Abstract
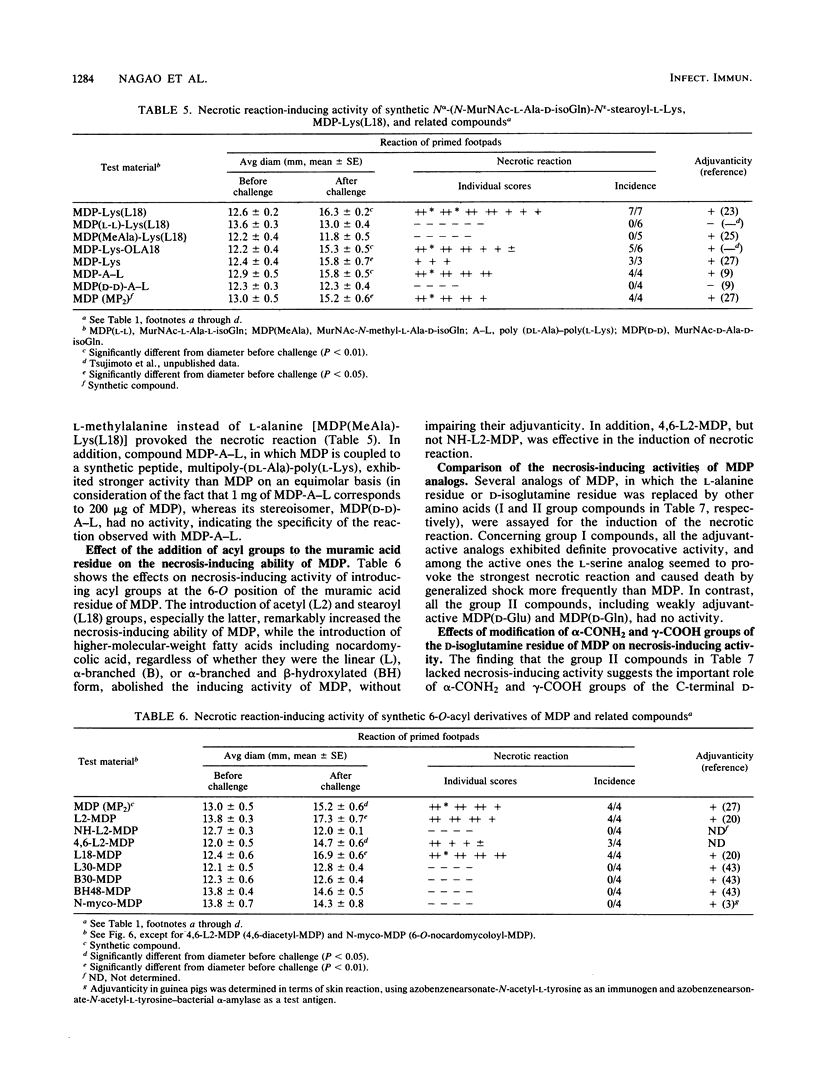
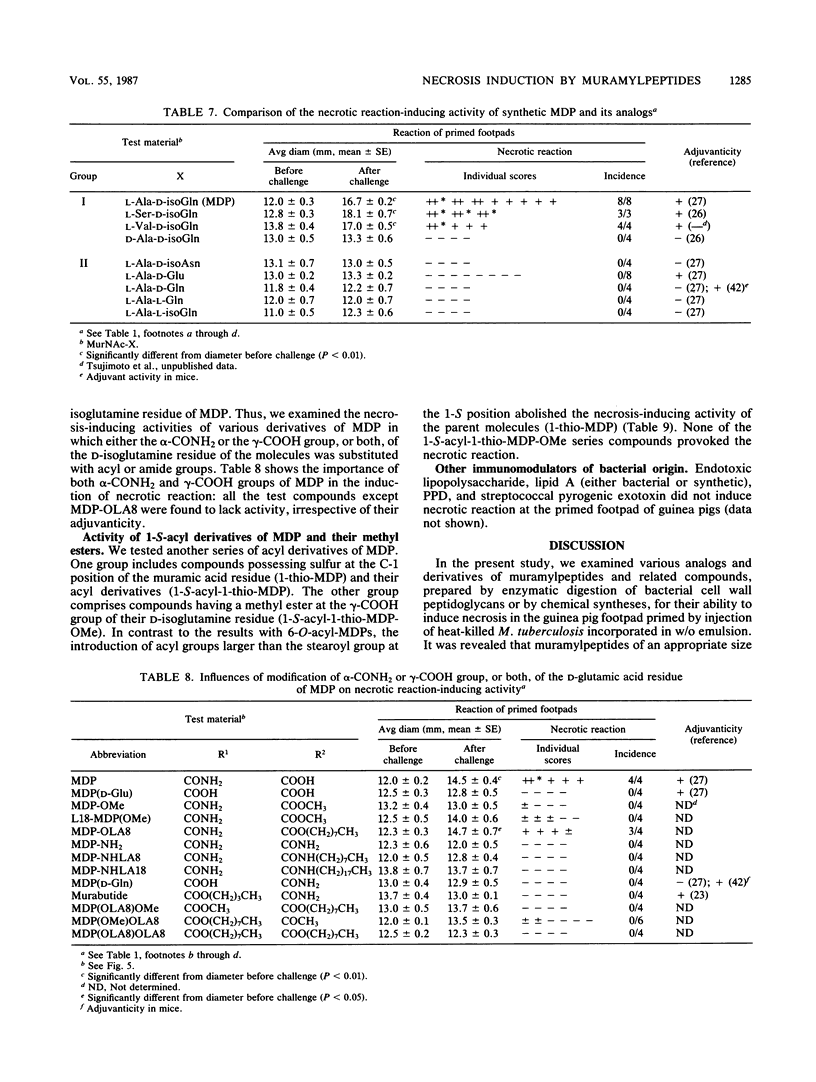
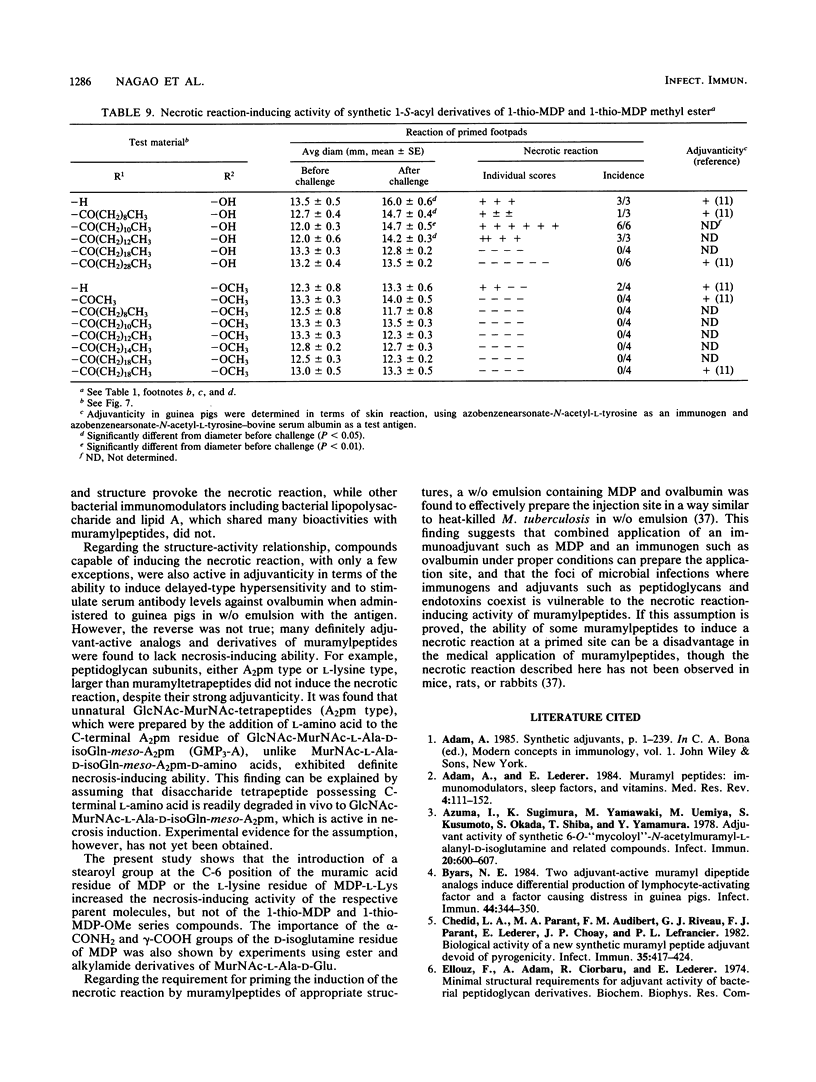
Intracutaneous injection of N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP) in guinea pigs caused an extensive necrotic reaction in footpads prepared by injection of heat-killed Mycobacterium tuberculosis in water-in-mineral-oil emulsion. We examined a variety of analogs and derivatives of muramylpeptides for their ability to provoke this reaction. A maximum and a minimum structure responsible for the necrotic reaction were found to be N-acetylglycosaminyl-beta(1-4)-N-acetylmuramyl-tripeptide (GlcNAc-MurNAc-L-Ala-D-isoGln-meso-A2pm) and MDP, respectively. An unexpected finding was that GlcNAc-MurNAc-tetrapeptides having L-amino acids at their C termini, unlike comparable compounds having C-terminal D-amino acids, exhibited definite necrosis-inducing activity, probably due to their tendency to undergo in vivo degradation to GlcNAc-MurNAc-tripeptide. Introduction of some acyl groups, especially the stearoyl group, to the 6-O position of the muramic acid or the peptide moiety of muramylpeptides increased the necrosis-inducing activity of the parent molecules. However, this was not observed with 1-thio-muramic acid analogs of MDP. Modification of the alpha- or gamma-carboxyl groups of the glutamic acid residues of muramylpeptides tended to decrease their necrosis-inducing ability. Analogs and derivatives of muramylpeptides which are capable of inducing necrosis at a primed site, with few exceptions, exhibited powerful adjuvanticity against ovalbumin in guinea pigs. However, the reverse was not necessarily true.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Lederer E. Muramyl peptides: immunomodulators, sleep factors, and vitamins. Med Res Rev. 1984 Apr-Jun;4(2):111–152. doi: 10.1002/med.2610040202. [DOI] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Yamawaki M., Uemiya M., Kusumoto S., Okada S., Shiba T., Yamamura Y. Adjuvant activity of synthetic 6-O-"mycoloyl"-N-acetylmuramyl-L-alanyl-D-isoglutamine and related compounds. Infect Immun. 1978 Jun;20(3):600–607. doi: 10.1128/iai.20.3.600-607.