Abstract
Rationale and Objectives
Improvements in the diagnosis of early breast cancers depend on a physician’s ability to obtain the information necessary to distinguish nonpalpable malignant and benign tumors. Viscoelastic features that describe mechanical properties of tissues may help to distinguish these types of lesions.
Materials and Methods
21 patients with nonpalpable, pathology-confirmed BI-RADS 4 or 5 breast lesions (10 benign, 11 malignant) detected by mammography were studied. Viscoelastic parameters were extracted from a time sequence of ultrasonic strain images and differences in the parameters between malignant and benign tumors were compared. Parametric data were color coded and superimposed on sonograms.
Results
The strain retardance time parameter, T1, provided the best discrimination between malignant and benign tumors (p<0.01). T1 measures the time required for tissues to fully deform (strain) once compressed, and therefore it describes the time-varying viscous response of tissue to a small deforming force. Compared to the surrounding background tissues, malignant lesions have smaller average T1 values while benign lesions have larger T1 values. This tissue-specific contrast correlates with known changes in the extracellular matrix of breast stroma.
Conclusion
Characterization of nonpalpable breast lesions is improved by the addition of viscoelastic strain imaging parameters. The differentiation of malignant and benign BI-RADS 4 or 5 tumors is especially evident with the use of the retardation time estimates, T1.
Keywords: breast cancer, elasticity, diagnosis
Introduction
Breast cancer is the fifth most common cause of cancer death worldwide, and the most frequently diagnosed cancer in women [1]. In the US during 2007, it was expected that approximately 178,480 women would develop invasive breast cancer and an estimated 40,910 patients will die from this disease. The combined efforts of early detection and improved treatment have steadily decreased the death rate in women from breast cancer since 1990. The earliest cancer signs are detectable by medical imaging often before symptoms appear. Diagnosis is currently based on information obtained from the clinical examination, anatomical imaging, and biopsy. Although histopathology is the gold standard for diagnosis, the biopsy procedure is invasive, expensive and carries some risk. Therefore, additional noninvasive diagnostic imaging method to increase specificity and reduce the need for biopsy would be beneficial.
Recent discoveries in molecular biology have triggered interest in developing new and potentially more specific imaging methods for breast cancer diagnosis [2]. These include techniques for (a) direct imaging of signaling molecules and/or receptors mediating malignant progression, and (b) indirect imaging of intrinsic tissue properties (e.g., biochemical, mechanical) that describe the tumor microenvironment controlling signaling pathways. We use the latter method to detect local changes in soft tissue. The alteration in elasticity properties is in part a result of inflammation that usually occurs during the early stages of disease development. The extracellular matrix (ECM) of breast stroma, which provides the solid consistency of parenchymal tissues, plays an active role in cancerous tumor growth [3]. Hence, breast stroma is a potentially valuable source of endogenous disease-specific contrast.
The term “elasticity imaging” refers to any application of imaging technologies to the spatiotemporal description of the mechanical properties of a medium [4]. Medical elasticity imaging is broadly categorized as dynamic or quasi-static, where in both cases a small applied force creates tissue deformations that may be tracked by ultrasonic, magnetic resonance, or optical imaging technologies. Dynamic methods for imaging viscoelastic breast features stimulate tissues with harmonic [5, 6] or impulse [7, 8] forces, typically in the micro-to-milliNewton (µN - mN) amplitude range and at frequencies above 50 Hz.
We employ quasi-static ultrasonic methods for viscoelastic breast imaging. Echo movements are tracked as an ultrasonic probe is gently pressed into the skin surface. Step-force amplitudes of 3–6 N1 are applied suddenly and handheld constant for 10–20 s while ultrasonic echo data are acquired to track movements in a series of strain images. Such methods are quasi-static because the frequency range for this force stimulus is less than about 1 Hz [9]. The slow movement of tissue under a load – called viscous creep – is characteristic of the viscoelastic (VE) properties of tissues. VE properties are found by analyzing a time series of elastic strain images. Because creep responses to mechanical forces vary in soft tissues and other hydropolymers with the frequency of the force stimulus, dynamic and quasi-static methods are thought to provide independent information. It is not yet known which part of the force bandwidth has the most diagnostic information.
Recent studies [10–13] suggest that elastic strain images of breast tissue can be effective at discriminating focal benign and malignant tumors. Thomas et al. [11] showed that strain and strain-rate images were most effective when diagnosing BI-RADS (Breast Imaging-Reporting and Data System) 3 lesions with lipomatous involution and somewhat less effective with BI-RADS 4 or 5 lesions. A sensitive diagnostic feature was the ratio of lesion size visualized in strain images versus that in spatially registered sonograms, suggesting that many of the malignancies studied had a palpable degree of desmoplasia thereby appearing larger on strain images than sonograms. Zhi et al [12] also showed that strain imaging was effective at malignant-benign discrimination for late-stage tumors. However, with strain images, early stage (I & II) infiltrating ductal carcinomas (IDCs) were misdiagnosed presumably because of the lack of desmoplasia. Although lesion palpability and size were not selection criteria for patients in these prior studies, more than half of the lesions were identified as palpable and the maximum tumor diameter ranged from 0.3 cm to 10 cm. The purpose of our study was to determine whether viscoelastic features improved benign vs. malignant differentiation for nonpalpable breast lesions discovered on mammographic scanners.
