Abstract
The authors examined the amount and durability of change in the cognitive content of 156 adult outpatients with recurrent major depressive disorder after treatment with cognitive therapy. The pre–post magnitude of change was large for the Attributional Style Questionnaire Failure composite (d = 0.79), Dysfunctional Attitudes Scale (d = 1.05), and Self-Efficacy Scale (d = 0.83), and small for the Attributional Style Questionnaire Success composite (d = 0.30). Changes in cognitive content were clinically significant, as defined by their 64%–87% scores overlapping with score distributions from community dwellers. Improvement was durable over a 2-year follow-up. Changes in negative cognitive content could be detected early and distinguished responders from nonresponders. In responders, continuation-phase cognitive therapy was associated with further improvements on only 1 measure of cognitive content. Early changes in negative cognitive content did not predict later changes in depressive symptoms, which the authors discuss in the context of methodological challenges and the cognitive theory of depression.
Keywords: depression, cognition, acute phase cognitive therapy, continuation phase cognitive therapy
The concept of cognition has played a prominent and influential role in the history of philosophy, religion, and psychology, including psychotherapy (e.g., Beutler & Guest, 1989), despite inconsistent and vague definitions that range from the organization of sensory input through the activation of behavior as a result of such input (Beutler & Guest, 1989; Kuiper & MacDonald, 1983). In the psychotherapy literature, the definitions and characterizations of cognition are important because cognition has played a central role in understanding and changing affect and disorders. Beck’s early descriptions of cognition included verbal or pictorial events in an individual’s stream of consciousness, which are the product of attitudes or assumptions developed by previous experiences, especially those occurring during sensitive learning periods (Beck, Rush, Shaw, & Emery, 1979). Later, Clark, Beck, and Alford (1999) elaborated on how the meaning that depressed people assign to experience influences affect and the course of depression. They offered heuristics such as “orienting schemas” (e.g., sensitivity to negative stimuli; personalizing), “cognitive structures” (e.g., negative views of self, loss, and depressive personality), and “cognitive products” (i.e., negative automatic thoughts, cognitive errors, negative appraisals, negative constructions and perspective) and described these heuristics’ roles in the cognitive model of depression (Clark et al., 1999, p. 78). This etiologic, multifactorial model recognizes interactions between genetic, biological, social, and psychological factors and advances the so-called “primacy hypothesis.” Specifically, Clark et al. (1999, p. 401) maintained that although negative views of the self, world, and future do not cause depression, they “are such a core feature of the depressive experience that they have a significant influence on the manifestations of other depressive symptoms.” A cognitive theory of depression advances disordered thought processes as a “final common pathway” to understanding depression (Clark et al., 1999, p. 34), which is described as more central than motivation or affect.
In cognitive therapy (CT), cognition includes both self-talk (i.e., thoughts and attitudes) and images (Beidel & Turner, 1986; Beutler & Guest, 1989). For the most part in the CT literature, assessing cognition means tracking the content of dysfunctional attitudes and attributional style. This is how we use the term in the current article in evaluating change in self-report measures of dysfunctional attitudes, attributional style, and self-efficacy. The research we describe here is based on what Clark et al. (1999) called “cognitive products.”
Historically, exploring the robust association between the emotional disorders and negative cognition propelled the development of both cognitive theories of depression (Abramson, Metalsky, & Alloy, 1989; Beck, 1976; Seligman, Abramson, Semmel, & von Bayer, 1979) and cognitive– behavioral therapy (CBT; Beck et al., 1979). Cognitive theory posits that changing dysfunctional cognitions or cognitive processes and their associated behaviors results in improvement in depression and other emotional disorders and even may reduce vulnerability for relapse and recurrence. Thus, cognitive therapists seek to reduce symptoms of psychiatric disorders by altering cognition and related behavior (Hollon & Beck, 1986). Moreover, clinicians use changes in cognitive content to monitor treatment progress (e.g., Dozois, Covin, & Brinker, 2003; Dozois & Dobson, 2002) and to decide how and when to change the course or focus of therapy. Both practitioners and theoreticians predict that vulnerability to future depression results in part from activating dysfunctional cognitions during distress. Thus, both theoretically and practically, modifying negative automatic thoughts and dysfunctional attitudes is seen as key to CT’s efficacy, over the short run as well as longitudinally.
A number of studies using a variety of measures have documented that substantial changes in cognitive content occur during acute-phase CT (A-CT; e.g., Barber & DeRubeis, 2001; DeRubeis et al., 1990; Free, Oei, & Appleton, 1998; Jacobson et al., 1996; Jamison & Scogin, 1995; Kolko, Brent, Baugher, Bridge, & Birmaher, 2000; Kwon & Oei, 2003; Rush, Kovacs, Beck, Weissenburger, & Hollon, 1981; Scogin, Hamblin, & Beutler, 1987; Simons, Garfield, & Murphy, 1984; Tang & DeRubeis, 1999; Tang, DeRubeis, Beberman, & Pham, 2005; Whisman, Miller, Norman, & Keitner, 1991). Whereas cognitive change has been shown to be greater in patients receiving CT (which reduces depressive symptoms) than in wait-list control conditions (which does not significantly reduce depressive symptoms; e.g., Jamison & Scogin, 1995; Scogin et al., 1987), studies designed to determine whether cognitive change is specific to CT compared with other treatments have produced mostly null results, raising the question of whether the degree of cognitive change may be related simply to the extent to which the treatment effectively reduces depressive symptoms.
For example, two studies yielded clearly mixed results. Whisman et al. (1991) reported less dysfunctional scores on measures of cognitive content when CT was added to pharmacotherapy plus milieu therapy for some, but not all, measures of cognitive content. However, they did not control for pretreatment differences between the groups, which were notable, but not statistically significant, with their small samples. Kolko et al. (2000) also found greater change in cognitive distortions, but not hopelessness, with CT compared with systematic behavioral family therapy and non-directive supportive therapy.
The most common finding, however, is that there is little to no difference in the degree of change in cognitive content when CT is compared with pharmacotherapy, either with or without additional noncognitive components, such as relaxation (Bowers, 1990; Imber et al., 1990; Rush et al., 1981; Simons et al., 1984). Similarly, in a component analysis of CBT, Jacobson et al. (1996) found that full combination CBT was no more effective for changing cognitions or reducing depression than those with only one or two components— behavioral activation (BA) alone or BA combined with activation and modification of dysfunctional thoughts (AT).
It is interesting to note that relatively few studies have evaluated cognitive change during CT beyond showing (as documented earlier) that dysfunctional cognitions reliably are reduced in A-CT and that changes in symptom levels and cognitive content are correlated. Few studies have included repeated assessments over a year or more. That is, relations between these changes have not been examined adequately. This is particularly surprising given the central nature of the relation between cognition and affect in both cognitive theory and practice.
Along these lines, Riskind (1995) reviewed the status of cognitive change in CT and other therapies and argued that the mechanism for change across different psychological treatments may be more similar than some think. Specifically, Riskind (1995, p. 195) argued that the primary difference between cognitive change in CT and other therapies was “meta-cognitive” (i.e., thinking about thinking), in that CT “focuses on bringing negative cognitions to scrutiny” whereas cognition plays a more peripheral role in other therapies. Of importance, he suggested that to delineate the mechanisms and degrees of change across modalities more clearly, future research would benefit from the inclusion of basic measurements of change in cognitive content over the course of treatment. That is, before studying mechanisms of change, we first need to measure adequately the degree to which changes in cognitive content and depressive symptoms are related during efficacious antidepressant treatments as well as the relative timing of these changes, regardless of the therapies’ labels or presumed targets of focus.
