Abstract
Both density-mediated and trait-mediated indirect biotic interactions may be important in structuring communities. Indirect interactions in many study systems remain unexplored; in part, because they are often difficult to detect, and in many instances, have been identified empirically only when unexpected results arise. Indirect effects induced by competition may be particularly important among organisms with complex life cycles, wherein competitive effects experienced in one life stage influence species interactions in one or more subsequent stages. We determined whether species-specific effects of larval competition in the mosquitoes Aedes albopictus and Aedes aegypti have indirect effects at the adult stage, specifically testing for effects on arboviral infection with Sindbis virus (SINV). For A. albopictus, but not for A. aegypti, competition resulted in greater infection, body titer, and dissemination rates compared to low-competition conditions. Whole body titers of virus increased with adult size irrespective of competition. However, between competitive treatments, mosquitoes from low-competition conditions had greater mean size, with lower infection rates and lower whole body titers than the smaller mosquitoes from high-competition conditions. These results suggest that larval competition, common in natural mosquito populations, has important indirect effects on adults by altering mosquito–virus interactions. Such indirect effects may change transmission parameters of pathogens.
Keywords: Aedes aegypti, Aedes albopictus, arborvirus model system, indirect effects, mosquitoes, Sindbis virus (SINV), susceptibility to infection, trait-mediated effects
Introduction
Biotic interactions among organisms play an important role in regulating population growth and in shaping communities. Among biotic interactions, competition has received a great deal of attention especially in the field of invasion biology where competitively superior invasive species displace or otherwise alter the distribution of established species (e.g., Petren et al. 1993, Holway 1999, Juliano et al. 2004). Although the most obvious effects of competition are reduced growth and survivorship, there are less obvious indirect effects mediated by competitively induced differences in life history traits (e.g., morphological or behavioral trait-mediated indirect effects; Abrams 1995).
Indirect effects describe interactions between two species mediated by a third species (e.g., exploitative competition, apparent competition, trophic cascades, indirect mutualism, interaction modifications; Wootton 1994). Although different authors have applied multiple terms to similar types of indirect interactions (e.g., Morrison 1999), there is a consensus on classifying indirect effects as “density mediated” or “trait mediated” (Altwegg 2002). Density-mediated indirect effects occur when abundance of one species indirectly alters the abundance of another species through effects produced by altering the abundance of an intermediate species. Trait-mediated indirect effects occur when one species alters traits (e.g., behavioral, morphological) in a second species in ways that change the interaction between the second and third species. The most frequently studied trait-mediated indirect effects involve predatory species that induce prey behavioral modification (e.g., reduced activity, increased use of refuges) that indirectly alters competitive interactions among those prey (Werner 1991, 1992, Werner and Anholt 1996, Relyea 2000).
Less attention has been given to indirect effects of competition among organisms with complex life cycles, where the impact of competition in one life stage has consequences for species interactions in subsequent stages (Altwegg 2002). Adult life history traits of organisms with complex life cycles are, to a large extent, products of their larval environment. For example, effects of competition include reduced growth, development, and survivorship. Competition-induced differences in adult life history traits such as size may alter species interactions with enemies, including predators, pathogens, and parasites. Although nutrient-limited conditions and physiological stress indirectly result in greater susceptibility to infection with pathogens or parasites in a single life stage (Matson and Waring 1984, Murray et al. 1998, Oppliger et al. 1998, Kiesecker and Skelly 2001), little is known about effects of competition in juvenile life history stages on susceptibility to infection in subsequent adult stages.
Water-filled containers are well suited to investigations of competitively induced indirect effects because they harbor simple communities subject to variable resource availability. Among the organisms occupying aquatic container communities, mosquitoes are the best studied because of the role of adults as vectors of pathogens. Resource availability and larval density in containers both influence mosquito survivorship, growth, and adult size (e.g., Lounibos et al. 2002, Juliano et al. 2004). Effects of competitive interactions among larval stages may carry over to the adult stage and affect vector competence, which describes the ability to become infected and subsequently to transmit a pathogen after imbibing an infectious blood meal (Hardy 1988).
Biological transmission of arboviruses includes acquisition of the virus by the vector from an infectious blood meal, replication, dissemination of virus to the salivary glands, and transmission to a host by bite (Hardy 1988, Higgs 2004). Successful completion of this process requires that infection and dissemination barriers within the mosquito be overcome (Hardy et al. 1983, Hardy 1988). For example, if arboviruses fail to pass through the midgut, then infection is limited to the midgut cells and, although the mosquito is “infected,” it cannot transmit virus (Hardy et al. 1983). Larval competition may have important consequences for adult arbovirus infection parameters. Typically, pupal and adult sizes of container-breeding mosquitoes are positively related to the feeding rate experienced by larvae (e.g., Christophers 1960). Resource competition, and associated low food availability, among larvae of the treehole mosquito Ochlerotatus triseriatus produced smaller adults that transmitted La Crosse encephalitis virus (LACV) at higher rates than did larger adults from well-fed larvae. Infection rates were independent of adult body size (Grimstad and Haramis 1984, Grimstad and Walker 1991), although when O. triseriatus reared from field-collected pupae were orally infected with LACV, disseminated infection and transmission rates were negatively correlated with adult size (Paulson and Hawley 1991). In contrast, large Aedes aegypti adults produced under varying conditions of larval crowding and food availability disseminated dengue-2 virus more efficiently than did smaller females (Sumanochitrapon et al. 1998). Thus, it appears that ecological conditions encountered by larvae can have variable effects on the interaction of mosquitoes with arboviruses. Investigations of competitive effects on pathogen transmission, other than size-related effects, remain rare.
The goal of our study was to determine the effects of larval competition on growth and survivorship of two well-known container mosquito species, Aedes albopictus and A. aegypti, as well as their subsequent competence for arboviral infection and dissemination using Sindbis virus (SINV). SINV is a model Alphavirus that cycles between reservoir bird hosts and Aedes and Culex vector species (Seabaugh et al. 1998, and references therein), and is widely used in experimental vector biology research (Dohm et al. 1995, Olson et al. 1996). A. albopictus is an invasive container- breeding mosquito native to Asia that became established in large areas of the United States, Europe, Africa, and South America during the last two decades (Lounibos 2002). In the southern United States, the spread of A. albopictus coincided with reductions in range and abundance of the resident exotic A. aegypti in artificial containers (reviewed by Juliano et al. 2004). A. albopictus is an important vector of several arboviruses affecting humans and second only to A. aegypti in global importance as a vector of dengue viruses (Gubler and Kuno 1997, Lounibos 2002). These species frequently encounter each other in artificial containers, in which interspecific competition has been well documented (Black et al. 1989, Ho et al. 1989, Barrera 1996, Braks et al. 2004, Juliano et al. 2004), which probably explains displacements of A. aegypti by A. albopictus (Juliano 1998). We tested whether the variations in population growth parameters known to arise from intra- and interspecific competition (Lounibos et al. 2002, Juliano et al. 2004) have carryover effects in the adult stage, and are associated with variation in susceptibility to SINV infection dynamics.