Materials and Methods
1. Patient Selection
Data were collected from 26 adult female patients through the breast clinic at UC Davis Medical Center in Sacramento, CA. Consent to participate was obtained from patients with a single, nonpalpable lesion identified by mammography just prior to undergoing a core needle biopsy procedure. The protocol was IRB approved at both UCD and UIUC. Data from 5 patients were rejected due to bad acquisition techniques that did not allow for further extractions of viscoelastic parameters needed for this study. Data from 21 patients were used for this clinical study. Out of these 21 patients, 10 patients were diagnosed with benign lesions and 11 patients with malignant lesions of various types. Patient ages ranged between 28 – 72 years, and tumor sizes ranged between 0.5 – 2.5 cm. The patients were selected randomly under the criteria that they have nonpalpbale lesions that were identified via mammography. All lesions were BI-RADS 4 or 5, which identified these lesions as potentially malignant and requiring tissue diagnosis.
Biopsy samples from patients with malignant tumors were assigned SBR (Scarff-Bloom-Richardson) scores based on three morphologic features: degree of tumor tubule formation, tumor mitotic activity and nuclear pleomorphism of tumor cells. SBR scale assigns 1–3 points for each feature; the final score is the sum of all 3 (minimum 3, maximum 9) where higher scores indicate greater likelihood of tumor growth and metastasis. Tumors scoring between 3 –5 are labeled Grade 1 (normal cell appearance); those with a score of 8 or 9 are labeled Grade 3 (poorly differentiated with tendency to grow and spread aggressively); and those with a score of 6 or 7 are in between. The SBR score and tumor grade aid physicians with treatment strategies and prognosis. Among the 11 patients with confirmed malignant tumors in our study, four were diagnosed as Grade 1 tumors, four as Grade 2, one as Grade 3, and 2 were not scored. Small size, low level of lymphatic invasion, low number of tumors per patient, lack of metastasis, and the tumor grades classify our patient group as having early stage malignancies.
2. Lesion Diagnoses
Benign and malignant lesions were further classified into four subtypes or combinations with the exceptions of one benign lesion diagnosed as dense collagenous stroma and one malignant lesion diagnosed as B-cell lymphoma.
a. IDC
Invasive/infiltrating ductal carcinoma (IDC) is the most common form of breast cancer, accounting for 65% to 80% of malignant mammary carcinomas [14]. Eight of 11 patients with malignant tumors were diagnosed with either IDC or IDC combined with DCIS (ductal carcinoma in-situ). All were Grade 1 or 2 and categorized as BI-RADS 4 or 5. While many IDC tumors have a stiff desmoplastic stroma surrounding the tumor, IDC tumors in our population were nonpalpable.
b. ILC
Invasive lobular carcinoma (ILC) accounts for about 10% to 15% of all breast cancers [14]. Two of the 11 patients with malignant tumors were diagnosed with ILC. One had a Grade 1 tumor and both were classified BI-RADS 5. Both ILC patients were in their mid 50’s to early 60’s. Both had only one breast involved and no signs of metastasis. Early-stage ILC lesions are usually nonpalpable due to lack of desmoplasia reaction, difficult to observe mammographically, and thus often larger in size at diagnosis.
c. Fibroadenoma
Seven of 10 patients with benign tumors were diagnosed with fibroadenoma. All of these patient images were categorized as BI-RADS 4.
d. Fibrocystic Change
Two of 10 patients with benign tumors were diagnosed with fibrocystic changes. Both were BI-RADS 4 lesions. The condition is characterized by stiff noncancerous regions containing a high-density of normal ECM collagen and increased interstitial fluid pressure. However, our patient group presented no palpable lesions at physical examination.
All four lesion types usually express increased levels of ECM proteins, which tend to stiffen the lesion to varying degrees relative to their surround medium. IDC lesions with desmoplasia are particularly stiff, often palpable, and frequently they appear larger in the elastic strain image than on the sonogram [15]. By limiting our test population for VE imaging parameters to nonpalpable lesions, our study focuses on early disease that can be difficult to diagnose.
3. Imaging Techniques
Techniques for imaging viscoelastic features of breast tissues have been described previously [9, 16]. A Siemens Sonoline Antares ultrasound scanner was used with a VF10-5 linear array transducer operated at 8 MHz. The scanner was configured with an Ultrasound Research Interface (URI) capable of recording radio-frequency (RF) echo data corresponding to the image displayed on the monitor for off-line processing. Patients were positioned supine and the breasts were scanned anterior-posterior with the chest wall as compression support. Patients were instructed to hold their breath during the 12 to 15 seconds data acquisition time to minimize breast motion. The RF acquisition frame rate was 17 fps. Scanning was repeated at least three times for each patient to ensure consistency in the compressive motion of the breasts.
