Abstract
Adoptive immunotherapy with antigen specific cytotoxic T lymphocytes (CTL) has been shown to be effective in restoring cellular immunity to cytomegalovirus (CMV) and preventing viral reactivation following allogeneic stem cell transplantation (SCT). In order to develop a cost-effective, relatively rapid method of CMV CTL expansion, we investigated the use of a pool of overlapping CMV peptides. Since the possibility exists of vaccinating CMV sero-negative donors, and these individuals may have T cell responses predominantly against IE-1, commercially available peptide mixes for pp65 as well as IE-1 were used to stimulate CTL from ten sero-positive donors. In four of these ten donors, responses were present to pp65 only, one donor did not respond to pp65 or IE-1, four donors responded to both pp65 and IE-1, and one donor to IE-1 only. These CMV specific T cells included a mixture of CD4+ and CD8+ effectors, and specific cytotoxicity correlated with IFN-γ production. The costs associated with a 28 day maintenance course of intravenous ganciclovir, cidofovir, foscarnet, and valganciclovir, as well as the preparation and shipping a single dose of CTL, were determined. The price of generating CMV CTL using this method was comparable to or less expensive than a 28-day maintenance course for these agents, not including the costs associated with drug administration, supportive care, and the treatment of drug-related complications. Considering the relative ease, low cost, and the fact that CTL administration can result in CMV specific immune reconstitution, this option should be considered for patients with CMV reactivation, or for prophylaxis in patients at high risk for infection.
Keywords: cytomegalovirus, pp65, IE-1, immunotherapy
INTRODUCTION
Most CMV infections following allogeneic stem cell transplantation (SCT) will respond to anti-viral medications, but the use of these agents can be associated with myelosuppression, nephrotoxicity, and impaired immune reconstitution.1, 2 This is particularly problematic for recipients of T cell depleted stem cell transplants, who are at higher risk for CMV infection and disease. There have been several previous reports of intervening with CMV specific cytotoxic T lymphocytes (CTL) following SCT, which can result in cellular immune reconstitution and suppression of viremia.3–5 Methods for growing these CTL include the use of genetically modified antigen presenting cells (APC) to achieve CMV pp65 expression, or pulsing APC with CMV peptides or viral lysates.6,7 Each of these strategies have their own intrinsic shortcomings. Genetic manipulation of APC will result in CMV antigens being naturally processed and presented, but is complicated by regulatory issues, high costs, and the time required for qualification of viral supernatants and cell therapy products. Depending upon the vector and APC, gene therapy approaches add variable amounts of time for transduction and qualification of APC, which may be difficult to achieve if CTL are needed on an urgent basis.8 Approaches using individual peptides are limited by our knowledge of HLA restricted CMV pp65 epitopes, and CMV T cells expanded using viral lysate may be predominantly CD4+.4 Other methods may be limited by the cost of isolating CTL and the need for specialized equipment, such as selecting CTL based on cytokine production,9 which could preclude widespread implementation. Thus far, the routine use of CMV CTL for treatment or prophylaxis of CMV infection has not been achieved, and virus specific immunotherapy is only offered at a few centers which have this as a research interest.
There are multiple potential benefits to intervening with virus specific CTL, including earlier immune reconstitution and avoidance of drug-related complications. Considering the costs of antiviral medications, drug administration, as well as monitoring and treating complications, the infusion of CMV CTL may be more cost-effective and directly beneficial to patients. Limitations to immunotherapy strategies targeting a single CMV protein include the fact that in some donors, dominant immune responses may be directed against antigens not used in CTL preparation. CMV pp65 is an important target for CMV specific CTL, with 70 to 90% of all CTL recognizing pp65 epitopes.10 There are other reports that would indicate that IE-1 specific T cells are also important in protective immunity to this virus,11, 12 including patients post-transplant. In addition, IE-1 specific immune responses are stronger and sustained over a longer period of time following vaccination with the Towne strain of CMV,13 impacting situations in which CTL are expanded from vaccinated donors. While the major focus in adoptive immunotherapy for CMV has been on pp65 specific immune reconstitution, we decided to determine whether CTL with broader antigenic specificity could be generated, and whether the costs associated with cell production could justify the routine use of cellular immunotherapy for CMV. In this paper we demonstrate that CMV pp65 and IE-1 specific CTL can be reliably expanded from the majority of normal donors, and that infusing these CTL can be a cost-effective strategy for patients with CMV reactivation.