Therefore, researchers need to examine whether change in dysfunctional cognition underlies improvement in depression—arguably the central tenet of cognitive theory and the basis of CT. Further research is needed (a) to replicate findings and extend understanding of how much cognition changes during A-CT (it is important to quantify the degree of change, to examine the valence of cognitive change [e.g., decreases in negative cognitions and/or increases in positive cognitions], to determine its statistical and clinical significance, and to examine the specificity of cognitive change in CT relative to other treatments), (b) to clarify relations between cognitive change and depressive symptoms during treatment (it is important to quantify the degree of relation, to examine the valence of cognitive change, and to determine whether cognitive change accounts for change in depressive symptoms, both contemporaneously and longitudinally, as predicated by cognitive theory), and (c) to examine relations between cognitive change and depressive symptoms after response to A-CT (it is important to examine again each of the issues examined over A-CT [i.e., whether there is statistically and clinically significant change in negative and/or positive cognitions, and, if so, whether and the degree to which this cognitive change is specific to CT and is associated with and accounts for change in depressive symptoms contemporaneously and longitudinally]).
We have already reviewed the literature concerning findings that cognitive content changes during CT. What evidence has accrued to date examining each of the other areas in question? We found few studies that directly addressed relations between changes in cognition and changes in depression during CT. Most studies that have reported on change in both cognitive content and depressive symptoms have shown simply that cognitive change occurs simultaneously with reductions in depressive symptoms (e.g., Barber & DeRubeis, 2001; Free et al., 1998; Kwon & Oei, 2003). Moreover, two investigations in the context of “sudden gains” (Tang & DeRubeis, 1999; Tang et al., 2005) reported that improvements in cognitive content preceded change in depressive symptoms. In contrast, Furlong and Oei (2002) found that change in depressive symptoms preceded cognitive change. Thus, this issue remains unresolved.
Turning to the relations between changes in cognition and changes in depression after CT, the existing treatment literature addressing longitudinal relations of changes in cognitive content on future depressive symptoms and episodes (i.e., the cognitive diathesis–stress treatment literature) is developing but is inconsistent. For example, Beevers, Keitner, Ryan, and Miller (2003) reported greater cognitive change during 6 months of pharmacotherapy with or without one of three psychosocial treatments—family, cognitive–behavioral, or their combination treatment. They found that greater cognitive change was correlated with a reduction in risk of recurrent depressive episodes over the following year. In contrast, Gortner, Gollan, Dobson, and Jacobson (1998) evaluated the efficacy of full combination CT compared with its components (BA and AT) for relapse prevention in a 2-year follow-up analysis of Jacobson et al.’s (1996) data. Concordant with Jacobson et al.’s findings, Gortner et al. (1998) found that adding cognitive components to BA techniques did not reduce relapse rates.
However, although the latter data indicate that a treatment focus on cognitive change is not necessary for effective therapy, they are silent on the actual functional relationship between cognitions and depressive symptoms. That is, BA-focused treatments may work through exposing patients to naturally reinforcing contingencies, which may produce cognitive change just as effectively as treatments, such as CT, that attempt to alter cognition explicitly or include the word “cognitive” in their label. Conversely, so-called cognitive strategies are associated with, and may effect, simultaneous changes in behavior. In other words, the specific effect of treatments must be demonstrated, as they may or may not correspond to the labels their developers have given them.
The current article reexamines the hypothesis that important and substantial improvements in cognitive content occur during CT and, more importantly, examines whether these improvements in cognitive content precede or occur simultaneously with reductions in depressive symptoms and whether they endure—or even increase—after CT ends. Measurement is repeated over more than 2 years. We hypothesized that (a) levels of self-reported negative cognition would decrease and positive cognition would increase over a 20-session course of A-CT; (b) these changes would be clinically significant, meaning that cognitive content would “normalize” after A-CT relative to the level of community samples (i.e., “normalizing” patients would report cognitive content more characteristic of community dwellers who do not present for treatment than of distressed or disordered individuals); (c) greater decreases in negative cognition would be observed in those patients who received both the acute and continuation phases of CT (i.e., 30 sessions, with the final 10 sessions focused on reducing residual symptoms and depressive relapse) relative to those receiving only A-CT; and (d) cognitive content would change before depressive symptoms, supporting a possible causal role for cognitions in depressive symptom change.
Method
Participants
The patients analyzed in this sample consented to enter the acute, experimental, and follow-up phases (described below) of the Institutional-Review-Board-approved randomized clinical trial conducted and described by Jarrett et al. (2001). Here, we abstract the method. A companion article (Vittengl, Clark, & Jarrett, 2004a) described the social and interpersonal functioning of the sample.
Patients were recruited through multiple avenues, including the media, printed announcements, and self- and practitioner referrals. Potential patients completed telephone screening and diagnostic interviews and provided informed consent prior to entering the protocol. Adult outpatients meeting the following inclusion criteria were eligible for the study: (a) presented with Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) nonpsychotic, recurrent, major depressive disorder with clear interepisode recovery (greater than or equal to 2 months of at least nearly normal functioning) and (b) had a score greater than or equal to 16 on the 17-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960). Patients were excluded when they presented with (a) concurrent medical disorders associated with depressive symptoms, organic mental disorders, psychotic disorders, active substance abuse or dependence, primary obsessive–compulsive or eating disorders, or borderline personality disorder; or (b) inability or unwillingness to complete questionnaires or to comply with the treatment protocol.
Approximately 3,500 potential participants were screened by research personnel via telephone, and 608 patients were evaluated in the diagnostic clinic. This process yielded 156 patients meeting the inclusion criteria and consenting to A-CT. From this sample, 155 participants (74.2% female) attended the first A-CT session. The mean age of participants was 41.3 years (SD = 11.0), the mean level of education was 15.4 years (SD = 2.8), and 7.1% were African American, 4.5% were Hispanic, 1.3% were Native American, and 87.1% were Caucasian. Patients’ mean age of onset of major depressive disorder was 19.9 years (SD = 9.6), and they had experienced a mean of 3.4 major depressive episodes (SD = 1.3). Treatment exposure data from the first, most recent two, and current major depressive episodes showed that 56.8% had been treated previously with pharmacotherapy, 59.4% with psychotherapy, 1.9% with electroconvulsive therapy, and 41.3% with at least two of these types of therapy. The number of current DSM–IV Axis I disorders in addition to the diagnosis of recurrent major depressive disorder ranged from 0–4 (M = 0.59, SD = 0.78) and included social phobia (20.0%), specific phobias (12.3%), panic disorder without agoraphobia (8.4%), posttraumatic stress disorder (7.7%), dysthymic disorder (5.2%), obsessive–compulsive disorder (1.3%), panic disorder with agoraphobia (1.3%), and 0.6% each of agoraphobia without a history of panic disorder, attention-deficit/hyperactivity disorder, bulimia nervosa, and hypochondriasis.
Study Phases
No protocol medications were prescribed during any phase of the study. Patients’ self-report of mood altering medications prior to relapse or recurrence was minimal, as reported in Jarrett et al. (2001).
A-CT
Five experienced therapists conducted A-CT (Beck et al., l979) within a 12–14 week protocol, including 20 individual sessions (50–60 min) held twice weekly for the first 8 weeks and once weekly for the last 4 weeks. No pharmacotherapy was provided. The objectives of A-CT are to teach patients to reduce depressive symptoms by (a) examining thoughts associated with negative affect, (b) evaluating the thoughts’ validity through logical and empirical methods, (c) generating alternatives to negative thoughts in the absence of supporting evidence, and (d) engaging in problem-solving when negative conclusions match the data. Therapists demonstrated competence on the Cognitive Therapy Scale (Young & Beck, 1980) completed by an off-site consultant (see Jarrett et al., 2001).
Using the intention-to-treat sample (N = 156), the response rate to A-CT (which was defined as the absence of DSM–IV major depressive disorder and an HRSD score of 9 or less when exiting A-CT) was 62.2% (n = 97). The HRSD scores were rated by the therapist when patients dropped out (n = 10) and by an independent clinician when the patient completed the post-A-CT assessment (n = 87). This response rate is consistent with the literature documenting the efficacy of A-CT in reducing the symptoms of major depressive disorder in adult outpatients (e.g., Hollon et al., 1992,2005; Jarrett et al., 1999; Rush, Beck, Kovacs, & Hollon, 1977).
