Abstract
A decade of empirical work in brain imaging, genomics, and other areas of research has yielded new knowledge about the frequency of incidental findings, investigator responsibility, and risks and benefits of disclosure. Straightforward guidance for handling such findings of possible clinical significance, however, has been elusive. In early work focusing on imaging studies of the brain, we suggested that investigators and institutional review boards must anticipate and articulate plans for handling incidental findings. Here we provide a detailed analysis of different approaches to the problem and evaluate their merits in the context of the goals and setting of the research and the involvement of neurologists, radiologists, and other physicians. Protecting subject welfare and privacy, as well as ensuring scientific integrity, are the highest priorities in making choices about how to handle incidental findings. Forethought and clarity will enable these goals without overburdening research conducted within or outside the medical setting.
Case reports, empirical investigations, and reviews of incidental findings—unexpected observations of possible clinical significance in healthy subjects or in patients recruited to research—have increased substantially in the literature on human research since the 1990s. Such findings are unexpected in the context of an individual’s medical history, may threaten health, and are distinguished from findings prompted by a neurologic complaint in a clinical situation. Past reports concerned with the incidental findings phenomenon and focusing on MRI, in particular, have covered a range of topics, including frequency and significance, subjects’ expectations and experiences, study protocols, and challenges of clinical follow-up. Findings with immediate clinical consequences are generally reported to be in the range of 2 to 8% of those detected in brain imaging research overall.1–6,24 Incidence may be as high as 40 to 50% in subjects over 65 years of age, although clinical urgency is not routinely as great in this age group as it is for the rare findings discovered unexpectedly in younger subjects.3 Subjects commonly assume that discovery of such findings will be disclosed to them,7,8 but protocols for handling such information vary significantly across laboratories.9,10 Most imaging studies do not regularly involve physician review of scans and, in many laboratories, students are permitted to scan independently.9 Risks of false positive findings and the burden of medical follow-up are ongoing sources of debate in the pursuit of best practices for handling incidental findings in imaging research.10–12
Early guidance on how unexpected findings in research might be handled is offered by the genetics literature,13,14 but it does not fully address the unique circumstances for detecting a clinical anomaly, such as a tumor or arteriovenous malformation in the brain (figure). In our own prior work,11 we raised awareness about the need for anticipating incidental findings in research and for articulating a plan for handling them in both Institutional Review Board (IRB) materials and in consent forms for subject recruitment. The present article synthesizes past work from the brain imaging and relevant genetics literature and contributes a specific series of options for developing and implementing management plans for incidental findings for the benefit of both patients and the research community. This analysis recognizes the special circumstances of brain imaging and site-specific variability; however, it is not intended either to establish definitely what ought to be done or to provide a single lens on best practices. The authors recognize that views may vary on the acceptability of some of the approaches proposed. Neurologists, along with clinicians in other specialties such as neuroradiology and psychiatry, have an important role to play in helping to design the research, referring subjects and patients to studies, consulting on findings that might be medically significant, and guiding follow-up.
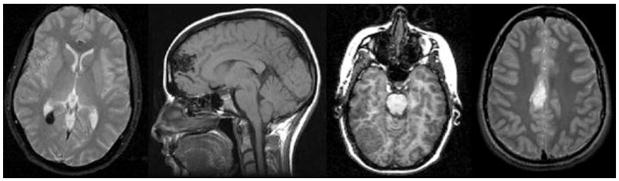
Figure. Series of brain abnormalities detected incidentally on research MRI scans acquired at 1.5 and 3 tesla.
From left to right: Cavernous hemangioma—This finding was treated neurosurgically and followed by MRI every 6 months. No interval changes have been noted and no intervention has been needed in 3 years since discovery. Arteriovenous malformation—This finding was treated successfully by embolization and surgical resection. Meningioma—This finding was treated by surgical resection. Further information about outcome is unavailable. Glioma—This finding was detected in an undergraduate student, with poor prognosis suspected by the consulting neurologist. The student opted to pursue medical management in his home city. Further information about outcome is unavailable. Courtesy of the Richard M. Lucas MR Imaging Center, Stanford University.