MATERIALS AND METHODS
Cell culture
Donor blood specimens were collected under a protocol approved by the Penn State Hershey Medical Center Human Subject’s Protection Office. Human leukocyte antigen (HLA) testing was performed serologically for HLA A, B, and DR at the Penn State Hershey Histocompatibility Laboratory. 60–80 mL of peripheral blood was collected from these donors, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation. 50–100×106 PBMC were placed in 15ml of RPMI (Gibco, Chicago, IL) with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) for 1 hour in T150 flasks (Corning, Corning, NY). Non-adherent peripheral blood lymphocytes (PBL) were removed and resuspended at 2×106/mL in RPMI 1640 / 10% FBS, and adherent cells were removed with a cell scraper (Corning). Adherent cells were washed in RPMI 1640 and placed at 10×106 in 0.5 mL RPMI in 50 mL conical centrifuge tubes, and then pulsed with either CMVpp65, IE-1, or both peptides simultaneously. The CMVpp65 and IE-1 peptide mixes (JPT Peptide Technologies, Berlin) consisted of 138 and 120 overlapping 15mers, respectively. Each of these peptides were suspended in 40µL of DMSO (Sigma Aldrich, St. Louis, MO) per the manufacturer’s instructions. 3µL (0.7mg/ml) of the peptide suspension was added to 10–20×106 adherent cells and the capped centrifuge tubes were incubated at room temperature for 2 hours, and then washed/centrifuged in RPMI 1640 three times. PBL were plated with peptide pulsed adherent cells at a responder: stimulator ratio of 10:1 in 24 well plates (Corning), 2 mL per well. CTL resulting from this method were analyzed by chromium release assays and flow cytometry for intracellular cytokine production.
Chromium release assays (CRA)
Targets for CRA included autologous and allogeneic B cell blasts (BB, used as a negative control) and BB pulsed with the pp65 or IE-1 peptide mixes. To determine whether these effector cells recognized naturally processed and presented pp65 and IE-1 epitopes, we also infected BB with vaccinia encoding pp65 and IE-1 (vacc-pp65 from Dr. William Britt, University of Alabama, Birmingham, and vacc-IE-1 from Dr. Don Diamond, City of Hope). B cell blasts were cultured from donor PBMC as previously described.14 Targets were labeled overnight with 51Cr (100 µCi/106 cells; from PerkinElmer Life and Analytical Science, Boston, MA), washed in PBS, and dispensed in triplicate into 96 well V-bottom plates (ICN, Costa Mesa, CA) at 4 × 103 cells/well, as previously described.6 CTL were added at a responder: target ratio (of 10:1), and after pelleting and incubation for 4 hours, the supernatant was counted in a gamma-counter. Spontaneous and total release for each target are used to calculate percent specific release with the following formula: % specific release = (experimental cpm-spontaneous cpm)/ (total cpm-spontaneous cpm).
Intracellular cytokine staining (ICS)
Flow cytometry for IFN-γ production was performed on a FACScan (Becton Dickinson) to detect pp65 and IE-1 -specific T cells. Multiple–color staining of immunophenotypic markers, both surface and intracellular, was performed as previously described.15 Surface markers of CTL were determined by staining with directly conjugated monoclonal antibodies specific for CD3, CD4, CD8, and CD56 (Becton Dickinson). Cultured T cells were incubated with equal numbers of stimulators, including autologous BB pulsed with pp65 and IE-1 overlapping peptides, autologous and allogeneic BB in RPMI 1640 with 10% FCS and in the presence of 10 µg/ml brefeldin A (Sigma) at 37°C for 5 hours. After incubation in FACS permeabilization buffer (Becton Dickinson) for 10 minutes, cells were aliquoted and stained with the following labeled antibodies (Becton Dickinson): CD4-FITC, CD8-peridinin chlorophyll protein and IFN γ-APC.