Randomization to the experimental phase
A-CT responders who completed the post-A-CT assessment and consented (n = 84) were randomized to either continuation-phase CT (C-CT; Jarrett, 1989; Jarrett & Kraft, 1997; n = 41) or an assessment-only control condition (n = 43). The C-CT protocol consisted of ten 60–90 min sessions of C-CT over 8 months (the first 4 sessions occurred twice monthly, and the remaining 6 sessions occurred once monthly) from the original therapist.
C-CT
C-CT is designed to prevent relapse and recurrence of depression by reducing residual symptoms, maintaining and generalizing skills learned in A-CT, and preparing for current or anticipated vulnerabilities. In C-CT, patients are taught to use emotional distress and symptoms to trigger the use of the skills learned in A-CT. Previous reports show that C-CT reduces relapse–recurrence over 8 months more than control procedures (Jarrett et al., 1998, 2001). From two nonrandomized cohorts, Jarrett et al. (1998) reported relapse–recurrence rates over 8 months of 45% (control) versus 20% (C-CT). Survival analyses of the current sample showed rates of 31% (control) versus 10% (C-CT; Jarrett et al., 2001).
Assessment-only control
The patients in the assessment-only control condition attended the 10 evaluation visits according to the same schedule as C-CT. Evaluators were prohibited from using psychosocial interventions with control patients.
Follow-up phase
All 84 patients entering the experimental phase were eligible for the follow-up phase, and 74 of the 84 entered. Follow-up consisted of 10 sessions scheduled monthly at Months 9–12 post-A-CT and bimonthly at Months 14–24 post-A-CT. The assessment-only period lasted 16 months beyond the experimental phase (24 months post-A-CT).
Measurement Occasions
Patients presented at the Psychosocial Research and Depression Clinic, Department of Psychiatry, at the University of Texas Southwestern Medical Center at Dallas, where trained evaluators completed rating scales (see below) and the Structured Clinical Interview for DSM–III–R (SCID, Outpatient Version; Spitzer, Williams, Gibbon, & First, 1989), with supplemental interview questions to assess comorbid DSM–IV disorders and subtypes. The measures reported here were completed at the following times: before A-CT Session 1 (or at pretreatment), at A-CT Sessions 9 and 17, post-A-CT/preexperimental phase (C-CT or assessment-only control), before experimental Session 6, postexperimental phase, and 12 and 24 months post-A-CT (4 and 16 months post-experimental phase; see Vittengl, Clark, & Jarrett, 2004b, Figure 2, for a summary of the study design). Patients who relapsed (according to the Longitudinal Interval Follow-up Evaluation or LIFE; Keller et al., 1987) during either the experimental or follow-up phase were asked to complete all sessions and assessments; they were referred for extra-protocol treatment if they were not actively participating in C-CT. To increase the generalizability of the findings, the current analyses were conducted with all available data, including those collected after some patients’ relapse or recurrence.
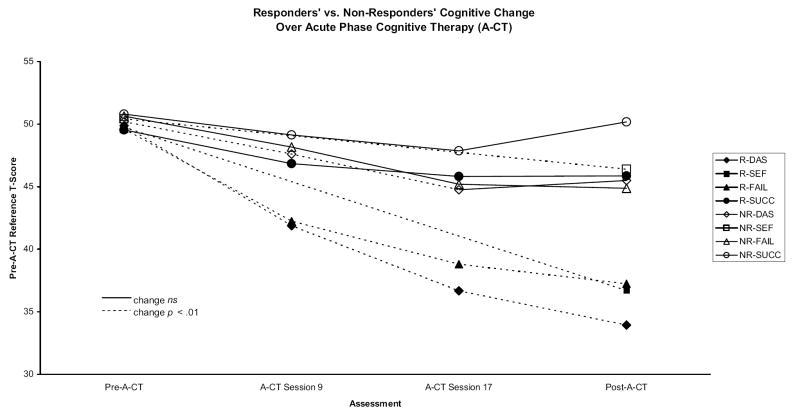
Figure 2.
Acute-phase cognitive therapy (A-CT) responders’ (R) and nonresponders’ (NR) standardized cognitive content and depressive symptoms scores. DAS = Dysfunctional Attitudes Scale; SEF = Self-Efficacy Scale; FAIL = Attributional Style Questionnaire Failure composite; SUCC = Attributional Style Questionnaire Success composite.
Measures
Evaluators
The first pretreatment assessments were conducted by a masters-level psychological associate, a psychiatric nurse, a doctoral candidate in clinical psychology, and a doctorallevel clinical psychologist. The second pretreatment assessments were conducted entirely by faculty-level diagnosticians, all holding doctorates in clinical psychology or medical degrees with training in psychiatry, to verify patients’ study eligibility or exclusion. All interviewers had received didactic training in use of the SCID. Ratings from the LIFE and of depressive symptom severity were calibrated among evaluators throughout the course of the data collections.
Depressive symptoms
Depressive symptoms were operationalized as the composite of two clinician-rated measures, the 17-item HRSD (Hamilton, 1960; Schwab, Bialow, Clemmons, & Holzer, 1967) and the Inventory for Depressive Symptomatology, Clinician Version (IDSC; Rush et al., 1986; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996), and two self-report measures, the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Inventory for Depressive Symptomatology, Self-Report Version (IDSR; Rush et al., 1986, 1996). These four measures demonstrated high convergence within and across time in the current sample, indicating that they measure the same symptom severity and change constructs (Vittengl, Clark, Kraft, & Jarrett, 2005). Consequently, the composite is more reliable and is likely to be more valid than the individual measures; moreover, using the composite reduced the number of statistical analyses necessary to test our hypotheses. Alpha internal consistency for the depressive symptom composite was high at all assessments (Mdn = .95, range = .89–.97; Vittengl et al., 2004a).
Attributional Style Questionnaire (ASQ)
The ASQ (Peterson et al., 1982) presents 12 hypothetical situations (6 negative, 6 positive). Participants generate a cause for each and rate the extent to which the cause reflects internal, global, and stable factors. The internal, global, and stable ratings for each negative situation (18 total items) and positive situation (18 total items) are averaged, respectively, to form a composite index of failure (FAIL) and success (SUCC) attributions relevant to depression (Peterson & Seligman, 1984). Alpha internal consistencies for the FAIL and SUCC composites have been moderate, but considered adequate, in past research (.72 and .75, respectively), as have their 5-week retest reliabilities (.64 and .70, respectively; Peterson et al., 1982). Validity of the FAIL and SUCC composites has been supported by moderate correlations with self-report measures of depressive symptoms and self-concept (SUCC R2s = .09–.20, FAIL R2s = .10–.13; Tennen, Herzberger, & Nelson, 1987). In the current study, alpha internal consistency was high at all assessments for the FAIL (Mdn = .88, range =.85–.89) and the SUCC (Mdn = .84, range = .81–.89) composites.
Dysfunctional Attitudes Scale (DAS)
The DAS (Form A; Weissman, 1979) is a 40-item self-report measure of attitudes hypothesized to relate to depression. Internal consistency has been high in past research (e.g., αs = .87–.90), and validity has been demonstrated by the DAS’s correlations with measures of depressive symptoms (e.g., rs = .33–.49 with the BDI; Dobson & Breiter, 1983; Haeffel et al., 2005; Harkin, Fraley, & Abela, 2005; Ilardi & Craighead, 1999), other measures of negative cognitive content (e.g., r = .43 with the Automatic Thoughts Questionnaire [Hollon & Kendall, 1980]; Dobson & Breiter, 1983; r = .47 with the Cognitive Content Questionnaire [Alloy et al., 2000]; Haeffel et al., 2005; r = .54 with the FAIL composite, Ilardi & Craighead, 1999), and personality pathology (e.g., r = .73 with a sum of Diagnostic and Statistical Manual of Mental Disorders [3rd ed., rev.; DSM–III–R; American Psychiatric Association, 1987] Axis II criterion ratings; Ilardi & Craighead, 1999). In the current study, internal consistency (Cronbach’s alpha) was very high at all assessments (Mdn = .95, range = .94–.96).