A small compressive force was applied manually to the breast surface by operators holding the transducer probe without restraint, the same as in clinical imaging. The operators apply a constant downward (compressive) force of approximately 4 N for the duration of the scan. Sridhar [9] showed that sonographers with limited training were able to keep the force constant within ±0.24 N. The recording of RF echo frames began just prior to compression. The entire force was applied within 1 s and held constant while RF frames were recorded for 12 to 15 s. Echoes were recorded at an average depth of 30 mm.
4. Curve fitting
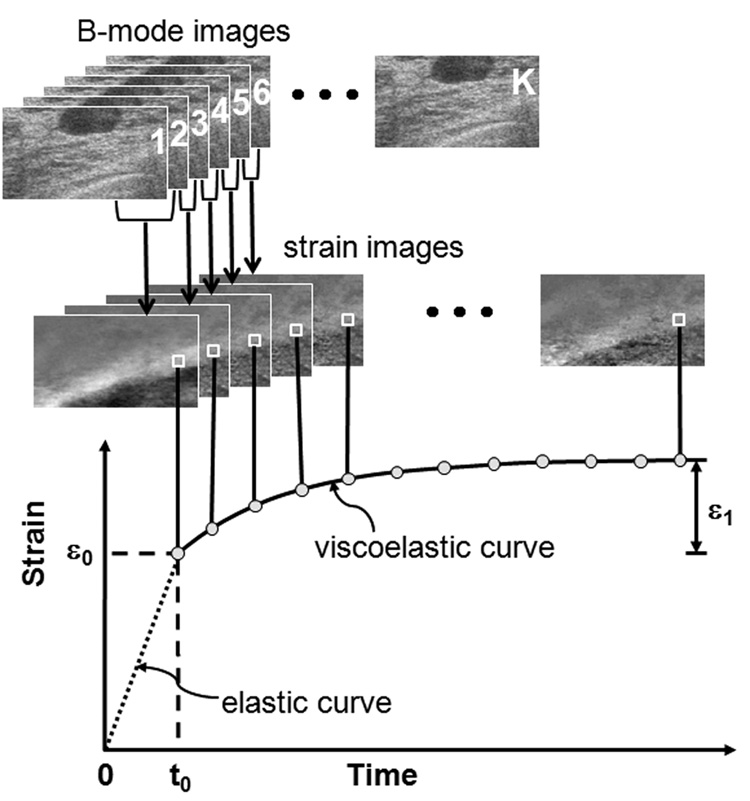
From the time series of RF frames recorded during the application of the force, strain images were formed using multicompression techniques [17] and a regularized optical flow algorithm [18]. Figure 1 illustrates how we analyze the RF echo frames (totaling K frames) recorded over time by applying a sliding reference frame to form a time series of K-1 strain images. The k-th strain image in the series is made from adjacent RF echo frame pairs, fk and fk+1 where k = 1, 2, 3, …, K-1. Each strain image is spatially registered to match the geometry of the pre-compression sonogram. Strain is plotted over the time series of images to generate the viscous creep curve as shown in the figure. The VE phase of the curve begins immediately after tissue compression at time t0. One curve is computed for each strain pixel, or small group of pixels, from which VE parameters are estimated as described below.
Figure 1.
Multicompression RF echo acquisition, strain image formation, and VE parameter estimation for a patient with a nonpalpable fibroadenoma. ε0 describes the instantaneous elastic strain. ε1 describes the viscoelastic strain amplitude. Compression is applied from time t = 0 until t0 during which the instantaneous elastic strain is measured. t0 is also the time at which computation of the viscoelastic response begins. The viscoelastic curve lasts 12 to 15 seconds.