RESULTS
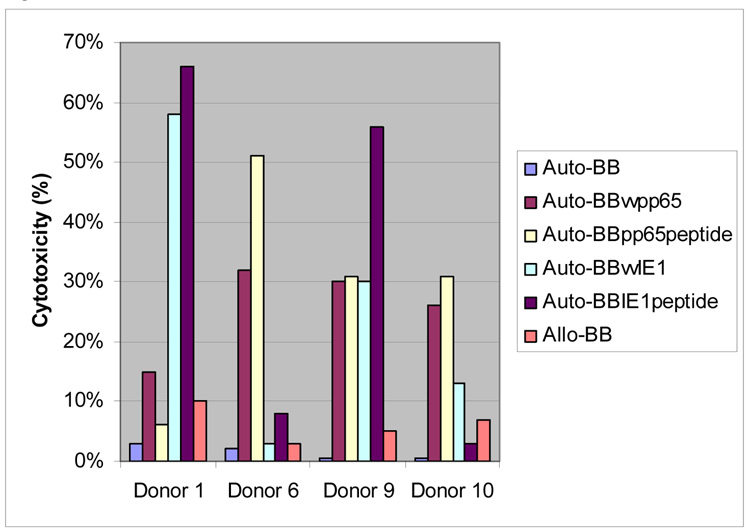
To determine whether CMV and IE-1 specific CTL could be regularly expanded from normal donors, we stimulated peripheral blood lymphocytes (PBL) with a peptide mix derived from IE-1 and pp65 simultaneously. Immunophenotyping shows that the majority of the cells are CD3+, with a mixture of CD4+ and CD8+ effector cells, as presented in Table 1. Cytotoxicity data (Figure 1) reveals a lack of auto- or allo-reactivity from these CTL. Of the ten donors tested, four had cytotoxicity to targets expressing both antigens, four had pp65 specific cytotoxicity only, one had IE-1 cytotoxicity only, and one donor responded to neither antigen. CRA data on CTL cultured separately with either pp65 or IE-1 peptide pulsed monocytes, or with both peptide mixes combined reveals that the simultaneous use of these peptides did not compromise cytotoxicity for the majority of donors (Table 2). To confirm the fact that these CTL recognized naturally processed pp65 and IE-1 epitopes, CRA were also performed using BB infected with vaccinia encoding either pp65 or IE-1, and this data is presented in Figure 2. Cytotoxicity against vaccinia pp65 and IE-1 infected BB was similar to that seen using peptides, demonstrating that these CTL recognize naturally processed and presented epitopes of both antigens.
Table 1.
Phenotype of CTL stimulated with combined pp65 and IE1 (%)
| CD3 | CD4 | CD8 | CD56 | |
|---|---|---|---|---|
| Donor 1 | 89 | 32 | 53 | 0.9 |
| Donor 2 | 95 | 28 | 65 | 1.5 |
| Donor 3 | 90 | 51 | 36 | 1.4 |
| Donor 4 | 90 | 50 | 38 | 0.7 |
| Donor 5 | 86 | 62 | 22 | 1.8 |
| Donor 6 | 88 | 42 | 45 | 0.6 |
| Donor 7 | 89 | 57 | 30 | 1 |
| Donor 8 | 83 | 54 | 29 | 1 |
| Donor 9 | 97 | 38 | 53 | 8.2 |
| Donor 10 | 81 | 32 | 40 | 3 |
Figure 1. Cytotoxicity of CTL.
Peripheral blood monocytes were pulsed with pooled CMV pp65 and IE-1 peptides and incubated with peripheral blood lymphocytes from 10 healthy CMV sero-positive donors. Targets include autologous B cell blasts (BB), BB pulsed with pp65 and IE-1 overlapping peptides, and allogeneic BB.
Table 2.
The cytotoxicity of CTL stimulated with pp65, IE1 alone, and combined pp65 and IE1 (%)
| Mixed CTLpp65&IE1 |
||||
|---|---|---|---|---|
| CTL-pp65 | CTL-IE1 | pp65 | IE1 | |
| Donor 1 | 8 | 60 | 6 | 66 |
| Donor 2 | 69 | 50 | 54 | 41 |
| Donor 3 | 37 | 4 | 24 | 12 |
| Donor 4 | 47 | 66 | 63 | 59 |
| Donor 5 | 50 | 37 | 62 | 30 |
| Donor 6 | 45 | 8 | 51 | 8 |
| Donor 7 | 46 | 5 | 41 | 6 |
| Donor 8 | −1 | −2 | −3 | −2 |
| Donor 9 | 53 | 69 | 31 | 56 |
| Donor 10 | 36 | −1 | 31 | 3 |
Figure 2. Cytotoxicity of CMV CTL.