OPTIONS FOR HANDLING INCIDENTAL FINDINGS
A range of options for handling incidental findings is shown in table 1. Little is known about the ways with which IRBs handle the issue of incidental findings, and these options are designed to accommodate varying disciplinary backgrounds and professional training of the research team, parameters of the scanning protocol including resolution and sampling, methods using automated or manual algorithms for analyzing the image data acquired, and the extent and immediacy of the involvement of neurologists or other medically trained staff. Option 1 is appropriate for research protocols in which the images are not of sufficient resolution or quality to provide a basis for reliably detecting atypical anatomy. Option 2 recognizes the limitations to disclosure of incidental findings in certain research settings, such as in psychology departments of liberal arts universities, in which personnel with the expertise to perform clinical analysis are not available. Options 3, 4, and 5 describe selective or routine expert image review. Option 3 has the benefit of having medical expertise available at least on an as-needed basis. Options 4 and 5 are more resource-intensive and require routine physician involvement. One consideration for this level of involvement in brain imaging research is the impact on resources and cost burden to already fragile research funding systems. A second is accessibility, which may be relatively straightforward when the research is housed in a medical center and considerably more complex when not. For all three options involving physician participation, it is important to note that the scope of responsibilities in research differs from responsibilities in the medical or diagnostic setting.15,16 Even without a full clinical workup, a clinical read of a research scan may impose some limited clinical duties that are separate from the duties of non-physician researchers in the researcher-subject relationship.17
Table 1.
A range of options for handling incidental findings
| Option | Implications/applications |
|---|---|
| 1. No action is taken beyond articulating a plan for handling incidental findings in the informed consent process. | Researchers do not have an obligation to actively screen for incidental findings, only to have a plan in place if an incidental finding is detected. With this option, researchers inform the participants that the scans will not be examined for abnormalities. This approach might be appropriate in those settings, and for those research protocols, in which the images obtained are not of sufficient resolution or quality to provide a basis for reliably detecting an atypical finding. |
| 2. Participants are informed that if a suspicious finding is discovered it will be reported to them, but images are not reviewed by an expert trained to perform a clinical evaluation. | This might be the approach of choice if the research team does not include personnel with the expertise to perform clinical analysis of a suspicious scan or does not have a pathway for obtaining a clinical evaluation. |
| 3. Expert review of scans with a medically suspicious abnormality is performed prior to communication to the participant. | Subjects are informed that incidental findings of potential clinical significance will receive expert review and the finding will be reported to them if the review indicates that clinical follow-up is warranted. This option requires the inclusion of an expert on the research team or the use of a consultant for expert clinical evaluation. |
| 4. Expert review of research scans is performed routinely; incidental findings that may have clinical significance are communicated. | This option entails a clinical read of all research scans. This differs from the option above in that all research scans in the study will be subject to clinical evaluation, not just those identified as presenting a possible incidental finding. Because this option necessitates a significant time commitment for a clinician, it is likely most practical for research conducted in a clinical setting. |
| 5. Both research and clinical-grade images are routinely acquired; incidental findings that may have clinical significance determined by expert review are communicated. | This approach may require longer scanning times or multiple scan sessions. This is the most resource-intensive of the options considered and is likely to be practical only in a clinical setting. |
Different options are appropriate for different research settings depending the nature of the research and the professional resources available to the research team.