Self-Efficacy Scale (SEF)
The SEF (Sherer et al., 1982) measures expectations for persistence and success in a variety of domains with 23 items (plus seven filler items) rated on a 5-point scale of agreement. Although items have been divided into General and Social subscales in some studies, we elected to use the total score because the subscales have shown similar validity coefficients (Sherer et al., 1982), a large normative data set was available for the total score (Lansford, Antonucci, Akiyama, & Takahashi, 2005), and internal consistency for the total scale has been high (.85–.87; Lansford et al., 2005). In the current study, internal consistency for the total scale was quite high at all assessments (Mdn = .92, range = .89–.92). The validity of the SEF has been supported by correlations with self-report measures of self-esteem (total scale rs = .56–.59; Lansford et al., 2005), interpersonal competency (subscale rs = .43–.45; Sherer et al., 1982), and assertiveness (subscale rs = .40–.41; Sherer & Adams, 1983).
Standardization of scores
To aid understanding of the magnitude of changes without altering statistical tests of change within measures (e.g., pre- vs. posttreatment), we placed the individual cognitive content and depressive symptom scales on a common metric (T scores; M = 50, SD = 10), as was done in Vittengl et al. (2004a). The four depressive symptom measures (BDI, HRSD, IDSC, IDSR), then, were averaged to form a single depressive symptoms composite at each assessment. The depressive symptom composite was then restandardized into T-score units to maintain a standard deviation of 10 at the first A-CT session. Although the primary analyses use the standardized measures, Table 1 contains descriptive statistics for the raw scales at each assessment. Appendix A contains correlations among measures during the acute phase, and Appendix B contains correlations during the experimental and follow-up phases.
Table 1.
Raw Scale Score Descriptive Statistics at Each Assessment
| A-CT
|
C-CT or control
|
Post A-CT follow-up
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scale and statistic | Pre | S9 | S17 | Post | Exit | Random | S6 | Post | 12 mon. | 24 mon. |
| BDI | ||||||||||
| M | 24.78 | 13.57 | 9.23 | 7.57 | 9.35 | 3.81 | 6.30 | 4.79 | 5.27 | 3.81 |
| SD | 8.04 | 8.79 | 7.77 | 7.37 | 8.87 | 4.35 | 9.17 | 6.51 | 6.56 | 6.41 |
| n | 152 | 137 | 132 | 126 | 154 | 84 | 77 | 71 | 63 | 56 |
| HRSD | ||||||||||
| M | 18.41 | 8.91 | 6.64 | 7.42 | 8.22 | 3.62 | 5.31 | 4.12 | 4.63 | 4.06 |
| SD | 3.83 | 4.90 | 5.05 | 6.45 | 6.88 | 2.85 | 6.62 | 5.39 | 5.67 | 4.77 |
| n | 155 | 138 | 135 | 128 | 155 | 84 | 78 | 74 | 71 | 63 |
| IDSC | ||||||||||
| M | 33.14 | 17.38 | 12.66 | 13.12 | 14.60 | 6.31 | 8.87 | 7.21 | 7.35 | 6.52 |
| SD | 7.41 | 10.01 | 9.99 | 11.44 | 12.41 | 5.05 | 11.18 | 9.06 | 8.56 | 7.60 |
| n | 155 | 138 | 135 | 128 | 155 | 84 | 78 | 73 | 71 | 63 |
| IDSR | ||||||||||
| M | 37.47 | 21.13 | 14.77 | 13.02 | 15.40 | 7.63 | 9.45 | 8.79 | 8.55 | 6.46 |
| SD | 9.23 | 11.78 | 10.98 | 10.62 | 12.67 | 6.41 | 10.73 | 9.02 | 7.73 | 7.05 |
| n | 151 | 137 | 133 | 126 | 154 | 84 | 77 | 71 | 62 | 56 |
| DAS | ||||||||||
| M | 157.16 | 133.84 | 116.88 | 110.87 | 115.59 | 97.37 | 108.71 | 99.12 | 100.87 | 95.40 |
| SD | 37.37 | 36.96 | 35.28 | 37.54 | 39.41 | 30.58 | 36.50 | 35.06 | 33.78 | 35.68 |
| n | 152 | 137 | 130 | 126 | 153 | 84 | 75 | 69 | 61 | 52 |
| SUCC | ||||||||||
| M | 4.55 | 4.76 | 4.85 | 4.79 | 4.82 | 4.89 | 4.76 | 4.81 | 4.64 | 4.81 |
| SD | 0.86 | 0.81 | 0.75 | 0.79 | 0.82 | 0.81 | 0.92 | 0.80 | 0.82 | 0.87 |
| n | 149 | 135 | 128 | 125 | 153 | 84 | 77 | 69 | 62 | 52 |
| FAIL | ||||||||||
| M | 4.92 | 4.38 | 4.07 | 3.95 | 4.08 | 3.73 | 3.95 | 3.84 | 3.85 | 3.84 |
| SD | 0.93 | 0.90 | 0.88 | 0.98 | 1.02 | 0.95 | 0.90 | 0.95 | 0.91 | 0.96 |
| n | 150 | 135 | 128 | 125 | 153 | 84 | 77 | 69 | 62 | 52 |
| SEF | ||||||||||
| M | 65.51 | 81.25 | 77.17 | 85.55 | 83.63 | 87.60 | ||||
| SD | 15.08 | 15.92 | 18.09 | 14.21 | 15.07 | 14.27 | ||||
| n | 151 | 122 | 152 | 84 | 64 | 50 | ||||
Note. Follow-ups occurred 12 and 24 months post-A-CT, which is equivalent to 4 and 16 months post-C-CT or assessment-only control. A-CT = acute-phase cognitive therapy; C-CT = continuation-phase cognitive therapy; Pre = pretreatment; S9 = Session 9; S17 = Session 17; Post = posttreatment; Exit = last available data point used in calculating effect sizes and health statistics; Random = at randomization; S6 = Session 6; mon. = months; BDI = Beck Depression Inventory; HRSD = Hamilton Rating Scale for Depression; IDSC = Inventory of Depressive Symptomatology, Clinician Version; IDSR = Inventory of Depressive Symptomatology, Self-Report Version; DAS = Dysfunctional Attitudes Scale; SUCC = Attributional Style Questionnaire Success composite; FAIL = Attributional Style Questionnaire Failure composite; SEF = Self-Efficacy Scale.
Appendix A.