Peripheral blood lymphocytes were stimulated with autologous monocytes pulsed with pp65 and IE-1 overlapping peptides. Specific cytotoxicity was measured using B cell blasts (BB) infected with either vaccinia encoding pp65 or IE-1 (vvp65, vvIE1), as well as BB pulsed with either peptide mix. Autologous and allogeneic BB were used as negative controls.
Figure 3 presents cytokine production by three of these donors, two having cytotoxicity and cytokine production to only one of the antigens, and one donor with IFN-γ production and cytotoxicity to both antigens. In general, the presence of cytotoxicity for either pp65 or IE-1 correlated with CD4+ and CD8+ T cells producing IFN-γ in response to these antigens as presented in Table 3. These donors had diverse HLA backgrounds and varying levels of response to these antigens. Donor 1 had IE-1 specific cytotoxicity, and CD4+ and CD8+ cells producing IFN-γ in response to this antigen. This donor lacked pp65 specific cytotoxicity as well as CD8+, IFN-γ specific T cells, but 0.6% of the cells were IFN-γ producing, CD4+, pp65 specific T cells. Donor 3 had IFN-γ producing CD8+ T cells specific for IE-1, but lacked significant cytotoxicity to this antigen. Donor 8, who was CMV sero-positive but lacked cytotoxicity to either antigen, did not have significant levels of IFN-γ producing T cells to pp65 or IE-1. Therefore, while there seems to be an expected close correlation between IFN-γ production and cytotoxicity, some donors without cytotoxicity may have IFN-γ production in response to a specific antigen.
Figure 3. IFN-γ production of T cells.
Peripheral blood monocytes were pulsed with pooled CMV pp65 peptides and IE1 peptides and incubated with nonadherent peripheral blood lymphocytes. IFN-γ producing T cells specific for these CMV peptides were analyzed at day 10 of culture by intracellular staining. A). CD8+ T cell response to CMV pooled peptides. B). CD4+ T cell response to the CMV peptides.
Table 3.
IFN-γ production by CTL stimulated with combined pp65 and IE1
| Response to pp65(%) | Response to IE1(%) | HLA Typing | |||||
|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | HLA- A | HLA- B | HLA-DR | |
| Donor 1 | 0.59 | 0.09 | 0.38 | 23 | 11 | 38, 54 | 04, 08 |
| Donor 2 | 0.98 | 19.08 | 0.39 | 3.96 | 02, 03 | 07, 55 | 04, 14 |
| Donor 3 | 0.81 | 2.42 | 0.12 | 0.44 | 11, 24 | 07, 51 | 01, 03 |
| Donor 4 | 2.2 | 5.3 | 0.36 | 6 | 02 | 27, 41 | 01, 08 |
| Donor 5 | 10.86 | 7.3 | 0.8 | 0.78 | 02, 26 | 41, 44 | 08, 11 |
| Donor 6 | 0.48 | 5.31 | 0.26 | 0.91 | 02, 26 | 46, 48 | 08 |
| Donor 7 | 10.4 | 5.25 | 0.26 | 0.02 | 01, 02 | 13, 60 | 07, 11 |
| Donor 8 | 0.17 | 0.04 | 0.15 | 0.05 | 02, 25 | 18, 60 | 01, 04 |
| Donor 9 | 16.48 | 1.82 | 1.53 | 21.61 | 11, 24 | 54, 60 | 14, 15 |
| Donor 10 | 0.71 | 2.81 | 0.13 | 0.19 | 11, 31 | 35, 60 | 04, 09 |
All CTL were cultured for 10 days, with a mean 1.8 ± 0.7-fold increase (range 1.1 to 2.5) in the number of cells at the end of the culture period. Therefore, for a typical 70 kg adult patient, a 100 mL blood draw from the stem cell donor would permit a cell dose of 5 × 105 CD3+ CTL/kg. Peggs et al5 demonstrated that CTL infusions with doses as low as 1 × 105 cells/kg could result in CMV specific immune reconstitution, although two of fourteen CTL recipients required a second infusion for subsequent CMV reactivation.