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars N. E. Two adjuvant-active muramyl dipeptide analogs induce differential production of lymphocyte-activating factor and a factor causing distress in guinea pigs. Infect Immun. 1984 May;44(2):344–350. doi: 10.1128/iai.44.2.344-350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINDLAY J., LEVVY G. A. Purification of beta-N-acetylglucosaminidase from the pig epididymis. Biochem J. 1960 Oct;77:170–175. doi: 10.1042/bj0770170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galelli A., Le Garrec Y., Chedid L., Lefrancier P., Derrien M., Level M. Macrophage stimulation in vitro by an inactive muramyl dipeptide derivative after conjugation to a multi-poly(DL-alanyl)-poly(L-lysine) carrier. Infect Immun. 1980 Apr;28(1):1–5. doi: 10.1128/iai.28.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Kotani S., Takada H., Tsujimoto M., Hirachi Y., Kusumoto S., Shiba T., Kawata S., Yokogawa K., Nishimura H. Liberation of serotonin from rabbit blood platelets by bacterial cell walls and related compounds. Infect Immun. 1982 Sep;37(3):1181–1190. doi: 10.1128/iai.37.3.1181-1190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A., Hioki Y., Kiso M., Okumura H., Azuma I. Synthesis and biological activities of N-acetyl-1-thiomuramoyl-L-alanyl-D-isoglutamine and some of its lipophilic derivatives. Carbohydr Res. 1983 Nov 25;123(2):183–199. doi: 10.1016/0008-6215(83)88476-6. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970 Mar 1;131(3):611–622. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Tanaka A., Kotani S., Shiba T., Kusumoto S., Yokogawa K., Kawata S., Ozawa A. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980 Jul;29(1):70–75. doi: 10.1128/iai.29.1.70-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Kinoshita F., Morisaki I., Shimono T., Okunaga T., Takada H., Tsujimoto M., Watanabe Y., Kato K., Shiba T. Immunoadjuvant activities of synthetic 6-O-acyl-N-acetylmuramyl-L-alanyl-D-isoglutamine with special reference to the effect of its administration with liposomes. Biken J. 1977 Dec;20(3-4):95–103. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Tsujimoto M., Koga T., Nagao S., Tanaka A., Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986 Oct;45(11):2534–2540. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Kinoshita F., Narita T. Immunoadjuvant activities of peptidoglycan subunits from the cell walls of Staphyloccus aureus and Lactobacillus plantarum. Biken J. 1975 Jun;18(2):93–103. [PubMed] [Google Scholar]
- Lefrancier P., Derrien M., Jamet X., Choay J., Lederer E., Audibert F., Parant M., Parant F., Chedid L. Apyrogenic, adjuvant-active N-acetylmuramyl-dipeptides. J Med Chem. 1982 Jan;25(1):87–90. doi: 10.1021/jm00343a018. [DOI] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Necrotic inflammatory reaction induced by muramyl dipeptide in guinea pigs sensitized by tubercle bacilli. J Exp Med. 1985 Aug 1;162(2):401–412. doi: 10.1084/jem.162.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Tsujimoto M., Kusumoto S., Shiba T., Kawata S., Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982 Feb;35(2):612–619. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. E., Cantrell J. L., Von Eschen K. B., Schwartzman S. M. Enhancement of endotoxic shock by N-acetylmuramyl-L-alanyl-(L-seryl)-D-isoglutamine (muramyl dipeptide). Cancer Res. 1979 Nov;39(11):4756–4759. [PubMed] [Google Scholar]
- Takada H., Galanos C. Enhancement of endotoxin lethality and generation of anaphylactoid reactions by lipopolysaccharides in muramyl-dipeptide-treated mice. Infect Immun. 1987 Feb;55(2):409–413. doi: 10.1128/iai.55.2.409-413.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Nagao R., Kotani S., Shiba T., Kusumoto S. Stimulation of the reticuloendothelial system of mice by muramyl dipeptide. Infect Immun. 1979 May;24(2):302–307. doi: 10.1128/iai.24.2.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M., Kotani S., Kinoshita F., Kanoh S., Shiba T., Kusumoto S. Adjuvant activity of 6-O-acyl-muramyldipeptides to enhance primary cellular and humoral immune responses in guinea pigs: adaptability to various vehicles and pyrogenicity. Infect Immun. 1986 Sep;53(3):511–516. doi: 10.1128/iai.53.3.511-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