COMMUNICATION AND DISCLOSURE
Language
The language of consent forms and the consent process should make the approach to be taken to incidental findings clear to prospective research subjects. The consent process can make explicit that the scans performed in the protocol are not intended to, and may not be suitable for, diagnosing a medical problem and, as applicable, that the purpose of the study is to gather research data and not to provide any diagnostic or therapeutic benefit. Furthermore, the risks section of the consent form is the place for investigators to clearly state the likelihood of an incidental finding in the type of research that will be performed, the kinds of incidental findings that may be discovered, and the plan to be followed for disclosure. This includes clarity about the types of findings that investigators might a priori not disclose. This concept has not been explored extensively for brain imaging, but lessons may be taken from the genetics literature in which a model has been proposed for assessing the risks and benefits of disclosure of findings based on the analytic validity and clinical utility of the possible result.18 Drawing further on the history of genetics research, a subject’s right to exercise autonomy and opt out of being informed about a finding is a fundamental tenet.19 This right extends to subjects participating in brain imaging research, and their understanding of both the immediate implications of their participation and potentially long-term ones is essential.
Responsibility for follow-up—both informational and financial—should also be clearly stated in the section of the consent forms in which the potential risks of the research are explained. Information is the responsibility of the research team. In the absence of funds in research budgets for assisting financially with follow-up, however, or any current agreement in the professional community on what the length or extent of such responsibility might be, this burden falls to the subject at the present time. Exculpatory language, i.e., language declining responsibility, is prohibited by federal regulations (see 45 CFR 46.116; 21). We suggest that any verbal explanation or expansion on the goals, risks, and benefits of the study follow the written text closely to minimize the possibility of misunderstanding (table 2). If a subject explicitly chooses to exercise the right to opt out, investigators may convey a sense of obligation to re-contact the subject, despite the consent agreement, if an anomaly that may be life-threatening is discovered.
Table 2.
Examples of key points for informed consent about incidental findings
|
Final construction of the language for consent will depend on the option selected for handling findings described in the text and in table 1.
Medium and timing of communication
It is essential that verbal communication is done in a timely fashion commensurate with the urgency of a finding and that the initial communication allows subjects to have questions answered in real time. Language in written follow-up can be tailored to express urgency. One contributing author’s institution uses the following language:
Thank you for participating in the research study entitled <study-title>. As part of your participation, you had an MRI scan performed on <scan-date>. As we discussed (in person, by phone) on <date>, we found a potentially significant abnormality on this scan, and we have confirmed with a radiologist that there is a finding that merits further evaluation. As disclosed in the informed consent document for this study, the research team cannot offer you an opinion as to its severity or degree of clinical significance, and the decision to proceed with further examination or treatment lies with you. We suggest you discuss this matter with a physician. If you do not have a physician, we would be happy to suggest one, or other avenues you might pursue to evaluate this finding. We will make the images demonstrating the incidental finding available to you or your physician, upon your request.
While overall management of the discovery of an incidental finding is the responsibility of the principal investigator, it is reasonable that information about a finding is communicated to the subject by another senior member of the team. This may be a neurologist, family physician, or other medical professional more accustomed to discussing issues of possible health significance. It is important that information about resources and procedures for following up are made available to the subject.
Bearing in mind that the incidence of false positives such as benign cysts has yet to be determined by large-scale studies, caution must be exercised when developing any framework for communication so that anxiety or undue burdens are not created. Answers to questions about the legal duty of the research team and risk management are not clearcut and may depend upon the circumstances surrounding the investigator– or physician–subject relationships.
SUBJECT SELECTION
Considerations of incidence
While it would be possible to reduce the likelihood of incidental findings in a study by excluding otherwise healthy volunteers from populations with known or increased risk factors,20 exclusion by ethnicity, gender, or age if stratified only by likelihood of incidental findings is not appropriate research practice. Such a strategy would also have the undesirable effect of compromising the scientific generalizability of results. This is in contrast, however, to research from which clinical populations may appropriately be excluded because of the scientific rationale of a study, such as individuals with a medical history of stroke in a brain imaging study.