Methods
Competition study
Aedes albopictus Lake Charles strain (Nasci et al. 1989) and A. aegypti Rockefeller strain used in the experiments were the progeny of genetically well-characterized strains. A. albopictus was obtained from a collection made at Lake Charles, Louisiana, USA, in 1987 and has been propagated under laboratory conditions since 1987. The A. aegypti Rockefeller strain was obtained from a long-standing colony at the University of Notre Dame (Notre Dame, Indiana, USA). The competition experiment between A. albopictus and A. aegypti used 5-L plastic containers filled with 4000 mL tap water, 500 mL oak leaf infusion water (O’Meara et al. 1989), and 0.2 g of larval food (1:1 albumin : yeast, by weight). Three days after adding the initial contents to containers, a supplemental 500 mL oak infusion and 0.2 g larval food were added. Initial food resources were soaked for five days before the addition to each container of first-instar (<24 h old) mosquitoes. Ten days later, we removed 50% of the liquid contents, except larvae, and added 0.1 g larval food, 250 mL oak infusion water, and 2250 mL tap water. Previous studies showed that this protocol provided sufficient resources for mosquitoes to complete development without negating the effects of larval competition (B. W. Alto, unpublished data). Competition treatments consisted of species density ratios of A. albopictus : A. aegypti (i.e., 160:0, 320:0, 160:160, 0:320, and 0:160). Ten replicates were used per treatment, for a total of 50 containers kept at 28° ± 1°C and 14:10 L:D photoperiod. Containers were checked daily, and pupae transferred to sealed 20-mL vials with tap water until adult emergence. Emerged adults were kept, by species, in cylindrical cages (11 cm high × 9.5 cm diameter) and provided with 10% sucrose and an oviposition cup. The experiment was maintained until the last adult had emerged.
Measurements of population growth correlates were used to estimate the effect of competition on female A. albopictus and A. aegypti population growth. Mean female size (wing length) and mean time to emergence were calculated for each replicate. Survivorship to emergence was calculated for each replicate by dividing the total number of adults by half the number of larvae of that species originally in the container (survivorship percentage assumes a 1:1 sex ratio). An estimated finite rate of increase (λ′) was also calculated for each replicate container:
| (1) |
where λ′ is a transformation of r′, a composite index of population performance (Juliano 1998); r′ is an estimate of r = dN/Ndt, which describes the per capita growth rate. N0 is the initial number of females in a cohort (assumed to be 50%), Ax is the number of females emerging on day x, wx is mean female size on day x, f(wx) is a function relating the number of eggs produced by a female to her size, and D is the time (in days) from emergence to oviposition. For A. albopictus and A. aegypti, D is assumed to be 14 and 12 d, respectively (Livdahl and Willey 1991, Juliano 1998). We used the following fecundity–size relationships (f(wx)) to calculate λ′: For A. aegypti (Briegel 1990),
| (2) |
(r2 = 0.875, N = 206, P < 0.001). For A. albopictas (Lounibos et al. 2002),
| (3) |
(r2 = 0.713, N = 91, P < 0.001). In both cases w is wing length, in millimeters. Effects of A. albopictus and A. aegypti competition were analyzed by individual Multivariate Analyses of Variance (MANOVA) to determine competitive treatment effects on the population growth correlates: time to emergence, survivorship to emergence, and adult size. Raw data adequately met assumptions of univariate normality and homogeneous variances for all correlates used in the MANOVAs. For all analyses, significant effects were further analyzed by contrasts of pairs of main effect multivariate means using a sequential Bonferroni adjustment (P = 0.05). Standardized canonical coefficients (SCC) were used to determine the relative contribution of each of the response variables to significant multivariate effects as well as their relationship to each other (e.g., positive or negative; Scheiner 2001). Competitive effects on A. albopictus and A. aegypti λ′ were analyzed using one-way ANOVAs with treatment as a categorical variable (SAS Institute 1989). Significant effects were further analyzed by pairwise comparisons of main effect means (Ryan-Einot-Gabriel-Welsch test, SAS Institute 1989).
Infection study
For each replicate from the competition study, newly emerged females and males were housed, by species, in cages (11 cm high × 9.5 cm diameter) and provided 10% sucrose solution and an oviposition cup. This arrangement facilitated mating and oviposition and enabled us to infect multiple females of approximately the same age with an infectious blood meal. Because larval competition increases developmental time, adults from the competition containers emerged over several weeks. Therefore, multiple cages were used to house adults for each replicate to ensure that the females given an infectious blood meal were of similar ages (4–10 d). SINV infection rates do not differ over the age range of 4–10 d for these Aedes species (Dohm et al. 1995). Thus, we were testing for the effects of larval competition on subsequent adult infection of the same individual mosquitoes. Adults were housed in cages within an incubator at 26° ± 1°C and 14:10 L:D photoperiod. Adult females of each species were deprived of sucrose but not water for 24 h, then allowed to blood feed for 30 min on a citrated bovine blood–SINV mixture maintained at 37° ± 1°C in a silicon membrane system (Butler et al. 1984). SINV (MRE-16 strain) titers used in bloodfeeding trials were 105.3 tissue culture dose required to infect 50% of wells (TCID50; Reed and Muench 1938). TCID50 is the quantity of virus that is required to infect 50% of the tissue cultures so that viral titers (number of virus particles/mL) can be determined. Viral titer refers to the amount of virus in solution. Virus titers were similar to those produced in wild bird reservoirs in nature (Ockelbo virus, a closely related strain of Sindbis virus; Lundstrom et al. 1993). Titers were determined by 10-fold serial dilutions in 96-well plates seeded with 6.0 × 105 Vero cells/mL (10 wells per dilution). TCID50 was determined by cytopathic effects after a 7-d incubation (Reed and Muench 1938). Vero cells infected with SINV virus exhibit stereotypical cytopathic effects, so infection is unambiguous. To avoid the possibility of reductions in titer with repeated thawing and freezing, all blood meals had virus derived from a single stock placed in 1.5-mL aliquots that were frozen (−80°C) and thawed only once. The infection study was conducted in a biosafety level-2 facility appropriate for SINV at the Florida Medical Entomology Laboratory in Vero Beach, Florida, USA.