Therefore, a cell dose of 2–5 × 105 CD3+ CTL/kg should be adequate for most patients. We compared the costs of preparing and shipping a single dose of 5 × 105 CD3+CTL/kg (for a 70 kg patient) with the costs of maintenance therapy with antiviral agents (cidofovir, ganciclovir, valganciclovir, and foscarnet), and this information is presented in Table 4. This cost analysis did not take into consideration the fees associated with laboratory monitoring, drug administration, or the cost of medications or fluids to treat or prevent complications. From this information it appears that the costs of preparing, qualifying, and shipping CMV CTL using the method outlined would be comparable to or less expensive than average wholesale prices for maintenance courses of these antiviral agents.
Table 4.
| Regimen, for 70 kg patient, 28 days | Average Wholesale Price (USD)* |
|---|---|
| Ganciclovir 5 mg/kg IV daily | 1,048.60 |
| Foscarnet 90 mg/kg IV daily | 1,234.80 |
| Valganciclovir 15 mg/kg oral daily | 1,915.20 |
| Cidofovir 5 mg/kg/week every 2 weeks | 1,657.60 |
| Single infusion of CMV CTL | 1,350.00 |
These figures include quality assurance testing, cryopreservation, and shipping of CTL, but do not account for IV administration costs, compounding costs of oral medications, charges for laboratory monitoring, or medications to treat or prevent drug-related complications. Drug costs from Cardinal Health Corporation, April 2008 (www.cardinal.com).
DISCUSSION
The decision to administer CMV CTL must be based on an understanding of the risks associated with CMV infection, the time frame, feasibility, and cost of CTL culture, as well as the risks associated with giving CTL. The methods used to generate CMV specific CTL in this report are simple to perform, and bypass requirements for gene therapy or the use of other techniques which may only be available at a few transplant centers. This method is also less expensive than other methods of CTL selection, such as cytokine capture. Due to the use of specialized reagents and labware, cytokine capture (including quality assurance testing of the final product) is over 3 times the cost of the method presented here, not including the expense of a cell selection device. Considering the relatively low cost, feasibility, and low risk for GVHD, cellular immunotherapy for CMV could be considered a reasonable option either at the time of reactivation or given as prophylaxis in high risk patients. As can be seen from the cost analysis, there is not a large difference in the cost of CTL preparation compared to standard courses of anti-virals, and the latter may need to be used over a prolonged period of time if viremia persists or recurs. In addition to costs, anti-viral drugs can result in multiple side effects, the most common being nephrotoxicity and myelosuppression. These toxicities are compounded by the fact that many SCT recipients may have cytopenias and most are receiving other nephrotoxic agents. The frequency of neutropenia ranges from 40–60% in SCT patients receiving ganciclovir,16, 17 and the use of this agent has been associated with an increase in bacterial sepsis and invasive fungal infections.16, 18, 19 In addition, the use of ganciclovir negatively impacts CMV specific cellular immune responses as well as lymphocyte function in general.2 The use of second line agents such as foscarnet and cidofovir can be limited by the potential for severe nephrotoxicity, both requiring intravenous fluid administration and careful monitoring of hydration and electrolyte status.20 Intervention with CMV specific CTL not only results in virus specific immune reconstitution and prevention of further viremia, but could also save on overall patient care costs.