VE parameters were extracted from each measured creep curve by least-square fitting of the patient data to a rheological model [19]. Our clinical imaging acquisitions are no longer than 15 s. Consequently the long-duration VE response terms are not engaged, and therefore a first-order discrete Kelvin-Voigt rheological model [9, 19] is appropriate,
| (1) |
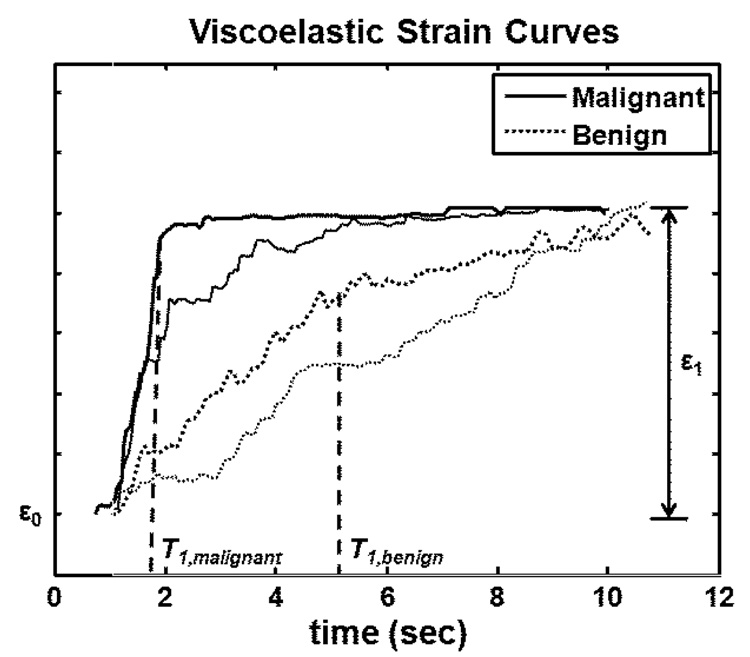
The term ε0 is the instantaneous elastic strain that occurs immediately after compression (Fig 1). In the second term, ε1 is the amplitude of the exponential creep curve. The constant T1 is the retardation time of the curve characterizing the delay in the full strain response. Strain delays in stroma are from frictional resistance due to movement of the ECM in viscous interstitial fluids [9]. Elastic strain ε0, viscoelastic strain amplitude ε1, and the strain retardance time constant T1 are the VE features analyzed in our study. Examples of malignant and benign VE curves are shown in Figure 2.
Figure 2.
Examples of malignant and benign viscoelastic strain curves. ε0 describes the instantaneous elastic strain. ε1 describes the viscoelastic strain amplitude. The retardance time constant T1 measures the time required for the VE curve to reach a plateau. T1 value for the malignant tumor is shorter than that for the benign tumor.
5. Pixel selection and averaging
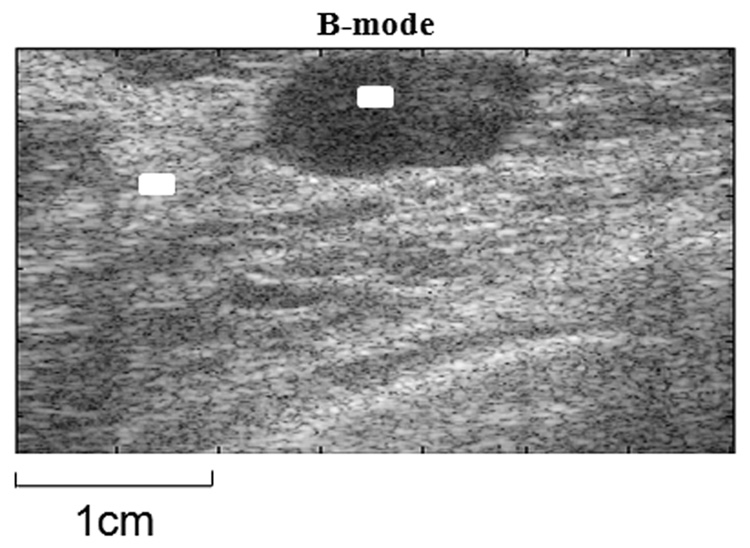
Small areas 10 × 30 pixels were selected by hand within the lesion and background regions of each patient image (Fig 3). In those regions, the average B-mode, ε0, ε1 and T1 values from the spatially registered images are estimated. In cases of tissue heterogeneity, the selection area was reduced to 10 × 15 pixels, but consistency of the mean parameter values was maintained by repeating the process for difference area selections.
Figure 3.
Example of pixel selection for statistical analysis. The selection boxes (marked in white) vary in size from 10 × 30 to 10 × 15 pixels.
6. Parametric contrast
Contrast is an important visual feature for diagnosis, more than the parameters values. The goal of VE imaging is to provide tissue-specific parametric contrast for diagnosis. Contrast is calculated using
| (2) |
where Xlesion and Xbackground represents any of the four parameters described above from the lesion and background tissue areas of a patient scan.