Females that failed to take a blood meal during the first trial were given a second trial 18 h later. After the second feeding attempt, unfed females were removed from the cages, and bloodfed females were held for a 16-d extrinsic incubation period (EIP). The time from initial ingestion of the infectious blood meal until the time the mosquito can transmit the arbovirus is the EIP. Females surviving the EIP were killed and individually stored at 80°C and, subsequently, their wings were removed (to be measured as an indicator of female size). Bodies and legs were ground into a powder separately in 1 mL diluent (Leibovitz L-15 media, 5% fetal bovine serum, and gentamicin), centrifuged at 21 000 m/s2 for 12 min at 4°C, and filtered (0.22 μm). We determined proportion infected, body titer (log10 TCID50), and proportion of infected females with disseminated infection (i.e., with legs infected) using 10-fold serial dilutions in triplicate wells of 96-well plates seeded with Vero cells by TCID50.
Infection was determined using a 1/10 dilution of the body stock solution, and body titer was determined using a full range of dilutions. When describing infection of mosquitoes, “negative” describes the absence of a viral infection, and “positive” describes a mosquito with a viral infection in the midgut, and perhaps other organs. An infection limited to the midgut is called an “isolated infection,” whereas an infection spread beyond the midgut, infecting secondary target organs (e.g., salivary glands, head, legs), is called a “disseminated infection.” Disseminated infection is a recognized indicator of a mosquito’s ability to transmit virus via biting (Gubler and Kuno 1997). So, dissemination of infection in positive females was determined by assaying undiluted leg stock solution (Turell et al. 1984). In this study, isolated infections refer to mosquitoes with positively infected bodies, but with absence of infection in legs, whereas disseminated infections refer to positively infected bodies and legs. Assaying salivary glands may be a more direct indicator of a mosquito’s ability to transmit virus. However, extraction of the salivary glands may result in contamination with surrounding tissue. Thus, we chose to assay legs, thereby avoiding this contamination problem and still obtaining a good indication of ability to transmit (Turell et al. 1984).
Prior to analyzing effects of competitive treatment on arboviral infection, we first examined interspecific differences in susceptibility using MANOVA and SCC on the response variables: proportion infected, body titer, and proportion with disseminated infection. Next, individual MANOVAs for A. albopictus and A. aegypti were used to determine the effect of larval competition on response variables: proportion infected, body titer, and proportion with disseminated infection. Multivariate contrasts with sequential Bonferroni adjustment for P = 0.05 (Rice 1989, Scheiner 2001) were used to compare high-density treatments (320:0, 160:160) with the low-density treatment (160:0), and then to compare the two high-density treatments.
For A. albopictus and A. aegypti, we tested for effects of mean female size on body titer by treating size as a covariate in an analysis of covariance (ANCOVA) testing for competitive treatment and competition × size interactions. Significant effects were further analyzed by all possible pairwise comparisons of treatment means (sequential Bonferroni adjustment; Rice 1989). We expected that effects of mean female size on body titer would be most pronounced in females with disseminated infections, and this analysis was our primary interest.
Product-moment correlation coefficients (r1,2) were used to describe the relationship between population growth measurements (time to emergence, survivorship, size, λ′) and infection parameters: proportion infected, body titer of females with isolated infection, body titer of females with disseminated infection, and proportion with disseminated infection among competitive treatments of A. albopictus and A. aegypti. These analyses enabled us to test the strength of positive or negative relationships among population growth measurements and infection.
Results
Competition study
For both A. albopictus and A. aegypti, competitive treatments significantly affected population growth measurements (Table 1), with uncrowded larval conditions consistently resulting in shorter time to emergence, greater survivorship, and greater adult size compared to crowded conditions (Figs. 1, 2). For A. albopictus, standardized canonical coefficients (SCC) showed that differences in adult size followed by survivorship to emergence contributed the most to the significant competition effect as well as to subsequent treatment differences (Table 1). For A. aegypti, SCC showed that differences in survivorship to emergence followed by time to emergence contributed the most to the significant competition effect as well as to pairwise differences (Table 1). For both species, competitive treatments significantly affected λ′ (A. albopictus, F2,26 = 191.84, P < 0.0001; A. aegypti, F2,19 = 51.94, P < 0.0001) and λ′ was significantly greater in the pattern: 160 larvae > 320 larvae > 160 + 160 larvae (Fig. 3). Thus, inter- and intraspecific competition had major population-level effects.
Table 1.
Multivariate ANOVA for main effects and multivariate pairwise contrasts of competitive treatment effects on female Aedes albopictus and A. aegypti population growth measurements: time to emergence, survivorship to emergence, and adult size.
| Standardized canonical coefficients
|
||||||
|---|---|---|---|---|---|---|
| Comparison | df | Pillai’s trace | P | Time | Survivorship | Size |
| A. albopictus | ||||||
| Competitive treatment | 6 | 1.02 | <0.0001 | −0.79 | 1.19 | 1.97 |
| 160:0 vs. 320:0 | 3 | 0.90 | <0.0001 | −0.88 | 1.18 | 1.89 |
| 160:0 vs. 160:160 | 3 | 0.91 | <0.0001 | −0.73 | 1.19 | 2.02 |
| 320:0 vs. 160:160 | 3 | 0.13 | 0.3274 | |||
| Error df | 26 | |||||
| A. aegypti | ||||||
| Competitive treatment | 6 | 0.93 | 0.0006 | −1.11 | 2.01 | 0.27 |
| 0:160 vs. 0:320 | 3 | 0.88 | <0.0001 | −1.12 | 1.99 | 0.28 |
| 0:160 vs. 160:160 | 3 | 0.76 | <0.0001 | −1.05 | 2.09 | 0.20 |
| 0:320 vs. 160:160 | 3 | 0.14 | 0.4472 | |||
| Error df | 19 | |||||
Fig. 1.