The primary motivation for expanding IE-1 and pp65 specific CD4+ and CD8+ T cells simultaneously is to provide CTL with reactivity against viral epitopes that are relevant for a broad group of patients. From our work and other studies,21 there is a wide diversity in CD4 and CD8 T cell responses to different CMV antigens in normal donors. Variability in the relative numbers of CD4 and CD8 effector cells and IFN-γ production among normal donors could reflect individual CMV specific cellular immunity as well as differences in HLA background and epitope dominance. These variables would likely result in differences in the degree of T cell responsiveness to epitopes presented in the peptide mix, some of which might not be optimally presented. Previous studies have demonstrated the importance of CMV specific CD4+ T cells for achieving long-term immune reconstitution to this virus.22 Our results indicate that both CD4+ and CD8+, pp65 and IE-1 specific effector cells are present following stimulation with these peptide mixes, and the ratios of CD4 and CD8 T cells are well-balanced. The patterns of antigen specific cytotoxicity are similar regardless of whether pp65 and IE-1 CTL are stimulated separately or simultaneously. While some donors respond to a given antigen but not to others, some individuals will have IFN-γ responses to an antigen but lack activity in CRA. It is possible that with further courses of stimulation these donors would develop cytotoxicity. Further study is needed to determine the role of cytokine producing effector cells for donors who lack cytotoxicity to a specific antigen.
CMV pp65 has been shown to be the immunodominant CMV antigen, but IE-1 specific immunity is also of importance in protection against CMV.11 Bunde et al23 demonstrated in organ transplant recipients that having high frequencies of IE-1 specific T cells in the early post-transplant course was protective against developing CMV disease. Khan et al11 reported that IE-1 specific T cells increased over time post-infection and may be effective for preventing low-level viral reactivation after an acute infection. Other studies have shown that a higher proportion of pp65 specific than IE-1 specific T cells made IFN-γ and TNF-α, and had greater cytotoxicity in stem cell transplant and organ transplant recipients.24 Studies are currently underway at our center and others examining the use of CMV vaccines for sero-negative stem cell donors. One such vaccine consists of the Towne strain of CMV, which has been shown to result in higher levels of IFN-γ producing, CD8+ T cells to IE-1 compared to pp65.13 While pp65 responses decrease over time in these vaccine recipients, IE-1 responses tend to be maintained. Therefore CTL with reactivity to more than one antigen is of potential benefit. While priming T cells with CMV lysate could result in cells with a broader range of specificity, this method of stimulation has been shown to favor CD4+ T cell expansion.4
An overriding concern with any use of cellular immunotherapy after allogeneic SCT is the efficacy of the product and the risks for GVHD, although this complication is generally not seen with adoptive immunotherapy using virus-specific CTL.4, 7 The cellular products that have been described in this report lack auto- or allo-reactivity in CRA, and two patients who have received pp65 specific CTL using this method have not developed GVHD (in press). With selective expansion of the CTL we would expect a decrease in potentially allo-reactive CTL and enrichment in CMV CTL over the 10 days in culture, and the administration of relatively low cell doses as in our current protocol (2–5×105 CD3+CTL/kg) would likely decrease the risk of GVHD. Situations in which there is no urgency for culturing CTL, as is the case when cells are cultured prospectively for prophylaxis, would allow for longer culture times, reducing the risk of GVHD. No one has clearly defined the minimal cell dose needed for immune reconstitution using CMV specific CTL, but this would depend upon the level of enrichment of virus specific cells. MacKinnon and group25 reported the use 1 × 105 CD3+ CMV specific T cells/kg, with no GVHD and the majority of recipients having no subsequent CMV reactivation. This group also described the selection of CTL based on IFN–γ capture,9 with a mean CMV reactive T cell dose of 3.4 × 106 total cells per patient. The administration of lower doses of CMV specific T cells in the future would reduce GVHD risk and permit cryopreserving several vials for subsequent infusions, if needed.
The use of both CMV IE-1 and pp65 peptides covers immunologically relevant CMV antigens for most individuals, although some donors may not respond to either antigen after 10 days of culture, and may need subsequent stimulations with these peptides. For subjects at high risk for CMV reactivation, prospectively culturing these CTL from sero-positive donors could be considered, with CTL infused prophylactically or at reactivation. With several studies demonstrating the efficacy of adoptive immunotherapy with CMV CTL, and with a practical method whereby these cells can be expanded, this form of therapy could be considered for more patients, particularly in light of the costs and deleterious side effects of most anti-viral agents. It is also possible that CTL with specificity to other viral, fungal, or tumor antigens could be cultured concurrently using this method, as has been described using gene therapy approaches.26 We will be implementing this new strategy to stimulate donor derived IE-1 and pp65 CTL for SCT patients with CMV reactivation, including those with donors who have received a CMV vaccine.
