioactivity.
Gastrointestinal (GI) tracts from each wild-type and KO mice were dissolved overnight in 10 ml of concentrated nitric acid and the dissolved samples were diluted with water to 25 ml. Serum from blood samples was obtained according to the method described above. One hundred μl GI tract solution and 50 μl of serum samples were counted with Biodegradable Counting Mixture Bio-Safe (Research Products Int.) in a PackardTri-Carb model 2300 TR Liquid Scintillation Analyzer (Global Medical Instrumentation) to determine the radioactivity remaining in the intestine and radioactivity accumulated in serum respectively. The radioactivity remaining in the intestine was deducted from the total radioactivity administered (100%) to determine the percent of 45Ca absorbed.
Experimental Design of Microarray Study.
Vitamin D-deficient rats were given a single i.v. dose of 90 ng/kg body weight 1,25(OH)2D3 in 0.1 ml ethanol or 700 ng of E analog/kg body weight in 0.1 ml ethanol or 0.1 ml ethanol vehicle alone (control). A sample of blood was taken immediately before the injection for serum calcium concentration measurement. Rats were deeply anesthetized with isoflurane and decapitated at 1, 3, 6, and 10 h after injection. There were three rats in each group for each time point in each of two separate experiments. Blood was collected at the same time for determination of changes in serum calcium concentration. For each rat, the first 15 cm of intestine (duodenum) was removed, slit open longitudinally, and scraped with the glass slide. The mucosa was placed into the vial with GTC extraction buffer supplemented with 2% of β-mercaptoethanol (PolyATtract System 1000, Promega), homogenized at high speed with PowerGen 700 (Fisher Scientific), flash frozen in liquid N2 and stored at –80°C. Experiments were done in duplicate.
Microarray Probe Preparation.
For each time point, Poly(A)+ RNA was isolated from pooled homogenized mucosa of three 1,25(OH)2D3 or three vehicle-treated rats. The mRNA was isolated by using the PolyATtract System 1000 (Promega) according to the manufacturer's instructions. The RNA was purified by using an RNeasy kit (Qiagen). The quality, integrity, and quantity of the Poly(A)+ RNA were determined by agarose gel electrophoresis and UV absorption spectrophotometry. Double stranded cDNA was synthesized from 3 μg of polyadenylated Poly(A)+ RNA by using the SuperScript Choice system (Invitrogen Life Technologies), all according to the Affymetrix Gene Expression manual (Affymetrix). After the clean-up procedure, a biotin-labeled in vitro transcription reaction was performed by using the cDNA template and BioArray High Yield In Vitro Transcription kit (Enzo Life Sciences) according to the manufacturer's instructions. The cRNA was fragmented at 1.0 μg/μl final concentration in 1× fragmentation buffer (40 mM Tris-acetate, pH 8.1, 100 mM potassium acetate, 30 mM magnesium acetate). The size range of cRNA before (0.5 kb and longer) and after (35–200 base fragments) fragmentation was checked by agarose gel electrophoresis.
Microarray Processing and Data Analysis.
The hybridization reaction and the automated hybridization procedure to Affymetrix Rat Expression Arrays 230 2.0 were performed by the Gene Expression Center at the Biotechnology Center of the University of Wisconsin-Madison as described (6, 26). Each probe sample was tested on an Affymetrix Test3 Array and the quality of the cDNA and cRNA syntheses was determined by the 3′/5′ ratio of housekeeping genes within the array (ubiquitin, rat glyceraldehyde 3-phosphate dehydrogenase, β-actin, and hexokinase). If the sample passed the quality control on the Affymetrix Test3 Array, it was hybridized to an Affymetrix high-density oligonucleotide arrays Rat Expression Arrays 230 2.0 per protocol recommendation in the Affymetrix GeneChip Expression Analysis Technical Manual (http://www.affymetrix.com/support/technical/manual/expressionmanual.affx).
Expression data were analyzed by using the Affymetrix Microarrray Suite software version 5.0 (MAS 5.0). Comparison tables for each time point for 1,25(OH)2D3 or E- vs. vehicle-treated rats were generated in EXCEL (Microsoft). For each comparison, e.g., 1,25(OH)2D3 treated relative to control (vehicle treated), and for each cDNA represented in the array, a ratio (e.g., 1,25(OH)2D3/control) and an absolute difference of intensities for 1,25(OH)2D3 and vehicle treated were calculated.
Statistical Analysis.
All measured values are presented as the mean +-SD. Statistical significance of differences was evaluated by using the unpaired, two-tailed t test when comparing two independent groups or paired, two-tailed t test when comparing two variables in one group.
Acknowledgments.