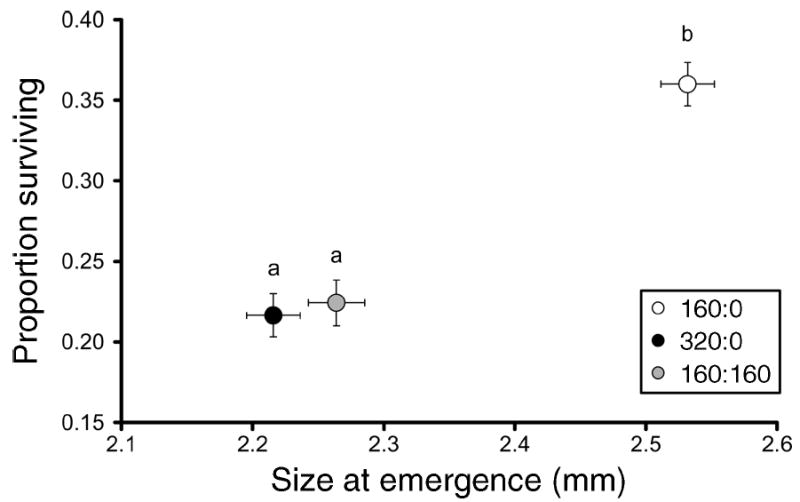
Aedes albopictus least-squares means (±se) for female survivorship and size at emergence. Different lowercase letters indicate significant differences between bivariate means. Competition treatments consisted of species density ratios of A. albopictus : A. aegypti—160:0, 320:0, and 160:160.
Fig. 2.
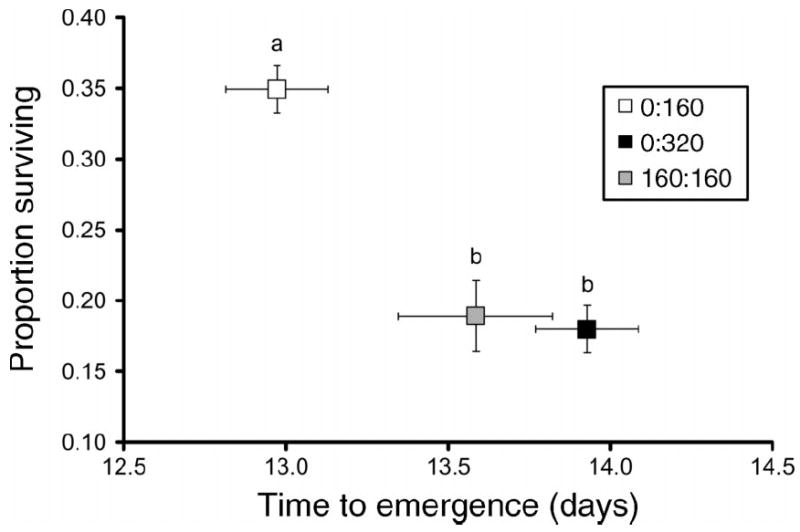
Aedes aegypti least-squares means (±se) for female survivorship and time to emergence. Different lowercase letters indicate significant differences between bivariate means. Competition treatments consisted of species density ratios of A. albopictus : A. aegypti—0:160, 0:320, and 160:160.
Fig. 3.
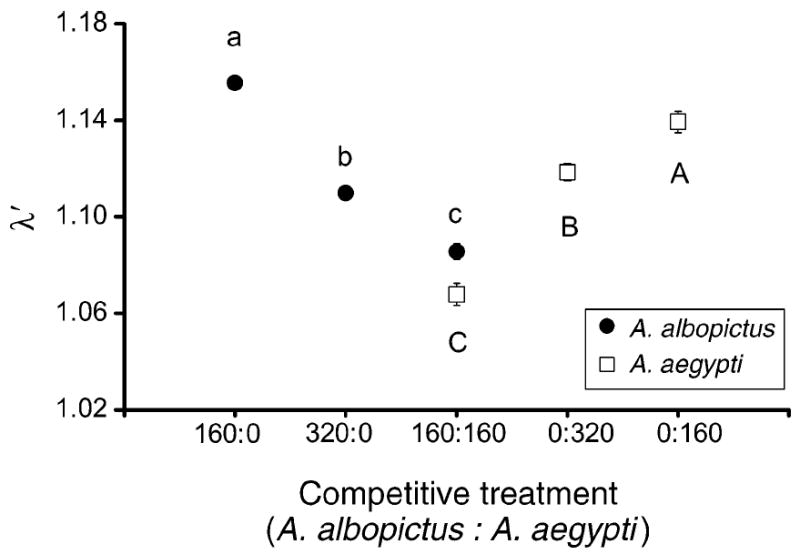
Least-squares means (±se) for estimated finite rate of increase, λ′, for Aedes albopictus and A. aegypti. Points without bars have standard errors too small to appear on the graph. Different lowercase and uppercase letters indicate significant differences between means for A. albopictus and A. aegypti, respectively.
Infection study
Prior to analyzing effects of competition on arboviral infection, we first examined interspecific differences in susceptibility. Proportions infected, whole body titer, and proportions with disseminated infection were significantly different between A. albopictus and A. aegypti (Pillai’s trace3,37 = 0.76, P < 0.0001). Proportion infected (SCC = 1.23) was the most important variable in the overall interspecific difference, followed by proportion with disseminated infection (SCC = −0.66), and whole body titer (SCC = −0.57). The opposite signs of the SCC showed that there was a negative relationship between the variables across the species, so that A. albopictus had a greater proportion of infected individuals, a lower body titer, and a lower proportion of disseminated infection compared to A. aegypti (least-squares means ± se for A. albopictus and A. aegypti, respectively: proportion infected, 0.94 ± 0.03 and 0.58 ± 0.04; body titer [TCID50], 4.08 ± 0.14 and 5.58 ± 0.22; and proportion with disseminated infection, 0.67 ± 0.03 and 1.00 ± 0).