Correlations Among Cognitive Content and Depressive Symptoms Composite Measures During Acute-Phase Cognitive Therapy
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAS | |||||||||||||||||||||||
| 1. Pre | |||||||||||||||||||||||
| 2. Session 9 | .63 | ||||||||||||||||||||||
| 3. Session 17 | .55 | .77 | |||||||||||||||||||||
| 4. Post | .47 | .70 | .88 | ||||||||||||||||||||
| 5. Exit | .48 | .69 | .89 | 1.00 | |||||||||||||||||||
| SUCC | |||||||||||||||||||||||
| 6. Pre | −.22 | −.10 | −.20 | −.06 | −.10 | ||||||||||||||||||
| 7. Session 9 | −.02 | −.22 | −.22 | −.16 | −.19 | .59 | |||||||||||||||||
| 8. Session 17 | .03 | −.12 | −.26 | −.17 | −.22 | .49 | .64 | ||||||||||||||||
| 9. Post | .18 | .00 | −.10 | −.14 | −.14 | .40 | .64 | .79 | |||||||||||||||
| 10. Exit | .05 | −.05 | −.15 | −.14 | −.22 | .50 | .64 | .81 | 1.00 | ||||||||||||||
| FAIL | |||||||||||||||||||||||
| 11. Pre | .57 | .35 | .33 | .32 | .31 | −.05 | .08 | .18 | .30 | .21 | |||||||||||||
| 12. Session 9 | .27 | .51 | .46 | .51 | .47 | .05 | −.07 | .01 | .02 | .06 | .53 | ||||||||||||
| 13. Session 17 | .19 | .38 | .52 | .48 | .47 | .10 | .05 | −.03 | .07 | .03 | .36 | .65 | |||||||||||
| 14. Post | .25 | .39 | .47 | .54 | .54 | .19 | .10 | .08 | .15 | .15 | .41 | .69 | .76 | ||||||||||
| 15. Exit | .21 | .36 | .45 | .53 | .55 | .18 | .10 | .04 | .15 | .10 | .44 | .63 | .78 | 1.00 | |||||||||
| SEF | |||||||||||||||||||||||
| 16. Pre | −.48 | −.36 | −.29 | −.23 | −.29 | .23 | .16 | .08 | .07 | .08 | −.31 | −.07 | −.12 | −.07 | −.14 | ||||||||
| 17. Post | −.25 | −.43 | −.50 | −.58 | −.58 | .16 | .24 | .28 | .33 | .33 | −.03 | −.33 | −.38 | −.41 | −.41 | .57 | |||||||
| 18. Exit | −.34 | −.44 | −.49 | −.57 | −.58 | .11 | .19 | .21 | .28 | .20 | −.14 | −.30 | −.36 | −.42 | −.45 | .67 | 1.00 | ||||||
| DEP | |||||||||||||||||||||||
| 19. Pre | .35 | .24 | .22 | .18 | .20 | −.23 | .00 | .01 | .11 | .10 | .35 | .16 | .14 | .04 | .12 | −.36 | −.10 | −.21 | |||||
| 20. Session 9 | .15 | .54 | .55 | .53 | .52 | −.15 | −.27 | −.17 | −.14 | −.16 | .20 | .49 | .37 | .29 | .28 | −.15 | −.37 | −.35 | .40 | ||||
| 21. Session 17 | .12 | .42 | .55 | .59 | .60 | −.06 | −.13 | −.20 | −.12 | −.16 | .10 | .40 | .46 | .39 | .40 | −.08 | −.35 | −.39 | .31 | .74 | |||
| 22. Post | .09 | .33 | .47 | .64 | .63 | −.02 | −.10 | −.09 | −.18 | −.15 | .10 | .45 | .42 | .46 | .46 | −.05 | −.50 | −.51 | .12 | .62 | .75 | ||
| 23. Exit | .00 | .34 | .48 | .64 | .55 | .05 | −.09 | −.14 | −.18 | −.08 | .05 | .40 | .45 | .46 | .48 | −.04 | −.50 | −.44 | .19 | .62 | .77 | 1.00 |
Note. Pairwise Ns = 116–155. DAS = Dysfunctional Attitudes Scale; Pre = pretreatment; Post = posttreatment; Exit = last available data point used in calculating effect size and health statistics; SUCC = Attributional Style Questionnaire Success composite; FAIL = Attributional Style Questionnaire Failure composite; SEF = Self-Efficacy Scale; DEP = depressive symptoms composite.
Appendix B.
Correlations Among Cognitive Content and Depressive Symptoms Composite Measures During Experimental and Follow-Up Phases
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAS | |||||||||||||||||||||||
| 1. Random | |||||||||||||||||||||||
| 2. Session 6 | .72 | ||||||||||||||||||||||
| 3. Post | .71 | .77 | |||||||||||||||||||||
| 4. 12 month | .76 | .72 | .90 | ||||||||||||||||||||
| 5. 24 month | .74 | .68 | .83 | .88 | |||||||||||||||||||
| SUCC | |||||||||||||||||||||||
| 6. Random | .00 | .03 | .08 | .04 | .16 | ||||||||||||||||||
| 7. Session 6 | .05 | −.10 | −.04 | −.11 | .16 | .65 | |||||||||||||||||
| 8. Post | .19 | .04 | .06 | .06 | .23 | .75 | .82 | ||||||||||||||||
| 9. 12 month | .16 | −.03 | .01 | .06 | .11 | .67 | .77 | .85 | |||||||||||||||
| 10. 24 month | .10 | .05 | .07 | .01 | .11 | .78 | .74 | .84 | .80 | ||||||||||||||
| FAIL | |||||||||||||||||||||||
| 11. Random | .44 | .39 | .32 | .38 | .40 | .33 | .23 | .28 | .35 | .26 | |||||||||||||
| 12. Session 6 | .40 | .38 | .30 | .39 | .40 | .17 | .04 | .12 | .23 | −.04 | .75 | ||||||||||||
| 13. Post | .41 | .30 | .37 | .44 | .40 | .22 | .14 | .20 | .26 | .10 | .74 | .81 | |||||||||||
| 14. 12 month | .32 | .23 | .36 | .42 | .54 | .21 | .18 | .24 | .32 | .24 | .67 | .75 | .81 | ||||||||||
| 15. 24 month | .33 | .25 | .28 | .40 | .44 | .22 | .19 | .23 | .23 | .25 | .68 | .75 | .82 | .82 | |||||||||
| SEF | |||||||||||||||||||||||
| 16. Random | −.47 | −.57 | −.40 | −.49 | −.43 | .20 | .19 | .15 | .01 | .14 | −.24 | −.27 | −.13 | −.17 | −.18 | ||||||||
| 17. Post | −.35 | −.55 | −.53 | −.47 | −.46 | .18 | .24 | .23 | .14 | .19 | −.06 | −.14 | −.10 | −.12 | −.12 | .85 | |||||||
| 18. 24 month | −.26 | −.42 | −.25 | −.29 | −.28 | .20 | .15 | .23 | .17 | .25 | .09 | −.02 | −.01 | .01 | .04 | .79 | .85 | ||||||
| DEP | |||||||||||||||||||||||
| 19. Random | .55 | .37 | .30 | .35 | .38 | −.09 | −.02 | .07 | .03 | .00 | .42 | .44 | .36 | .30 | .28 | −.36 | −.19 | −.04 | |||||
| 20. Session 6 | .27 | .53 | .34 | .39 | .36 | .05 | −.36 | −.14 | −.15 | −.18 | .22 | .39 | .20 | .17 | .18 | −.32 | −.41 | −.28 | .35 | ||||
| 21. Post | .35 | .39 | .50 | .54 | .44 | −.01 | −.31 | −.15 | −.09 | −.06 | .26 | .27 | .36 | .21 | .27 | −.43 | −.51 | −.26 | .37 | .64 | |||
| 22. 12 month | .10 | .20 | .35 | .48 | .38 | .07 | −.09 | −.15 | −.15 | −.21 | −.04 | .10 | .20 | .15 | .09 | −.13 | −.34 | −.29 | .11 | .35 | .43 | ||
| 23. 24 month | .32 | .39 | .48 | .50 | .60 | .04 | −.19 | −.07 | .00 | −.04 | .35 | .41 | .40 | .35 | .45 | −.33 | −.33 | −.28 | .38 | .56 | .65 | .60 |
Note. Pairwise Ns = 43–84. DAS = Dysfunctional Attitudes Scale; Random = at randomization; Post = posttreatment; 12 month = 12 months after completing acute-phase cognitive therapy; 24 month = 24 months after completing acute-phase cognitive therapy; SUCC = Attributional Style Questionnaire Success composite; FAIL = Attributional Style Questionnaire Failure composite; SEF = Self-Efficacy Scale; DEP = depressive symptoms composite.
Identification of Healthy Participants
Identification of participants in the “healthy” range of cognitive content was based on available norms and a cutoff of 1.28 standard deviations from the normative mean as the limit for health (i.e., for a normally distributed variable, about 90% of the normative population would be considered healthy and 10% unhealthy). This value is more conservative (i.e., identifies fewer healthy persons) than the traditional cutoff of 2 standard deviations from the normative mean for marking clinically significant dysfunction (about 2% of the population would be considered unhealthy using this index; e.g., see Jacobson & Truax, 1991). The prevalence of major depressive disorder, however, is much greater than 2% in the general adult population, perhaps 5%–7% over 12 months and 13%–16% over the lifetime (e.g., Hasin, Goodwin, Stinson, & Grant, 2005; Kessler et al., 2003). Assuming that depressive cognitive content precedes or accompanies major depressive disorder, we expected the normative prevalence of unhealthy levels of depressive cognitive content to be closer to 10%, although this specific cutoff is somewhat arbitrary.