Vulnerable populations
Although scientific concerns drive most protocol designs, regulatory and ethical considerations play an important role when planning for research with special populations. NIH policies require the inclusion of women, minorities, and children, as scientifically appropriate, in studies that the NIH funds. However, there are populations for which special considerations exist for incidental findings. These are, for example, children, subjects receiving medical intensive care, and subjects with limited access to health care and health insurance.
Children
There is currently a lack of neuroim-aging data that can provide baseline images of “normal” brains at various ages and upon which to evaluate the possible clinical significance of incidental findings. Projects are under way to address this need,21 but predicting the significance of an incidental finding may still prove to be difficult given the complexity and variability of human brain development. According to Food and Drug Administration guidelines, MRI studies employing the most commonly used protocols are not considered to pose a significant risk. A limitation in pediatric brain studies is the fact that some types of neuroimaging studies rise above the level of minimal risk, such as PET protocols that use ionizing radiation, and can be performed only with careful and specific justification. As with any other subject population, children should be included in neuroimaging studies only to the extent that the risks and benefits are justified. Appropriate to age and cognitive ability, their assent to participate in research is necessary.
Other special populations
Certain categories of incidental findings will be particularly sensitive in cases where there is CNS evidence, for example, of physical abuse.22 When research has the potential to uncover such findings, an explicit plan for handling them appropriately is needed, guided by IRB requirements and recommendations, for ensuring that subjects as well as parents or guardians are informed of that plan as part of the consent process.
For studies that include subjects with limited decision-making capacity, it is important to clarify who is empowered to make decisions about the person’s involvement in the research. This may not be the same person who is authorized to make surrogate treatment decisions. Once the person who can make decisions about participation in research is identified, it is important that researchers and IRBs consider who among this group will be informed of incidental findings and who is authorized to seek follow-up evaluation for participants requiring neurologic evaluation.
The participation of employees or students of a member of the research team also raises special issues. As is the case for any other participant, they need to be made aware of the possibility of incidental findings, who will learn of those findings, and the plan for handling them. Under almost all circumstances, participating in research will inevitably yield information that would otherwise not be shared. Expectations of special care, on the one hand, or coerced participation in hierarchical relationships such as these, on the other, cannot be overlooked. Some institutions have developed specific policies to limit student and employee participation in research within the institution.
Recruitment
An important consideration for subject selection precedes selection itself. Although explicit exclusion–inclusion criteria theoretically describe a population to be studied, the way in which the study is advertised and the way in which subjects are provided incentives to participate strongly influence who will ultimately be enrolled. Subjects may have a natural curiosity to participate in a study that will yield a brain picture that they may take home, and this has at times been used as a recruitment incentive. Providing actual scans, however, may encourage participants to try to interpret their own images or compare them with those of others (a phenomenon known to occur in college dormitories of universities where undergraduates often participate in imaging research). Site-specific practices for offering images to subjects are reasonable but, in general, providing highly processed generic images such as three-dimensional cortical renderings is a more appropriate incentive than raw image data.
Possible discovery of a clinically significant finding can be an incentive for some participants to volunteer with the expectation that the outcome will either be an entitlement to free medical care10 or reassurance of good health.23 One way to minimize this eventuality is to restrict mention of incidental findings to the informed consent process, keeping it strictly apart from the advertising and recruiting phase of a study.
DISCUSSION
We have combined a growing body of evidence from the peer-reviewed literature with open dialogue among a wide range of professionals from different specialties to analyze different approaches for handling incidental findings in brain imaging. In considering the various scenarios for our analyses, we remain mindful of the distinction between research subjects who volunteer after recruitment and are compensated, and neurologic patients who have a therapeutic relationship with a caregiver. Strict standards for handling incidental findings are not appropriate given the present state of knowledge about them. Investigators must be guided by their IRBs, be clear about the approaches they choose, and work closely with the medical community, as appropriate, to achieve a beneficial result. We conclude, therefore, with the points summarized in table 3, which provide practical guidelines for achieving these goals.