Interspecific competition had significant effects on proportion infected, whole body titer, and proportion of A. albopictus with disseminated infection (Pillai’s trace6,24 = 0.52, P = 0.025). Proportion infected (SCC = 1.12) made the greatest contribution to the multivariate differences among treatments, and body titer (SCC = 0.23) and proportion with disseminated infection (SCC = −0.06) contributed less. Aedes albopictus at low density alone (160 per container) had a significantly lower proportion infected, lower proportion with disseminated infection, and lower body titer compared to high density treatments (Pillai’s trace3,25 = 0.38, P = 0.011; Fig. 4A, B). Proportion infected was the major contributor to this effect (SCC = 1.14), whereas titer (SCC = 0.27) and proportion with disseminated infection (SCC = −0.08) contributed little. The two high density treatments did not differ significantly (Pillai’s trace3,24 = 0.14, P = 0.319; Fig. 4A, B). For the infection study, mortality during the extrinsic incubation period resulted in few A. aegypti females from the 160:160 treatment. Therefore, only means for intraspecific density treatments are reported for A. aegypti. There were no significant effects of competition on infection parameters for A. aegypti (Pillai’s trace2,11 = 0.23, P = 0.301). Mean ± se females assayed per treatment replicate were; 160:0 (10.0 ± 1.84), 320:0 (11.89 ± 1.15), 160:160 (A. albopictus) (6.44 ± 0.63), 0:320 (6.11 ± 1.12), and 0:160 (5.20 ± 0.92).
Fig. 4.
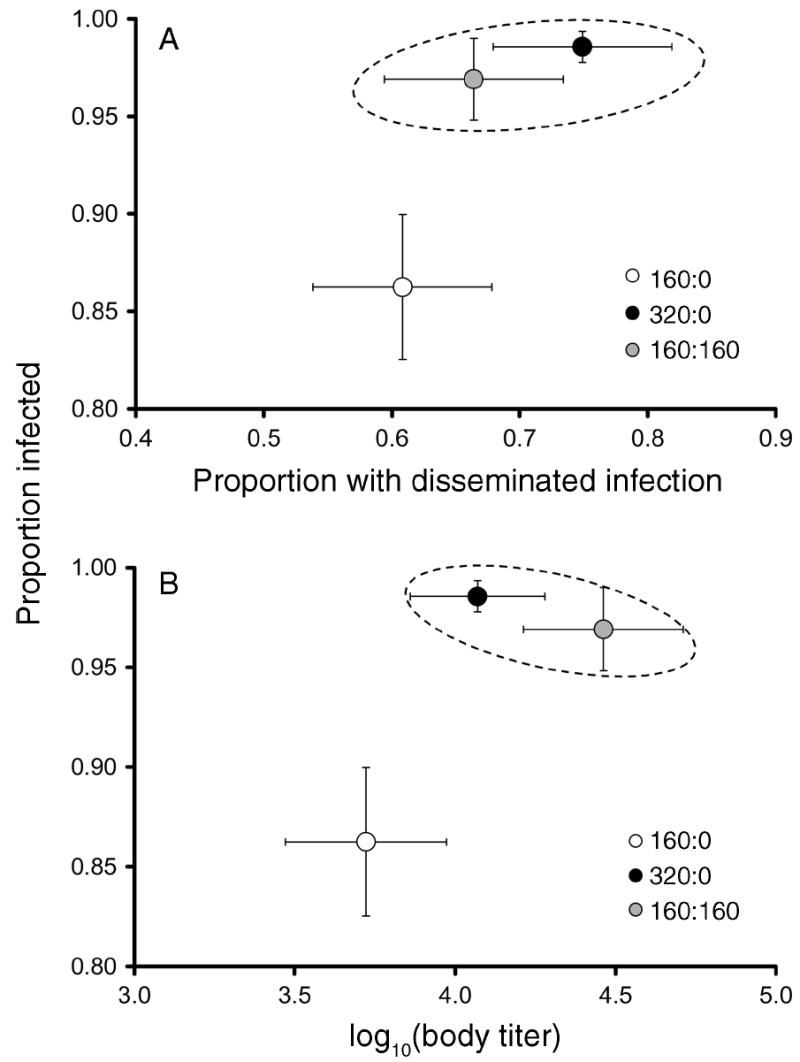
Bivariate plots of least-squares means (±se) for three dependent variables for Aedes albopictus females fed on a Sindbis virus blood meal. (A) Proportion of infected females vs. proportion with disseminated infection. (B) Proportion of infected females vs. body titer. In both graphs, the dashed ellipse indicates multivariate means that are not significantly different. Numbers in the figure key represent the ratio of A. albopictus to A. aegypti.
Females with disseminated infections are capable of transmitting virus and therefore are of epidemiologic significance. For these females, an analysis of covariance with mean female size as a covariate showed significant effects of size and competition on whole body viral titer for A. albopictus with disseminated infections, but no significant size × competition interaction (Table 2). Thus, effects of mean body size and of competition are independent. Estimated slopes were positive, indicating that within a competitive treatment body titer increased with size for mosquitoes with disseminated infection (Fig. 5). Pairwise comparisons of adjusted means among treatments showed that significant differences in body titer followed the pattern 160:160 > 320:0 > 160:0 (mean ± se: 5.80 ± 0.23, 5.13 ± 0.22, 3.23 ± 0.32 TCID50, respectively). For A. aegypti with disseminated infections, there were no significant competitive treatment or covariate effects (Table 2).
Table 2.
ANCOVA for the effects of competitive treatment and size covariate on body titer for Aedes albopictus and A. aegypti females with disseminated infections.
| Source | df | F | P |
|---|---|---|---|
| A. albopictus, disseminated | |||
| Size | 1 | 18.38 | 0.0002 |
| Competitive treatment | 2 | 15.20 | <0.0001 |
| Size × competition | 2 | 0.26 | 0.7750 |
| Error df | 23 | ||
| A. aegypti, disseminated | |||
| Size | 1 | 1.74 | 0.2170 |
| Competitive treatment | 2 | 0.74 | 0.5036 |
| Size × competition | 2 | 2.62 | 0.1336 |
| Error df | 8 |
Note: An infection spread beyond the midgut, infecting secondary target organs (e.g., salivary glands, head, legs), is called a “disseminated infection.”
Fig. 5.