We obtained normative data for identification of patients with healthy cognitive content from the published literature. For the DAS, Dozois et al. (2003) provided general adult norms (M = 115.0, SD = 26.7) derived by pooling data from a number of published reports, primarily community control and normal comparison groups. For the ASQ, we pooled data from several published reports on data from community control groups. We pooled data using methods summarized by Kendall and Sheldrick (2000) that weight studies’ mean and standard deviation statistics by sample size to derive a population estimate. We pooled data from three reports (Fear, Sharp, & Healy, 1996; Heimberg et al., 1989; Jack & Williams, 1991) to derive SUCC norms (M = 4.78, SD = 0.59) and from these reports plus three additional reports (Eaves & Rush, 1984; Peselow & Fieve, 1992; Zimmerman, Coryell, Corenthal, & Wilson, 1986) to derive FAIL norms (M = 3.94, SD = 0.62). For the SEF, we used norms from a large representative sample in the United States (M = 90.62, SD = 12.65; Lansford et al., 2005).
Results
How Much Does Cognitive Content Improve During A-CT?
Average change in cognitive content
Figure 1 shows the changes in the standardized measures over A-CT. (It is important to note that SUCC and SEF are reverse scored for this figure, so that all decreases reflect improvement in depression and cognitive content.) Relative to the pre-A-CT distribution, depression decreased markedly; SUCC decreased a little; and FAIL, DAS, and SEF decreased an intermediate amount. Because of using multiple measures and analyses, we selected a conservative alpha of .01 (two tailed) for significance in all statistical tests. The standardized DAS, FAIL, and SUCC measures were greater than the depressive symptoms composite at the Session 9, Session 17, and 1-week post-A-CT assessments, and SEF was greater than the depressive symptoms composite at the post-A-CT assessment (note that SEF was not measured at A-CT Sessions 9 or 17; all ps < .0001, two-tailed t tests), indicating that cognitive functioning improved less than depression.
Figure 1.
Changes in standardized cognitive content and depressive symptoms scales during acute-phase cognitive therapy (A-CT) represent improved functioning. SUCC and SEF are reverse scored for this figure, so all decreases reflect improvement in depression and cognitive functioning. Ns for each assessment point are shown in Table 1. Means based only on patients with complete data yielded an identical pattern of results; therefore, the table and figure present all available data for each assessment point. DAS = Dysfunctional Attitudes Scale; SEF = Self-Efficacy Scale; FAIL = Attributional Style Questionnaire Failure composite; SUCC = Attributional Style Questionnaire Success composite; DEP = composite measure of depressive symptoms.
Intention-to-treat analyses were performed by comparing the pre- and last available A-CT assessments. Very similar to the pattern shown in Figure 1, all measures changed significantly ( ps < .0004, two-tailed t tests) during A-CT. The effect size d (mean change divided by the standard error of mean change) was computed to characterize the magnitude of change. Cohen (1988) suggested that d values greater than or equal to 0.2, 0.5, and 0.8 be interpreted as small, medium, and large effects, respectively. SUCC (d = 0.30, n = 149) changed a small amount; FAIL (d = 0.79, n = 150), DAS (d = 1.05, n = 152), and SEF (d = 0.83, n = 151) changed large amounts; and depressive symptoms changed an even larger amount (d = 1.55, n = 155).
Proportions of patients with “healthy” cognitive content scores
The proportion of healthy participants, defined as scoring within 1.28 standard deviations of the normative mean (90th percentile), was calculated at pre-A-CT and exit from A-CT. As shown in Table 2, on the DAS, 38.2% of patients were healthy pre-A-CT and 78.3% were healthy at exit, a significant increase ( p < .0001 by sign test). On the SEF, 29.8% were healthy pre-A-CT, and this proportion increased significantly to 63.6% at exit ( p < .0001 by sign test). For FAIL, 44.0% were healthy pre-A-CT, and this improved to 75.3% at exit ( p < .0001 by sign test). For SUCC, 75.8% were healthy pre-A-CT, and, consistent with the small change in dimensional scores, this proportion increased nonsignificantly to 84.6% at exit ( p = .035 by sign test).
Table 2.
Changes in Health Status of Cognitive Content During Acute-Phase Cognitive Therapy
| Not healthy at exit
|
Healthy at exit
|
|||
|---|---|---|---|---|
| Measure and health status | n | % | n | % |
| DAS | ||||
| Not healthy at entry | 32 | 21.1 | 62 | 40.8 |
| Healthy at entry | 1 | 0.7 | 57 | 37.5 |
| SUCC | ||||
| Not healthy at entry | 13 | 8.7 | 23 | 15.4 |
| Healthy at entry | 10 | 6.7 | 103 | 69.1 |
| FAIL | ||||
| Not healthy at entry | 31 | 20.7 | 53 | 35.3 |
| Healthy at entry | 6 | 4.0 | 60 | 40.0 |
| SEF | ||||
| Not healthy at entry | 54 | 35.8 | 52 | 34.4 |
| Healthy at entry | 1 | 0.7 | 44 | 29.1 |
Note. Healthy participants were defined as those scoring within 1.28 standard deviations of the best available normative mean (approximately 90th percentile). Percentages reflect the proportion of the sample completing the measure at entry and exit. DAS = Dysfunctional Attitudes Scale; SUCC = Attributional Style Questionnaire Success composite; FAIL = Attributional Style Questionnaire Failure composite; SEF = Self-Efficacy Scale.
Notable minorities of patients (a) never scored in the healthy range (DAS = 21.8%, FAIL = 20.7%, SUCC = 8.7%, SEF = 35.8%), (b) got better during A-CT (DAS = 40.8%, FAIL = 35.3%, SUCC = 15.4%, SEF = 34.4%), or (c) were always well (DAS = 37.5%, FAIL = 40.0%, SUCC = 69.1%, SEF = 29.1%). Only a few patients were cognitively healthy but became unhealthy (DAS = 0.7%, FAIL = 4.0%, SUCC = 6.7%, SEF = 0.7%). The clinical importance of most of these deteriorations is questionable, however. None of the patients shifting into an unhealthy range of cognitive content did so on more than one measure (i.e., there was no overlap among these four subsets of patients). Further, all patients shifting into an unhealthy range of cognitive content showed at least nominal decreases (i.e., improvements) in composite depressive symptoms from pre-A-CT to the last available A-CT assessment (in T-score points; M = 22.34, SD = 11.79, range = 5.24–37.80), with the exception of 1 patient who increased 1.50 raw score points on the FAIL and increased 9.65 T-score points on the depressive symptom composite.
How Are Changes in Cognitive Content and Change in Depressive Symptoms Related During A-CT?
Differences in change in cognitive content among responders versus nonresponders
Response was defined as the absence of DSM–IV major depressive disorder and an HRSD score ≤ 9 when exiting A-CT. Responders’ and nonresponders’ cognitive content became increasingly distinct over the course of A-CT (see Figure 2). A-CT responders and nonresponders did not differ significantly at the pre-A-CT assessment on the DAS, FAIL, SUCC, or SEF ( ps > .45, two-tailed t tests). By A-CT Sessions 9 and 17, responders had lower DAS and FAIL scores than nonresponders ( ps < .002, two tailed) but SUCC scores did not vary between these groups ( ps > .19, two tailed). SEF was not measured at Sessions 9 or 17. At the post-A-CT assessment, responders had significantly lower DAS and FAIL scores and higher SEF scores ( ps < .0001, two tailed) than nonresponders. In addition, responders had higher SUCC scores than nonresponders at a trend level of significance ( p = .015, two tailed). These results indicate that pre-A-CT cognitive content did not predict response to A-CT. Instead, by the middle of treatment, improvements in cognitive content may reflect a developing response to A-CT.