Results
a. Statistical Analysis
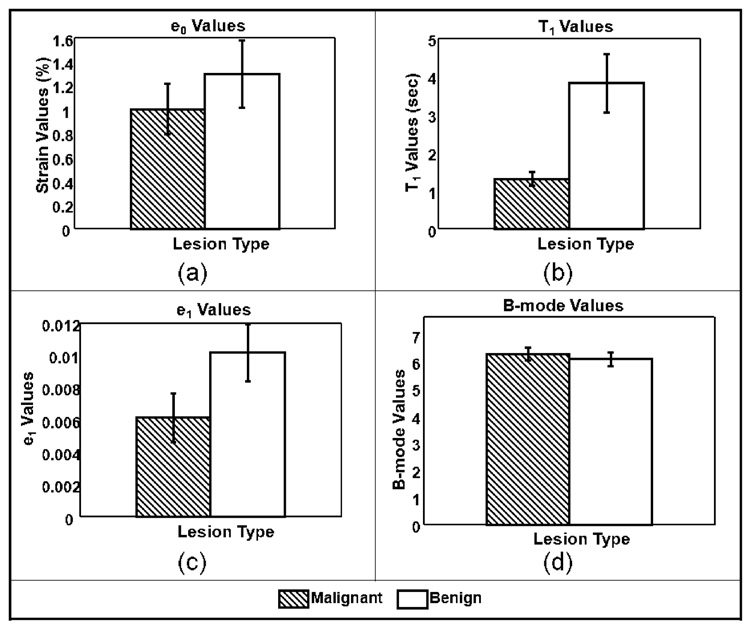
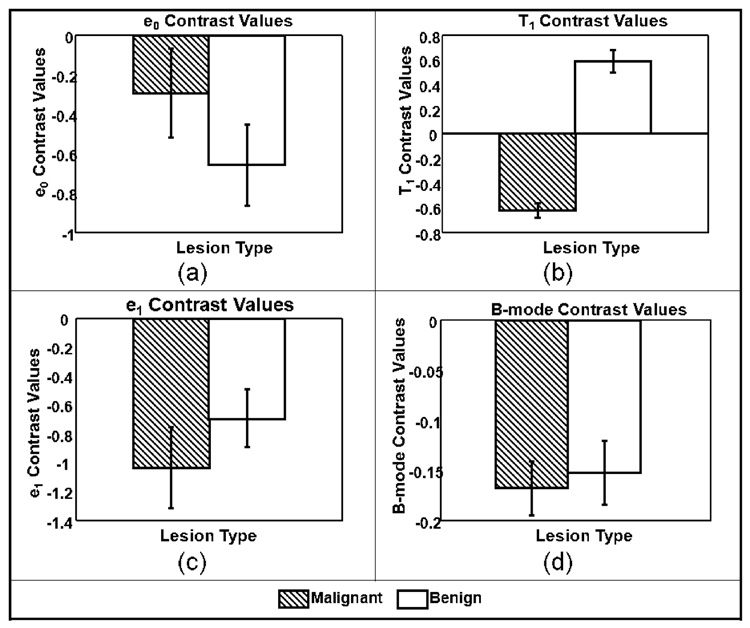
Average VE parameter values for the 21 patient studies are displayed in Figure 3 as histograms with error bars indicating standard errors. Due to the low number of patient studies in each lesion subtype, data were grouped into malignant and benign classes only. Two-sided t-tests were performed for each of the four parameters to test the hypothesis that data from the malignant and benign groups are really just one population. Parameters for which p values were less than 0.05 were considered able to distinguish between the two lesion groups. The t-tests results from data summarized in Figure 3 are shown in Table 1.
Table 1.
t-test results from viscoelastic parameters
| Parameter | p-values (95% significance) |
|---|---|
| ε0 | 0.4213 |
| T1 | 0.0098 |
| ε1 | 0.0986 |
| B-mode | 0.5830 |
Only the VE retardance time constant, T1, was found to be separable and at the 99% significance level (p < 0.01). The histogram of Figure 4(b) shows the difference of means for T1 clearly exceed the standard errors. For comparisons, we refer to the viscoelastic parameters found for normal glandular breast tissue from a previous study, T1 = 3.2 ± 0.8 s [9]. The current study summarized in Figure 4(b) shows an average T1 value of 3.9 ± 0.7 s for benign tumors and 1.4 ± 0.2 s for malignant tumors. Therefore the difference in average T1 values between normal parenchyma and malignant lesions is also highly significant. We also looked for correlations between T1 image contrast, Eq (2), and tumor grade value (1–3), and we found the linear relationship CT1 = −0.18×Grade − 0.35 The norm of the residuals for the equation fit was 0.014, suggesting strong correlation. However, there are too few samples to obtain the statistical power required for significance; therefore the result should be considered preliminary.
Figure 4.
Average lesion parameters. (a) Elastic strain values ε0 (p<0.5); (b) Time constants T1 (p<0.01); (c) creep-curve amplitudes ε1 (p<0.1); and (d) B-mode pixel values (p<0.6). Error bars denote ±1 standard error.
b. Contrast histograms and scatter plot
While the t-test for retardation time T1 revealed a statistically significant difference between the mean values for benign and malignant tumors, it is important for image-based diagnosis to also explore contrast differences with respect to background areas. Equation (2) was applied to calculate patient contrast for each of the four parameters, B-mode, ε0, ε1, T1; the results are shown in Figure 5, where we see that only T1 provided statistically significant contrast.
Figure 5.
Contrast values for (a) elastic strain, (b) T1, (c) ε1 and (d) B-mode images. Error bars denote ±1 standard error.