Table 3.
Practical guidelines for researchers and clinicians conducting brain imaging research
|
Substantial work remains to be done toward fully understanding the challenges of incidental findings and the details of responding to them. We did not, for example, consider studies involving secondary uses of clinical or research scans in which previously undetected findings are uncovered. These will require separate and thoughtful consideration given the time lapse and likelihood that scans may be obtained for such a project under a waiver of consent.
The development of a database of incidental findings and an atlas of different types of incidental findings would be a valuable medical resource in advancing our knowledge of incidental findings. Given the non-invasiveness of many neuro-imaging methods, it is now possible to scan the same subjects repeatedly. However, this possibility represents a source of consideration for future work on incidental findings, as it may lead to an underestimation of the occurrence of incidental findings if people with normal scans are enrolled in multiple studies while others with findings only enter one study. Taking into consideration the logistics and complexities of confidentiality, a mechanism for tracking repeat vs single-time volunteers is needed to ensure that further data on neurologic incidental findings accurately reflect their occurrence.
Protecting human subjects and patients in research is of paramount importance, and key to the discussion of incidental findings are trust and reciprocity in a scientific process that has made important advances in furthering the understanding of mind and behavior and in realizing immeasurable benefits in the diagnosis and treatment of neurologic and psychiatric disease. To preserve this trust, researchers and physicians involved in brain research have an ethical duty to consider the psychological, social, and medical contexts subjects bring to the research setting. It is imperative that the design of brain imaging research and other applications accommodate these extended dimensions of brain research.
Acknowledgments
The Detection and Disclosure of Incidental Findings in Neuroimaging Research workshop was supported by the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Drug Abuse (NIDA), the National Institute of Biomedical Imaging and BioEngineering, the National Institute on Aging (NIA), and the National Institute of Mental Health, and NINDS grant R01 NS045831 (J. Illes, PI). Support for early work on incidental findings from The Greenwall Foundation is also acknowledged. Continuing work by S.M. Wolf, J. Illes, and L. Parker on incidental findings in neuroimaging and other domains is supported by 1R01 HG003178 (S. Wolf, PI).
Footnotes
Disclosure: The authors report no conflicts of interest.
NOTE ADDED IN PROOF
New reference added: Vernooij et al.24
Publisher's Disclaimer: This article summarizes the views of a multidisciplinary group of professionals from the neuroscience, bioethics, law, and humanities communities who participated in the Detection and Disclosure of Incidental Findings in Neuroimaging Research meeting and the ongoing work of members of this group, but it does not reflect endorsement by or an official position of National Institute of Neurological Disorders and Stroke, National Institute of Mental Health (NIMH), National Institute of Biomedical Imaging and BioEngineering, the NIH, or any other federal agency. It is intended to promote discussion of the issues only. Views perceived by the authors to be held by most participants, as well as alternative views expressed at the Detection and Disclosure of Incidental Findings in Neuroimaging Research meeting, are described. The full list of workshop participants is available at http://www.ninds.nih.gov/news_and_events/proceedings/if-execsummary.htm. See also the NIMH Council workgroup on MRI research practices “MRI research safety and ethics: Points to consider” at http://www.nimh.nih.gov/council/advis.cfm.
This article is based on the Detection and Disclosure of Incidental Findings in Neuroimaging Research workshop (NIH and Stanford University); Bethesda, MD; January 6–7, 2005.