Correspondence of change in depressive symptoms and cognitive content
From entering to exiting A-CT, changes (difference scores) in depressive symptoms were significantly positively correlated with changes in cognitive content as measured by the DAS, FAIL, SUCC, and SEF (rs = .60, .50, .31, and .57, respectively; ps < .004, two tailed). Thus, patients who improved more in cognitive content also tended to improve more in depressive symptoms. These correlations were only moderate, however, and, as described above, cognitive content changed less than depressive symptoms (smaller decreases in T scores and smaller effect sizes). Consequently, we estimated the magnitude of residual change in cognitive content after accounting for change in depression, and vice versa, using the intercept t test in ordinary least squares regressions. As shown in Table 3, there was no significant residual change in the cognitive measures after controlling for change in depressive symptoms ( ps > .23, two tailed), and the residual effect sizes were small. In contrast, residual change in depressive symptoms remained significant when controlling for change in the four cognitive content measures individually (in bivariate regressions) and collectively (in a multiple regression). The residual effect sizes for change in depressive symptoms were moderate to large. Extending the finding that cognitive content changed less than depressive symptoms, these results indicate the expected amount of change in depressive symptoms is moderate to large even if there is no change in cognitive content from entering to exiting A-CT. In contrast, the expected amount of change in cognitive content is small if there is no change in depressive symptoms from entering to exiting A-CT.
Table 3.
Changes in Depressive Symptoms and Cognitive Content From Entering to Exiting Acute-Phase Cognitive Therapy
| Effect size (d)
|
||
|---|---|---|
| Entry minus exit scores (Δ) | Raw change | Residual change |
| Δ DEP | 1.55* | |
| Controlling Δ DAS | 0.80* | |
| Controlling Δ FAIL | 1.03* | |
| Controlling Δ SEF | 1.03* | |
| Controlling Δ SUCC | 1.45* | |
| Controlling Δ all | 0.74* | |
| Δ DAS | 1.05* | |
| Controlling Δ DEP | 0.08 | |
| Δ FAIL | 0.79* | |
| Controlling Δ DEP | 0.01 | |
| Δ SUCC | 0.30* | |
| Controlling Δ DEP | −0.10 | |
| Δ SEF | 0.83* | |
| Controlling Δ DEP | −0.05 | |
Note. Ns = 147–155. Effect sizes (d) were computed from intercept t tests in regression analyses. DEP = depressive symptoms composite; DAS = Dysfunctional Attitudes Scale; FAIL = Attributional Style Questionnaire Failure composite; SEF = Self-Efficacy Scale; SUCC = Attributional Style Questionnaire Success composite.
p < .01, two tailed.
Relations among early and late change in cognitive content and depressive symptoms
We computed concurrent and cross-time correlations of change in cognitive content and depressive symptoms. For these analyses, we computed change from early (pre-A-CT minus A-CT Session 9) to late (A-CT Session 9 minus 1-week post-A-CT) treatment. (SEF could not be included in these analyses because data on this variable were collected only at the pre- and post-A-CT assessments.) As shown in Table 4, early and late in A-CT, decreases in depressive symptoms correlated with concurrent improvements in cognitive content.
Table 4.
Correlations Among Early and Late Change in Cognitive Content and Depressive Symptoms During Acute-Phase Cognitive Therapy
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. DEP Δ1 | |||||||||
| 2. DAS Δ1 | .60 | ||||||||
| 3. SUCC Δ1 | −.37 | −.41 | |||||||
| 4. FAIL Δ1 | .46 | .56 | −.31 | ||||||
| 5. DEP Δ2 | −.10 | −.20 | .06 | .06 | |||||
| 6. DAS Δ2 | .09 | −.23 | .08 | .02 | .45 | ||||
| 7. SUCC Δ2 | .03 | .05 | −.24 | −.06 | −.25 | −.21 | |||
| 8. FAIL Δ2 | −.09 | −.16 | .02 | −.21 | .34 | .27 | −.08 | ||
| 9. DEP post | −.55 | −.28 | .06 | −.34 | −.54 | −.42 | .11 | −.07 |
Note. N = 120. Correlations ≥ ∣23∣, p ≤ .01, two tailed. Concurrent changes appear in italics; predictive correlations appear in bold. DEP = depressive symptoms composite; Δ1 = difference between scores at Session 1 and Session 9; DAS = Dysfunctional Attitudes Scale; SUCC = Attributional Style Questionnaire Success composite; FAIL = Attributional Style Questionnaire Failure composite; Δ2 = difference between scores at Session 9 and 1 week post-acute-phase cognitive therapy; post = single assessment at 1 week post-acute-phase cognitive therapy.
However, early change in cognitive content did not significantly predict late change in depressive symptoms, nor did early change in depressive symptoms significantly predict late change in cognitive content. Early improvements in negative cognitive content (DAS, FAIL) and depressive symptoms, but not positive cognitive content (SUCC), did predict lower depressive symptoms assessed 1 week post-A-CT. However, when early change in depressive symptoms was controlled, early change in cognition (DAS partial r = .08, p = .39; FAIL partial r = −.12, p = .19) no longer predicted post-A-CT depressive symptoms significantly. Together with the above regression analyses, this pattern of correlations suggests that change in cognitive content (especially negative cognitive content) parallels but does not account for or predict change in depressive symptoms. This result challenges the primacy hypothesis (or at least a simple, straightforward version thereof) within the cognitive theory of depression. That is, if the primacy hypothesis is correct, then it appears that the effects of cognitive content on affect do not occur on a timescale of weeks or months, as assessed in this and most studies. Rather, they may occur on a more “micro” timescale of days or perhaps even moments. Specifically, it may be that CT is effective not because there is a global change in the frequency of patients’ cognitive content but because patients learn to pay attention to their cognitive content and become more skilled in both noticing and taking steps to change each negative cognition as it arises.
Does C-CT Improve Cognitive Content Among Responders to A-CT?
Three types of statistical analyses (pairwise t tests, repeated-measures analyses of variance, and mixed-effects repeated-measures models) were used to evaluate the effect of C-CT on cognitive functioning during the experimental (Months 0–8 post-A-CT) and follow-up (Months 9–24 post-A-CT) phases. All three sets of analyses led to substantively equivalent conclusions, so we present only the widely understood t tests here. No effect of C-CT on the DAS, SUCC, or SEF measures was detected 0, 4, 8, 12, or 24 months post-A-CT ( ps > .08, two tailed). Consequently, we pooled the C-CT and control groups before plotting changes in SUCC, DAS, and SEF. As shown in Figure 3, the mean SUCC, DAS, and SEF scores were relatively stable over the experimental and follow-up phases.
Figure 3.
Cognitive content after acute-phase cognitive therapy (A-CT) for pooled or separate continuation-phase cognitive therapy (C-CT) and control groups. SUCC and SEF are reverse scored for this figure, so all decreases reflect improvement in depression and cognitive functioning. DAS = Dysfunctional Attitudes Scale; SEF = Self-Efficacy Scale; FAIL = Attributional Style Questionnaire Failure composite; SUCC = Attributional Style Questionnaire Success composite; mo. 3 months.
In contrast, the C-CT and assessment-only groups did differ on the FAIL at some time points. The C-CT and control groups did not differ on the FAIL at the post-A-CT assessment (as one would expect with randomization) or at 4 months post-A-CT ( ps > .24, two tailed). At 8 ( p = .013, two tailed) and 12 ( p = .011, two tailed) months post-A-CT, however, there was a trend for those in the C-CT group to score lower on the FAIL, and this difference was significant 24 months post-A-CT ( p = .003, two tailed). The development of this difference on the FAIL appears to be due to the stability of the control group between 4 and 24 months post-A-CT ( p = .81, two tailed), whereas the C-CT group demonstrated a decrease in the FAIL over this period ( p = .008, two tailed). However, both groups of patients providing these FAIL scores showed nonsignificant decreases in depressive symptoms between 4 and 24 months post-A-CT (in T-score points, C-CT M = 6.05, SD = 11.71, p = .02; control M = 3.58, SD = 13.89, p = .19). Changes (4-month scores minus 24-month scores) in FAIL and depressive symptom scores were not significantly correlated (r = −.11, p = .62) in the C-CT group, but these changes were positively correlated (r = .49, p = .009) in the control group.
Discussion
The process of changing cognitions during CT is assumed to be a basic component of relieving emotional distress and improving depressed mood. For example, some hypothesize that increased cognitive change is specific to CT (compared, for example, with pharmacotherapy; e.g., DeRubeis et al., 1990). Others implicate cognitive change in relapse prevention (e.g., Segal et al., 2006).