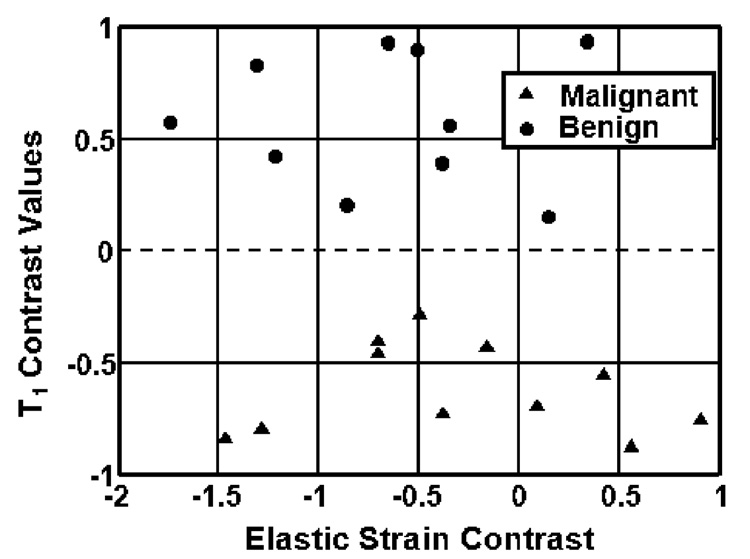
Figure 6 shows a scatter plot of elastic strain (ε0) contrast versus T1 contrast for each of the 21 patients. In this figure, T1 contrast is negative for all malignant tumors and positive for benign tumors, thus clearly providing lesion discrimination. None of the other features provided such strong discriminability.
Figure 6.
Scatter plot of patient contrast values for two parameters, ε0 and T1. A dotted line drawn at T1 contrast = 0 divides malignant and benign lesions. However, ε0 contrast offers no significant discriminability.
c. Color imaging
The previous section showed that spatially averaged T1 contrast allows us to clearly discriminate nonpalpable malignant and benign breast lesions. This section explores spatial variations in the retardance time constant using color mapping of the T1 contrast as an overlay on the sonogram. The concept is analogous to color-flow imaging where blood velocity is mapped onto the sonographic anatomy.
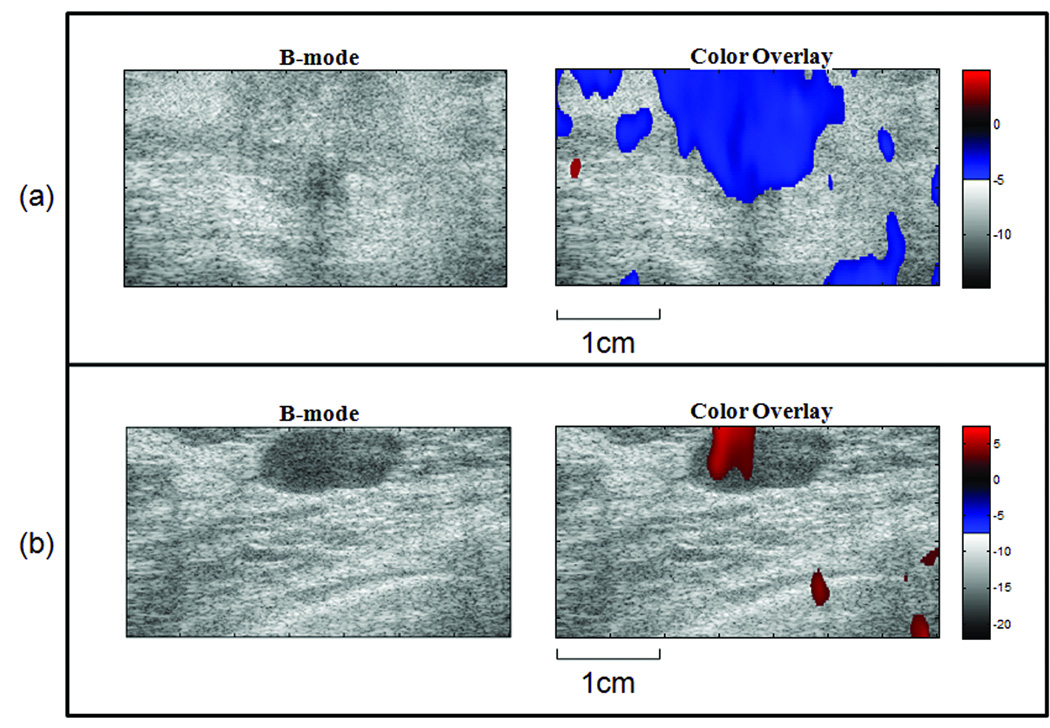
Consider each image as a matrix of size I × J. For each pixel p(i, j) in the matrix, i= 1,…,I and j=1,…,J, we compare its T1 value to the spatially averaged T1 value near the periphery of the image, T̄1 (selection box size 10×30 or 10×15 pixels). For pixels where T1 (i, j) < T̄1, we assign an overlay color in the blue spectrum where the shade indicates the negative difference value T1(i, j)−T̄1. For pixels where ,T1(i, j) > T̄1, we assign an overlay color in the red spectrum where the shade indicates the positive difference value T1(i, j)−T̄1 For example, see the color bars in Figure 7 where an an isoechoic IDC lesion and a hypoechoic fibroadenoma are shown. These color pixels were spatially smoothed and amplitude thresholded in a manner similar to color-flow imaging.