We thank Wendy Hellwig and Colleen Jones for analytical work and animal care, our animal research facilities personnel, the Gene Expression Center Core Facility for help with the microarray study, and Pat Mings for help with manuscript preparation and submission. We thank Dr. M. Hediger (University of Berne Institute of Biochemistry and Molecular Medicine, Bern, Switzerland) for providing TRPV6 mutant breeder pairs. This work was supported in part by the Wisconsin Alumni Research foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Nicolaysen R. Studies upon the mode of action of vitamin D. III. The influence of vitamin D on the absorption of calcium and phosphorus in the rat. Biochem J. 1937;31:122–129. doi: 10.1042/bj0310122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schachter D, Rosen SM. Active transport of Ca45 by the small intestine and its dependence on vitamin D. Am J Physiol. 1959;196:357–362. doi: 10.1152/ajplegacy.1959.196.2.357. [DOI] [PubMed] [Google Scholar]
- 3.Harrison HE, Harrison HC, Park EA. Vitamin D and citrate metabolism. Effect of vitamin D in rats fed diets adequate in both calcium and phosphorus. Am J Phyiol. 1958;192:432–436. doi: 10.1152/ajplegacy.1958.192.2.432. [DOI] [PubMed] [Google Scholar]
- 4.DeLuca HF. Vitamin D: The vitamin and the hormone. Fed Proc. 1974;33:2211–2219. [PubMed] [Google Scholar]
- 5.Nicolaysen R, Eeg-Larsen N, Malm OJ. Physiology of calcium metabolism. Physiol Rev. 1953;33:424–444. doi: 10.1152/physrev.1953.33.3.424. [DOI] [PubMed] [Google Scholar]
- 6.Kutuzova GD, DeLuca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D3 stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserman RH. Vitamin D and the dual processes of intestinal calcium absorption. J Nutr. 2004;134:3137–3139. doi: 10.1093/jn/134.11.3137. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman RH, Feher JJ. Vitamin D-dependent calcium-binding proteins. In: Wasserman RH, et al., editors. Calcium Binding Proteins and Calcium Function. New York: Elsevier-North-Holland; 1977. pp. 292–302. [Google Scholar]
- 9.Kowarski S, Schachter D. Intestinal membrane calcium-binding protein. Vitamin D-dependent membrane component of the intestinal calcium transport mechanism. J Biol Chem. 1980;255:10834–10839. [PubMed] [Google Scholar]
- 10.Cai Q, Chandler JS, Wasserman RH, Kumar R, Penniston JT. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc Natl Acad Sci USA. 1993;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R. Calcium transport in epithelial cells of the intestine and kidney. J Cell Biochem. 1995;57:392–398. doi: 10.1002/jcb.240570304. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, et al. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 14.Akhter S, Kutuzova GD, Christakos S, DeLuca HF. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys. 2007;460:227–232. doi: 10.1016/j.abb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Kutuzova GD, et al. Calbindin D9k knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci USA. 2006;103:12377–12381. doi: 10.1073/pnas.0605252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benn BS, et al. Active intestinal calcium transport in the absence of TRPV6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng JB, Brown EM, Hediger MA. Apical entry channels in calcium-transporting epithelia. News Physiol Sci. 2003;18:158–163. doi: 10.1152/nips.01440.2003. [DOI] [PubMed] [Google Scholar]
- 18.Okunade GW, et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 19.Chirayath MV, et al. Vitamin D increases tight-junction conductance and paracellular Ca2+ transport in Caco-2 cell cultures. Am J Physiol. 1998;274:G389–G396. doi: 10.1152/ajpgi.1998.274.2.G389. [DOI] [PubMed] [Google Scholar]
- 20.Shultz TD, Bollman S, Kumar R. Decreased intestinal calcium absorption in vivo and normal brush border membrane vesicle calcium uptake in cortisol-treated chickens: Evidence for dissociation of calcium absorption from brush border vesicle uptake. Proc Natl Acad Sci USA. 1982;79:3542–3546. doi: 10.1073/pnas.79.11.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita H, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between Enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanhooke JL, Tadi BP, Benning MM, Plum LA, DeLuca HF. New analogs of 2-methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 with conformationally restricted side chains: Evaluation of biological activity and structural determination of VDR-bound conformations. Arch Biochem Biophys. 2007;460:161–165. doi: 10.1016/j.abb.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Bianco SD, et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suda T, DeLuca HF, Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970;100:1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- 25.Halloran BP, DeLuca HF. Intestinal calcium transport: Evidence for two distinct mechanisms of action of 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1981;208:477–486. doi: 10.1016/0003-9861(81)90534-8. [DOI] [PubMed] [Google Scholar]
- 26.Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol. 2007;218:37–44. doi: 10.1016/j.taap.2006.10.005. [DOI] [PubMed] [Google Scholar]