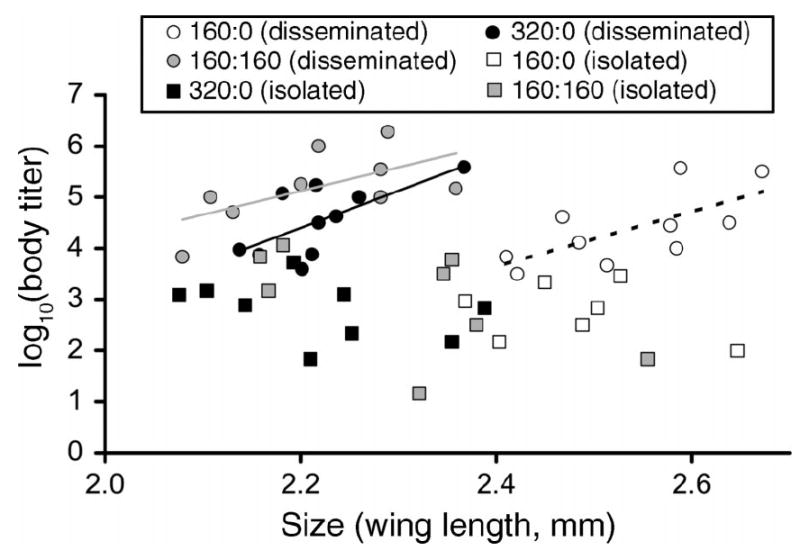
Least-squares means for body titer and size of adult Aedes albopictus females with disseminated (i.e., infection spread beyond the midgut, infecting secondary target organs such as body, legs) and isolated (i.e., infection limited to the midgut) Sindbis virus (SINV) infections. The size effect on females with disseminated infections gives a slope of 5.48 (se = 1.28). Solid and dashed lines drawn through bivariate means show the best fit for A. albopictus with disseminated infections in three competitive treatment conditions. Numbers in the figure key represent the ratio of A. albopictus to A. aegypti.
Product-moment correlations showed significant relationships between infection and all correlates of population growth for A. albopictus (Table 3). In particular, increased time to emergence, a result of intra- and interspecific competition, was positively correlated with infection rate for A. albopictus, but survivorship, size, and λ′ were negatively correlated with infection rate (Table 3). Also, survivorship and λ′ were significantly negatively correlated with mean A. albopictus body titer for females with disseminated infections. All correlations between population growth parameters of A. aegypti and infection parameters were not significant (Table 3).
Table 3.
Product-moment correlation coefficients (r1,2) for the relationship between population growth measurements (time to emergence, survivorship, size, and λ′) and infection parameters.
| Infection parameters and growth correlates | A. albopictus r1,2 | A. aegypti r1,2 |
|---|---|---|
| Infection | ||
| Time to emergence | 0.50** | −0.08 |
| Survivorship | −0.50** | −0.07 |
| Size | −0.64** | 0.17 |
| λ′ | −0.55** | 0.10 |
| Titer (infection isolated) | ||
| Time to emergence | 0.13 | |
| Survivorship | −0.34 | |
| Size | −0.20 | |
| λ′ | −0.35 | |
| Titer (infection disseminated) | ||
| Time to emergence | 0.26 | −0.24 |
| Survivorship | −0.38* | 0.38 |
| Size | −0.00098 | 0.13 |
| λ′ | −0.40* | 0.19 |
| Dissemination | ||
| Time to emergence | −0.04 | |
| Survivorship | −0.22 | |
| Size | −0.20 | |
| λ′ | −0.13 |
Notes: Infection parameters include infection, body titer of females with isolated infection, body titer of females with disseminated infection, and dissemination for Aedes albopictus (df = 25) and A. aegypti (df = 12). An infection limited to the midgut is called an “isolated infection,” whereas an infection spread beyond the midgut, infecting secondary target organs (e.g., salivary glands, head, legs), is called a “disseminated infection.” Asterisks denote significant correlation coefficients. No r1,2 values are reported for A. aegypti dissemination and body titer (isolated) since all infected individuals had disseminated infections.
P < 0.05;
P < 0.01
Discussion
The two experiments in this study were designed to quantify the effects of intra- and interspecific larval competition, and then to determine whether competitive effects carried over into the adult stage and influenced competence for arbovirus infection. For both Aedes species in the competition experiment, all population growth measurements clearly showed that higher larval densities resulted in poorer performance (Figs. 1-3). Analyses of survivorship, time to emergence, and size at emergence suggested that the effects of intra-and interspecific competition were similar. However, for both Aedes species, a synthesis of multiple growth measurements (λ′) showed that interspecific competition was more intense than intraspecific competition (Fig. 3).
A variety of model systems have shown that the outcome of interspecific competition depends on resource type (e.g., Tilman 1982, Sanders and Gordon 2003). Contrasting outcomes have been obtained with these two Aedes species, A. aegypti having the competitive advantage over A. albopictus with nutritious larval food (e.g., liver powder, yeast), but not with low nutrient, more natural resources (e.g., leaf litter; Black et al. 1989, Barrera 1996, Juliano 1998, Braks et al. 2004). The current experiment used a combination of natural (leaf infusion) and supplemental (albumin, yeast) resources and, for both Aedes species, interspecific competition was greater than intraspecific competition as measured by λ′. Our intention in designing the competition experiment was not to mimic natural resources, but rather to use a resource base known to maximize the production of Aedes females for the infection study, without negating the effects of competition. We achieved these objectives in that we detected competitive interactions and obtained sufficient numbers of adults for the infection study.
Although our experimental design was constrained to maximize adult production without negating competition, mosquito densities and sizes conformed to observations from field conditions. In the current experiment, densities were 0.032 and 0.064 larvae/mL for the 160 and 320 larvae treatments, respectively. Sampling of the entire contents of water-holding golf cart tires in Broward, Indian River, and Monroe counties in Florida (from December 1996 or January 1997 to April 1998) showed that larval densities were within the range observed in tires occupied by A. albopictus, A. aegypti, or both species (N = 790, mean ± se, 0.17 ± 0.02, range 0.00083–3.08 larvae/mL; G. F. O’Meara, unpublished data). Also, A. albopictus adult female wing lengths (Fig. 1) were within the range of A. albopictus collected at tire sites in East St. Louis, Illinois, USA (N = 180, mean ±se, 2.43 ± 0.02 mm, range 1.84–2.95; B. W. Alto and S. A. Juliano, unpublished data). Similarly, both A. albopictus and A. aegypti female wing lengths in the current study were within the range of field-collected females of these species from tire sites in southwestern Louisiana (mean [±se] 2.68 ± 0.02 mm, range 2.04–3.12 mm, N = 150; mean 2.64 ± 0.03 mm, range 1.92–3.12 mm, N = 115, respectively; Nasci 1990). Wing length, as a surrogate of adult size, is a good indicator of larval environmental conditions (e.g., food resources, larval density; Juliano 1998, and references therein). Thus, our experimental setup produced adult females that parallel those sizes found in nature.