Figure 7.
Sonograms with T1 parametric color overlays. The example in (a) is from a patient with a malignant (IDC) lesion; the negative T1 contrast values are shown in blue. The example in (b) is from a patient with a benign (fibroadenoma) lesion; the positive T1 contrast values are shown in red.
Discussion
This preliminary clinical study tentatively confirms the hypothesis that viscoelastic parameters from patients with nonpalpable breast lesions can be used to differentiate between malignant and benign breast tumors. A statistical test performed on four parameters, ε0, ε1, T1, B-mode, showed a significant difference between the retardation time constants T1 for the two lesion types. In addition, T1 contrast was found to be negative for malignant lesions and positive for benign lesions in all 21 patient studies. The observed contrast differences are consistent with known changes in the collagenous stromal ECM near the lesion [20]. Benign tumors have increased normal collagen density; that is, the associated proteoglycan content increases in proportion. Dense ECM increases both the cross linking among collagen fibers and the viscosity of interstitial fluids, which increase the resistance to creep and lengthen T1 values. Very similar results were found in VE phantom studies where hydropolymers density was locally increased [19]. Malignant tumors also have increased collagen density; however these collagen fibers are structurally different from those of normal ECM stroma. Malignant stroma has reduced proteoglycan content for loose and dense connective breast tissues that then reduces the frictional forces resisting creep. Consequently, T1 values in nonpalpable lesions are less than with that of normal breast stroma. In other words, IDC and ILC lesions are generally more fluidic in their mechanical response than the more solid-responding fibroademomas. We point out that lesion palpability is determined very subjectively. A study with more patients is required to reach conclusion about clinical efficacy.
As we discovered previously by comparing VE results from breast tissues of normal volunteers [9] and hydropolymers phantoms [19] over much longer acquisition times (200 s and 3600 s, respectively), the RF acquisition time significantly influences lesion contrast. Eigenanalysis of normal breast tissues [9] suggests there are at least two VE components in the rheological model for 200 s breast acquisitions using our quasi-static elasticity imaging method. The VE retardance time constants are T1 = 3.2 ± 0.8 s and T2 = 42 ± 28 s. By truncating the acquisition time to practical values <20 s, as in the current study, we can ignore the effects of T2 on T1, and we can expect a coefficient of variation for T1 estimates of 25% due mostly to jitter from the hand-held force application [9]. The current study did not examine the T2 component because 200 s acquisitions were impractical. Also there is greater relative error for T2 estimates, which is a tissue response at a much lower force bandwidth (<0.1 Hz) and which requires development of new scanning techniques. Nevertheless, we are encouraged by the diagnostic performance of T1 estimates for malignant-benign discrimination.
Potential sources of error in viscoelasticity imaging are patient movement (respiratory and cardiac) and hand-held transducer movement during force application. Small transducer movements increase decorrelation errors in strain images [17, 18], which then propagate into large noise variations in VE parametric images. Changing to the high-frequency force stimuli used in dynamic imaging methods [4–7] reduces VE parameter estimation errors for several reasons. However, we know from the polymer mechanics literature that the frequency of the applied force influences the time-varying strain response and therefore VE parameters. To visualize this point, consider movement of stroma: an ECM polymer embedded within a viscous interstitial fluid. Slow application of a compressive force allows fluid motion with little frictional resistance, and therefore cross linking among ECM fibers determines the VE response. However, dynamic friction increases with the velocity of the applied force, depending on the viscosity of the fluid, until fluid flow dominates the time-varying strain response. Consequently, there is merit in pursing both quasi-static and dynamic elasticity imaging methods. Both methods are currently limited for clinical applications by our ability to precisely apply forces to the tissue.
Results from this preliminary study have demonstrated that viscoelastic imaging of breast tumors can effectively aid in the characterization of nonpalpable breast lesions. The greatest advantage of this novel imaging method is that the viscoelastic parameters obtained can be used to clearly differentiate between malignant and benign lesions for patients with nonpalpable, early stage, BI-RADS 4 and 5 breast tumors. The addition of viscoelastic features into the diagnosis feature space can aid the physicians in making a more accurate diagnosis of early breast cancer patients.
Acknowledgements
This work was supported by the NCI under award No. CA082497 and by the Beckman Institute for Advanced Science and Technology at the University of Illinois.