Acknowledgments
This work was supported by grants NIH R01CA106319-2 and the Four Diamonds Foundation for Cancer Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997 Sep 15;90(6):2502–2508. [PubMed] [Google Scholar]
- 2.Battiwalla M, Wu Y, Bajwa RP, et al. Ganciclovir inhibits lymphocyte proliferation by impairing DNA synthesis. Biol Blood Marrow Transplant. 2007 Jul;13(7):765–770. doi: 10.1016/j.bbmt.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 4.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002 Jun 1;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003 Oct 25;362(9393):1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Burton RL, Dai LJ, Britt WJ, Lucas KG. B lymphoblastoid cell lines as efficient APC to elicit CD8+ T cell responses against a cytomegalovirus antigen. J Immunol. 2000 Oct 1;165(7):4105–4111. doi: 10.4049/jimmunol.165.7.4105. [DOI] [PubMed] [Google Scholar]
- 7.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 8.Lucas KG, Sun Q, Burton RL, et al. A phase I–II trial to examine the toxicity of CMV- and EBV-specific cytotoxic T lymphocytes when used for prophylaxis against EBV and CMV disease in recipients of CD34-selected/T cell-depleted stem cell transplants. Hum Gene Ther. 2000 Jul 1;11(10):1453–1463. doi: 10.1089/10430340050057521. [DOI] [PubMed] [Google Scholar]
- 9.Thomson K, Julie M, Pang K, et al. Direct isolation of donor-derived antigen-specific T cells and their adoptive transfer for treatment or prophylaxis of CMV infection following allogeneic transplantation. Blood. 2006;108:177a. [Google Scholar]
- 10.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996 Nov;70(11):7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan N, Best D, Bruton R, Nayak L, Rickinson AB, Moss PA. T cell recognition patterns of immunodominant cytomegalovirus antigens in primary and persistent infection. J Immunol. 2007 Apr 1;178(7):4455–4465. doi: 10.4049/jimmunol.178.7.4455. [DOI] [PubMed] [Google Scholar]
- 12.Gibson L, Piccinini G, Lilleri D, et al. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J Immunol. 2004 Feb 15;172(4):2256–2264. doi: 10.4049/jimmunol.172.4.2256. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson MA, Sinclair E, Bredt B, et al. Antigen-specific T cell responses induced by Towne cytomegalovirus (CMV) vaccine in CMV-seronegative vaccine recipients. J Clin Virol. 2006 Mar;35(3):332–337. doi: 10.1016/j.jcv.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997 Dec 1;100(11):2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao L, Sun Q, Lucas KG. Rapid generation of CMV pp65-specific T cells for immunotherapy. J Immunother. 2007 Jul–Aug;30(5):557–561. doi: 10.1097/CJI.0b013e31803b945b. [DOI] [PubMed] [Google Scholar]
- 16.Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993 Feb 1;118(3):173–178. doi: 10.7326/0003-4819-118-3-199302010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Riddell SR, Greenberg PD. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 18.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996 Nov 15;88(10):4063–4071. [PubMed] [Google Scholar]
- 19.Winston DJ, Ho WG, Bartoni K, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993 Feb 1;118(3):179–184. doi: 10.7326/0003-4819-118-3-199302010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006 Sep;71(2–3):154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005 Sep 5;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995 Oct 19;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 23.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005 Apr 4;201(7):1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey SF, La Rosa C, Zhou W, et al. Functional comparison of T cells recognizing cytomegalovirus pp65 and intermediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006 Nov 15;194(10):1410–1421. doi: 10.1086/508495. [DOI] [PubMed] [Google Scholar]
- 25.Peggs KS, Mackinnon S. Augmentation of virus-specific immunity after hematopoietic stem cell transplantation by adoptive T-cell therapy. Hum Immunol. 2004 May;65(5):550–557. doi: 10.1016/j.humimm.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Lucas KG, Filo F, Heilman DK, Lee CH, Emanuel DJ. Semiquantitative Epstein-Barr virus polymerase chain reaction analysis of peripheral blood from organ transplant patients and risk for the development of lymphoproliferative disease. Blood. 1998 Nov 15;92(10):3977–3978. [PubMed] [Google Scholar]