The infection component revealed that larval competition altered adult mosquito susceptibility to arboviral infection and potential for virus transmission. In particular, competitively stressed A. albopictus females were more likely to become infected and have higher Sindbis virus (SINV) titers and dissemination than females reared with less competition. Results are consistent with other model systems where competition, in the form of nutrient limitation or stressors, enhanced susceptibility to infection with pathogens or parasites (Matson and Waring 1984, Murray et al. 1998, Oppliger et al. 1998, Kiesecker and Skelly 2001). In the current study, infection rate was the variable most sensitive to the impact of larval competition. Intra- and interspecific competition altered subsequent A. albopictus interactions with SINV, suggesting that biotic interactions in early developmental stages may be important in determining adult arboviral infection parameters among mosquitoes. This type of indirect effect may be viewed as an interaction modification since “a change in density of one species alters the nature of a direct interaction between two other species” (Wootton 1993). On the other hand, effects of competition on A. aegypti infection parameters were not observed, and reasons for differences between the two Aedes species in responses to competitive treatments are unknown. Although there was less statistical power in the A. aegypti tests due to lower sample sizes, biological explanations could include species-specific qualitative differences in the availability of midgut receptor sites used by SINV or escape barriers (e.g., midgut escape barrier) that may be differentially affected by competition. These results suggest species-specific differences in how larval competition affects adult competence for arboviral infection parameters. Similarly, in plant communities, studies have demonstrated species-specific responses to indirect effects (e.g., indirect facilitation), most likely attributable to differences among species life history traits (e.g., Levine 1999, Pages et al. 2003).
The correlation coefficients demonstrating that infection rates were significantly associated with all correlates of population growth (Table 3) represent the first evidence that life history traits, in addition to adult size, change parameters associated with vector competence. Furthermore, correlations between life history traits and infection parameters in A. albopictus showed that negative effects of competition on population growth are associated with enhanced vector potential (Table 3). The observation that larger A. albopictus females with disseminated infections had significantly greater body titer can be explained as simply a size phenomenon (i.e., more tissue is available for virus propagation). For body titer, there were independent effects of both mean adult size and competition. The lack of a significant size × competition interaction showed that the effect of size on body titer was similar among competitive treatments (Fig. 5). More importantly, we found density-dependent differences in body titer, with greater mean body titer among A. albopictus reared under competitive conditions. Significant density-dependent differences in body titer were identical to significant density-dependent differences in λ′, except in the opposite direction (Figs. 3, 5). Specifically, more intense competition as measured by a lower λ′ resulted in greater body titer. Our results demonstrate both size-dependent and size-independent effects of competition on infection dynamics that have opposite effects across competitive treatments. Within a competitive treatment, larger mosquitoes have greater body titer, but between competitive treatments larger mosquitoes from low competition larval rearing conditions have lower body titer and a lower proportion infected, compared to smaller mosquitoes emerging from high competition conditions. Overall, our results demonstrate that competitive stress experienced by A. albopictus larval stages carried over to the adult stage and significantly influenced susceptibility to infection and dissemination.
Over and above the effects of competition, the two Aedes species differed in susceptibility to infection and dissemination. Other studies have shown interspecific differences for quantitative aspects of infection (Gubler et al. 1979, Turell et al. 2001). However, previous research using these Aedes species, as well as other mosquito species, did not quantify the variables most important for interspecific differences (e.g., infection, body titer, dissemination) or the positive and negative interrelationships among these variables across species (i.e., see standardized canonical coefficients [SCC]). Aedes albopictus was significantly more susceptible to infection than was A. aegypti, as seen in other studies (Gubler et al. 1979, Turell et al. 2001). Conversely, although A. aegypti had lower infection rates, those females that were infected had significantly higher body titer and dissemination rates compared to infected A. albopictus. Infection contributed approximately twice as much as body titer and dissemination to interspecific differences. Factors limiting body titer and dissemination (e.g., midgut escape barrier) were less efficient (or not expressed) in A. aegypti compared to A. albopictus under these conditions. These results suggest fundamental differences in physiology between these Aedes species that alter their susceptibility to arboviral infection and dissemination, and these differences are likely to have important epidemiological consequences.
If effects of competition on vector infection with SINV apply to arboviruses such as dengue and West Nile, these results may have important implications for human health. The current study suggests that competition experienced by larval A. albopictus may enhance the threat posed by this species in pathogen transmission. Uncrowded larval rearing at low densities, used in most laboratory studies of vector competence, do not accurately reflect conditions in nature where competition is often strong and widespread (Juliano et al. 2004). Our study suggests that indirect effects are important in determining mosquito vector ability, and that the effect may be species specific. Failure to consider larval conditions may result in misleading estimates of relative susceptibility to infection for A. albopictus and A. aegypti, and by extension, other arboviral vectors. This report is the first to quantify how larval competition may affect arbovirus infection in adult mosquitoes, and demonstrates the species specificity of the process from infection to dissemination. Future assessments of vector potential should consider the species-specific effects of larval conditions that reflect competitive conditions observed in nature.
Acknowledgments
We thank P. Grimstad and R. Nasci for providing us with Aedes eggs; R. Escher and B. Wagner for daily maintenance of the competition study and for measuring wing lengths; J. Maruniak for invaluable support with cell culture and SINV assay protocols; G. O’Meara for providing data on mosquito larval densities from field collections; D. Bowers, C. Lord, and W. Tabachnick for advice with cell culture and SINV assays; J. Butler, B. Coon, and K. McKenzie for assistance with the membrane bloodfeeding system; C. Jenning for supplying citrated blood; and C. Lord, M. Braks, and W. Tabachnick for helpful reviews and discussion. This research was supported by grants from Sigma XI Grants-in-Aid of Research and the National Institutes of Health (R01-AI-44793). This is Florida Agriculture Experiment Station Journal Series R-10533.
Footnotes
Corresponding Editor: O. J. Schmitz.