Special thanks to Pamela Phelps and Tonya Sheppard as the sonographers.
All authors have reviewed and approved the manuscript.
Supporting Grant: This work was supported by the NCI under award No. CA082497 and by the Beckman Institute for Advanced Science and Technology at the University of Illinois.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For comparison, mammographic compressional forces are 100–200 N.
Contributor Information
Yupeng Qiu, Department of Bioengineering and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana IL 61801. Current Address: Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Room 5237, 405 North Mathews, Urbana, IL 61801.
Mallika Sridhar, Department of Biomedical Engineering, University of California, Davis CA 95616. Current Address: Department of Radiation Oncology, University of California, San Francisco,, 1600 Divisadero Street, San Francisco, CA 94115
Jean K. Tsou, Department of Biomedical Engineering, University of California, Davis CA 95616. Current Address: 405 Safflower Place, West Sacramento, CA 95691
Karen K. Lindfors, Department of Radiology, University of California-Davis Medical Center, Sacramento, CA. Current Address: University of California, Davis School of Medicine, Suite 3100, ACC 4860 Y Street, Sacramento, CA 95817
Michael F. Insana, Department of Bioengineering and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana IL 61801. Current Address: Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Room 4247, 405 North Mathews, Urbana, IL 61801
References
- 1.American Cancer Society. Cancer Facts & Figures 2007. 2007 [Google Scholar]
- 2.Insana MF, Wickline SA. Multimodality biomolecular imaging. Proc IEEE. 2008;96:378–381. [Google Scholar]
- 3.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratscheck M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Can Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 4.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annual Review of Biomedical Engineering. In: Yarmush Martin., editor. Annual Reviews. Vol. 5. Palo Alto: 2003. pp. 57–78. [DOI] [PubMed] [Google Scholar]
- 5.Lerner R, Parker K. Sonoelasticity imaging. Acoustical Imaging. 1988;17:317–327. [Google Scholar]
- 6.Sinkus R, Tanter M, Xydeas T, Catheline S, Bercoff J, Fink M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn Reson Imaging. 2005;23:159–165. doi: 10.1016/j.mri.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 7.Fatemi M, Greenleaf J. Ultrasound stimulated vibro-acoustic spectrography. Science. 1998;280:82–85. doi: 10.1126/science.280.5360.82. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Soo M, Trahey G, Nightingale K. Acoustic radiation force impulse imaging of in vivo breast masses; Proc. IEEE Ultrason. Symp; 2004. pp. 728–731. No. 078038412. [Google Scholar]
- 9.Sridhar M, Insana MF. Ultrasonic measurements of breast viscoelasticity. Med Phys. 2007;34:4757–4767. doi: 10.1118/1.2805258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas A, Kümmel S, Fritzsche F, Warm M, Ebert B, Hamm B, Fischer T. Real-time sonoelastography performed in addition to B-mode ultrasound and mammography: improved differentiation of breast lesions. Acad Radiol. 2006;13:1496–1504. doi: 10.1016/j.acra.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Thomas A, Warm M, Hoopmann M, Diekmann F, Fischer T. Tissue Doppler and strain imaging for evaluating tissue elasticity of breast lesions. Acad Radiol. 2007;14:522–529. doi: 10.1016/j.acra.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhi H, Ou B, Luo BM, Feng X, Wen YL, Yang HY. Comparison of ultrasound elastography, mammography, and sonography in the diagnosis of solid breast lesions. J Ultrasound Med. 2007;26:807–815. doi: 10.7863/jum.2007.26.6.807. [DOI] [PubMed] [Google Scholar]
- 13.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 14.Rosen PP. Rosen’s Breast Pathology. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 15.Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, Pennanen MF. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 16.Insana MF, Pellot-Barakat C, Sridhar M, Lindfors K. Viscoelastic imaging of breast tumor microenvironment with ultrasound. J Mammary Gland Biol & Neoplasia. 2004;9:393–404. doi: 10.1007/s10911-004-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H, Liu J, Barakat C, Insana MF. Optimizing multicompression approaches to breast elasticity imaging. IEEE Trans Ultrason Ferroelec Freq Control. 2006;53:90–99. doi: 10.1109/tuffc.2006.1588394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellot-Barakat C, Frouin F, Insana MF. Ultrasound elastography based on multiscale estimations of regularized displacement fields. IEEE Trans Medical Imaging. 2004;23:153–163. doi: 10.1109/TMI.2003.822825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridhar M, Liu J, Insana MF. Viscoelasticity imaging using ultrasound: parameters and error analysis. Phys Med Biol. 2007;52:2425–2443. doi: 10.1088/0031-9155/52/9/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losa G, Alini M. Sulphated proteoglycans in the extracellular matrix of human breast tissues with infiltrating carcinoma. Int. J. Cancer. 1993;54:552–557. doi: 10.1002/ijc.2910540406. [DOI] [PubMed] [Google Scholar]