References
- 1.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;281:36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 2.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. Am J Neuroradiol. 2002;23:1674–1677. [PMC free article] [PubMed] [Google Scholar]
- 3.Illes J, Rosen AC, Huang L, et al. Ethical consideration of incidental findings on adult brain MRI in research. Neurology. 2004;62:888–890. doi: 10.1212/01.wnl.0000118531.90418.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamourian A. Incidental findings on research functional MR images: Should we look? Am J Neuroradiol. 2004;25:520–522. [PMC free article] [PubMed] [Google Scholar]
- 5.Weber F, Knopf H. Incidental findings in magnetic resonance imaging of the brain of healthy young men. J Neurol Sci. 2006;240:81–84. doi: 10.1016/j.jns.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Hentschel F, Klix WE. Management of incidental findings in neuroimaging in diagnosis and pathophysiological research. Fortschr Neurol Psychiatr. 2006;74:651–655. doi: 10.1055/s-2006-932200. [DOI] [PubMed] [Google Scholar]
- 7.Kirschen MP, Jaworska A, Illes J. Subjects’ expectations in neuroimaging research. J Magn Reson Imaging. 2006;23:205–209. doi: 10.1002/jmri.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilgenberg S. Transformation: From medical student to patient. Ann Intern Medicine. 2006;144:779–780. doi: 10.7326/0003-4819-144-10-200605160-00015. [DOI] [PubMed] [Google Scholar]
- 9.Illes J, Kirschen MP, Karetsky K, et al. Discovery and disclosure of incidental findings on brain MRI in research. J Magn Reson Imaging. 2004;20:743–747. doi: 10.1002/jmri.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illes J, Desmond JE, Huang LF, Raffin TA, Atlas SW. Ethical and practical considerations in managing incidental findings in functional magnetic resonance imaging. Brain Cogn. 2002;50:358–365. doi: 10.1016/s0278-2626(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 11.Illes J, Kirschen MP, Edwards E, et al. Incidental findings in brain imaging research. Science. 2006;311:783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illes J. On the contents of Pandora’s Box of incidental findings in brain imaging research. Nat Clin Pract Neurol. 2006;2:60–61. doi: 10.1038/ncpneuro0119. [DOI] [PubMed] [Google Scholar]
- 13.Report and Recommendations of the National Bioethics Advisory Commission. Rockville, MD: National Bioethics Commission; 1999. Research involving human biological materials: ethical issues and policy guidance. [Google Scholar]
- 14.Working Group on Reporting Genetic Results in Research Studies Meeting Summary. Bethesda, MD: National Heart, Lung and Blood Institute; 2004. Reporting genetic results in research studies meeting summary. [Google Scholar]
- 15.Richardson HS, Belsky L. Medical researchers’ ancillary clinical care responsibilities. BMJ. 2004;328:1494–1496. doi: 10.1136/bmj.328.7454.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson HS, Belsky L. The ancillary-care responsibilities of medical researchers. An ethical framework for thinking about the clinical care that researchers owe their subjects. Hastings Cent Rep. 2004;34:25–33. [PubMed] [Google Scholar]
- 17.Mastroianni AC, Kahn JP. Risk and responsibility: ethics, Grimes v Kennedy Krieger, and public health research involving children. Am J Public Health. 2002;92:1073–1076. doi: 10.2105/ajph.92.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravitsky V, Wilfond B. Disclosing individual genetic results to participants. Am J Bioethics. 2006;6:8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- 19.National Human Research Protections Advisory Committee (NHRPAC) IRB Guidebook Chapter on Human Genetics Research. Washington, DC: Department of Health and Human Services; 2002. [Google Scholar]
- 20.Gunel M, Awad IA, Finberg K, et al. A founder mutation as a cause of cerebral cavernous malformation in Hispanic Americans. N Engl J Med. 1996;334:946–951. doi: 10.1056/NEJM199604113341503. [DOI] [PubMed] [Google Scholar]
- 21.Evans AC. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 22.Prasad MR, Kramer LA, Ewing-Cobbs L. Cognitive and neuroimaging findings in physically abused pre-schoolers. Arch Dis Child. 2005;90:82–85. doi: 10.1136/adc.2003.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho MK. Conflicts of interest in magnetic resonance imaging: issues in clinical practice and research. Top Magn Reson Imaging. 2002;13:73–77. doi: 10.1097/00002142-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]