Literature Cited
- Abrams PA. Implications of dynamically variable traits for identifying, classifying and measuring direct and indirect effects in ecological communities. American Naturalist. 1995;146:112–134. [Google Scholar]
- Altwegg R. Trait-mediated indirect effects and complex life-cycles in two European frogs. Evolutionary and Ecology Research. 2002;4:519–536. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Black WC, IV, Rai KS, Turco BJ, Arroyo DC. Laboratory study of competition between United States strains of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1989;32:847–852. doi: 10.1093/jmedent/26.4.260. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lounibos LP, Lourenco-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and A. albopictus (Diptera: Culicidae) in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990;36:165–172. [Google Scholar]
- Butler JF, Hess WR, Endris RG, Holscher KH. In vitro feeding of Ornithodoros ticks for rearing and assessment of disease transmission. Acarology. 1984;VI:1075–1081. [Google Scholar]
- Christophers SR. Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Cambridge University Press; Cambridge, UK: 1960. [Google Scholar]
- Dohm DJ, Logan TM, Barth JF, Turell MJ. Laboratory transmission of Sindbis virus by Aedes albopictus, Ae. aegypti, and Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 1995;32:818–821. doi: 10.1093/jmedent/32.6.818. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Haramis LD. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition-deprived mosquitoes. Journal of Medical Entomology. 1984;21:249–256. doi: 10.1093/jmedent/21.3.249. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Walker ED. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. Journal of Medical Entomology. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Kuno G. Dengue and dengue hemorrhagic fever. CABI Publishing; New York, New York, USA: 1997. [Google Scholar]
- Gubler DJ, Nalim S, Tan R, Saipan H, Saroso JS. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. American Journal of Tropical Medicine and Hygiene. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- Hardy JL. Susceptibility and resistance of vector mosquitoes. In: Monath TP, editor. The arboviruses: epidemiology and ecology. CRC Press; Boca Raton, Florida, USA: 1988. pp. 87–126. [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annual Review of Entomology. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Higgs S. How do mosquito vectors live with their viruses? In: Gillespie SH, Smith GL, Osbourn A, editors. Microbe–vector interactions in vector-borne diseases. Cambridge University Press; Cambridge, UK: 2004. pp. 103–137. [Google Scholar]
- Ho BC, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus, and Ae. triseriatus (Diptera: Culicidae): larval development in mixed cultures. Journal of Medical Entomology. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Holway DA. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology. 1999;80:238–251. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker JM, Skelly DK. Effects of disease and pond drying on gray tree frog growth, development, and survival. Ecology. 2001;82:1956–1963. [Google Scholar]
- Levine JM. Indirect facilitation: evidence and predictions from a riparian community. Ecology. 1999;80:1762–1769. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Suarez S, Menendez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? Journal of Vector Ecology. 2002;27:86–95. [PubMed] [Google Scholar]
- Lundstrom JO, Turell MJ, Niklasson B. Viremia in three orders of birds (Anseriformes, Galliformes and Passeriformes) inoculated with Ockelbo virus. Journal of Wildlife Diseases. 1993;29:189–195. doi: 10.7589/0090-3558-29.2.189. [DOI] [PubMed] [Google Scholar]
- Matson PA, Waring RH. Effects of nutrient and light limitation on mountain hemlock: susceptibility to laminated root rot. Ecology. 1984;65:1517–1524. [Google Scholar]
- Morrison LW. Indirect effects of phorid fly parasitoids on the mechanisms of interspecific competition among ants. Oecologia. 1999;121:113–122. doi: 10.1007/s004420050912. [DOI] [PubMed] [Google Scholar]
- Murray DL, Keith LB, Cary JR. Do parasitism and nutritional status interact to affect production in snowshoe hares? Ecology. 1998;79:1209–1222. [Google Scholar]
- Nasci RS. Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae) Journal of Medical Entomology. 1990;27:716–719. doi: 10.1093/jmedent/27.4.716. [DOI] [PubMed] [Google Scholar]
- Nasci RS, Hare SG, Willis FS. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and laboratory. Journal of the American Mosquito Control Association. 1989;5:416–421. [PubMed] [Google Scholar]
- Olson KE, Higgs S, Gaines PJ, Powers AM, Davis BS, Kamrud KI, Olson JO, Blair CD, Beaty BJ. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Vose FE, Carlson DB. Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. Journal of Medical Entomology. 1989;26:528–534. doi: 10.1093/jmedent/26.6.528. [DOI] [PubMed] [Google Scholar]
- Oppliger AJ, Clobert J, Lecomte P, Lorenzon K, Boudjemadi, John-Alder HB. Environmental stress increases the prevalence and intensity of blood parasite infection in the common lizard Lacerta vivipara. Ecology Letters. 1998;1:129–138. [Google Scholar]
- Pages J-P, Pache G, Joud D, Magnan N, Michalet R. Direct and indirect effects of shade on four forest tree seedlings in the French Alps. Ecology. 2003;84:2741–2750. [Google Scholar]
- Paulson SL, Hawley WA. The effect of body size on the vector competence of field and laboratory populations of Aedes triseriatus for La Crosse virus. Journal of the American Mosquito Control Association. 1991;7:170–175. [PubMed] [Google Scholar]
- Petren K, Bolger DT, Case TJ. Mechanisms in the competitive success of an invading sexual gecko over an asexual native. Science. 1993;259:354–358. doi: 10.1126/science.259.5093.354. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Relyea RA. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology. 2000;81:2278–2289. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Sanders NJ, Gordon DM. Resource-dependent interactions and the organization of desert ant communities. Ecology. 2003;84:1024–1031. [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. Fourth. Vol. 2. SAS Institute; Cary, North Carolina, USA: 1989. Version 6. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. Second. Oxford University Press; Oxford, UK: 2001. pp. 99–115. [Google Scholar]
- Seabaugh RC, Olson KE, Higgs S, Carlson JO, Beaty BJ. Development of a chimeric Sindbis virus with enhanced per os infection of Aedes aegypti. Virology. 1998;243:99–112. doi: 10.1006/viro.1998.9034. [DOI] [PubMed] [Google Scholar]
- Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapoing P, Innis B. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. American Journal of Tropical Medicine and Hygiene. 1998;58:283–286. doi: 10.4269/ajtmh.1998.58.283. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton, New Jersey, USA: 1982. [PubMed] [Google Scholar]
- Turell MJ, Gargan TP, II, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. American Journal of Tropical Medicine and Hygiene. 1984;33:176–181. doi: 10.4269/ajtmh.1984.33.176. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. Journal of Medical Entomology. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Werner EE. Nonlethal effects of a predator on competitive interactions between two anuran larvae. Ecology. 1991;72:1709–1720. [Google Scholar]
- Werner EE. Individual behavior and higher-order species interactions. American Naturalist. 1992;140:S5–S32. [Google Scholar]
- Werner EE, Anholt BR. Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology. 1996;77:157–169. [Google Scholar]
- Wootton JT. Indirect effects and habitat use in an intertidal community: interaction chains and interaction modifications. American Naturalist. 1993;141:71–89. [Google Scholar]
- Wootton JT. The nature and consequences of indirect effects in ecological communities. Annual Review of Ecology and Systematics. 1994;25:443–466. [Google Scholar]