The purpose of this article was to quantify hypothesized negatively and/or positively valenced changes in cognitive content and to reveal any change patterns during CT in adult outpatients who presented with recurrent major depressive disorder. Cognitive content included measures of attributions for failure (FAIL) and success (SUCC), dysfunctional attitudes (DAS), and self-efficacy (SEF). We examined relations between changes in cognitive content and changes in depressive symptoms over the course of 20 sessions of A-CT (3–4 months). For A-CT responders, we asked whether 10 sessions of C-CT (over 8 months) produced more improvement in cognitive content than A-CT alone (i.e., 10 sessions of assessment-only control over 8 months). For either and both conditions, we evaluated the extent to which changes in cognitive content lasted over 24 months. We used rudimentary, unprimed self-report measures and clinical ratings of depressive symptoms and cognitive content, which, although primitive, are typical in contemporary clinical research.
As hypothesized, intention-to-treat analyses showed that changes from baseline to the end of A-CT were both statistically and clinically significant on standardized scores reflecting attributional style regarding failure events, dysfunctional attitudes, and self-efficacy. These results confirm previous reports that statistically significant changes occur during A-CT and add to the existing literature by demonstrating that the amount of change was typically large (on all of the measures except for attributions regarding success) and the mean-level change was always clinically significant. Specifically, most (64%–85%) patients’ scores post-A-CT fell into the distribution of scores found for community dwellers who are not seeking treatment, which we labeled as “healthy” for the sake of simplicity. Of these, 35%–41% and 15%–34% began treatment in the “unhealthy” range of negative and positive cognitions, respectively, and developed healthy levels over the course of A-CT. The smaller changes observed in attributions of success reflects the fact that 76% of the patients scored in the healthy range prior to A-CT and highlights previous observations that negatively valenced cognitions and positively valenced cognitions do not necessarily follow similar patterns (Clark et al., 1999). Further, it is reassuring that only rarely (i.e., 0.7% to 6.7%) did the change in cognitive content involve deterioration (i.e., the score moved from the healthy to unhealthy range). When scores deteriorated, this was not seen on all measures; rather, only one measure of cognitive change per patient did so. At the same time, however, and similar to the response rate of depressive symptoms in A-CT, 9%–36% of patients never scored in the healthy range on some cognitive measure.
All measures of cognitive change correlated moderately with changes in depressive symptoms concurrently. The pattern of change in attributions for failure and dysfunctional attitudes was larger for responders compared with nonresponders and was discernible by the midpoint of A-CT (i.e., Session 9, with early sessions occurring twice a week).
Over A-CT, and compared with measures of cognitive content, the effect size for the depression factor (which combined four commonly used measures of depressive symptoms) was the largest. We computed concurrent and cross-time correlations to describe the relations among early (through Session 9) and late (from Session 9 to post A-CT) change in negative cognitive content and depressive symptoms. Contrary to the primacy hypothesis in its most basic form, regression analyses showed that reductions in depressive symptoms accounted for changes in cognitive content rather than the other way around. Moreover, predictive power was limited to concurrent change. That is, whereas change in depressive symptoms over A-CT did account for change in cognitions (but not vice versa), neither early change in depressive symptoms nor early change in cognition predicted later change in the other. Further, whereas early change in negative cognitions did predict post-A-CT levels of depressive symptoms, when early change in depressive symptoms was controlled this correlation was not significant, again suggesting the primacy of change in depressive symptoms over change in cognitive content over A-CT. It is difficult to know why these results were observed. One possibility is that processes distinct from cognitive change explain change in depression. Alternatively, it is possible that cognitive change is central to change in depression and the methods currently available may have prevented observation of this relation.
Of course, all of the results reported here are limited by our methods. Specifically, the correlations between early A-CT change in cognitive content and late depressive symptoms are moderate for negative cognitions (Mdn = −.31) and not significant (.06) for positive cognitions. The assessment of cognitive content was not primed. Emotionally primed measures of cognitive reactivity show promise in predicting later depression (e.g., see Segal et al., 2006) and were not used here. Further, the timing and interval of assessment could have influenced our results. Nevertheless, if these results are replicable (particularly with improved assessments of cognitive processes, rather than simply cognitive content), then this result would challenge a simple straightforward version of the primacy hypothesis within cognitive theory.
Compared with the changes in cognitive content over A-CT, notably less change occurred thereafter in the A-CT responders who were followed for 24 months. Whereas a few of these changes were statistically significant (e.g., the DAS increased significantly from randomization to Month 4, then decreased significantly from Months 4 to 8), there was no strong clear pattern of change as there was over A-CT. This suggests that the statistically and clinically significant changes in cognitive content occur early in A-CT and are largely durable over 2 years.
Contrary to our hypothesis, patients randomized to C-CT did not show significantly more improvement on most measures of cognitive content compared with the patients who only had A-CT. Again, this result suggests that most of the change in cognitive content occurs during the acute phase and can be maintained over time. Previous analyses have shown that 8 months of C-CT reduced relapse rate significantly more than A-CT alone (i.e., 10% compared with 31% relapse; Jarrett et al., 2001), but, for the most part, it does not appear that this can be attributed to greater change in cognitions. Rather, it appears that for responders to A-CT, most of the important change in cognitive content occurs early during the therapeutic process (i.e., in A-CT) rather than later (i.e., in C-CT). The one exception to this finding was that patients who received C-CT showed significant decreases in attributions regarding failure compared with those who only had A-CT, whose failure attributions were stable with no significant change following A-CT. If this result is replicated, then it may link concurrent changes in attributions for negative events to relapse prevention, at least for responders to A-CT who then receive C-CT.
Because all patients in this sample received CT (either A-CT alone or combined with C-CT), the design and the within-subject comparisons we made during A-CT do not allow comment on the extent to which the substantial and durable changes in cognitive content are unique to or greater with CT compared with no treatment or with another “brand” or modality of antidepressant intervention (e.g., interpersonal psychotherapy or pharmacotherapy). To allow such examinations, it will be important for future studies that compare treatments to include measures of negative and positive cognitive content as well as to assess relations with cognitive and other related processes (described below).
Changing cognition can involve not only changing what people think but more importantly the way they think or process or analyze information. The rudimentary measures available in the field today and used here represent the content of people’s thoughts rather than the actual process of thinking. A limitation of these results is that it is hard to know how similar endorsement strategies on self-report questionnaires are to the moment-to-moment thinking that occurs before, simultaneous with, and after affect shifts. Similarly, it is unclear how the process of thinking (e.g., faulty processing of emotionally laden, self-referent information) is linked to the content of thinking (e.g., “I failed at x, thus, I’m unworthy/unlovable.”). In summary, the external validity of cognitive measurement used in contemporary clinical research on depression is unproven, raising the question of what technologies might be better.
Specifically, if self-report questionnaires using endorsement strategies (which are subject to response biases) are only a proxy for how cognitive processes or thinking change or remain stable, then what other methods might offer more optimal tests of cognitive theory in depressed patients who engage in CT? We think that ultimately it will be important to link the surface manifestations of cognitions targeted during CT and evaluated here (i.e., cognitive content or products) to (a) underlying processes available through interdisciplinary, translational studies and emerging technologies from such fields as cognitive science, neuroscience, and basic biology; and (b) basic behavioral and affective processes occurring outside therapy (particularly those related to important life roles, e.g., partner, parent, worker, community member). We acknowledge that this is a tall order, even for an interdisciplinary team, but the nature of the questions our field is asking require improved technologies for measuring the underlying constructs of interest and testing complex hypotheses.
We are encouraged by interdisciplinary teams who are beginning to combine strategies from imaging, psychology, clinical science, and neuroscience to describe the brain changes that occur after CT (see Siegle, Carter, & Thase, 2006; Mayberg, 2003; Goldapple et al., 2004). It will be important to determine how these brain changes are linked to the moment-to-moment changes in thinking, affect, and behavior inherent in important life roles. We think that clinical psychology and clinical science will contribute to these translational efforts by designing new paradigms to study the relations between cognition, other behavior, affect, and psychopathology.
In conclusion, the results we report here are from unprimed measures documenting that improvements in negative cognitive content and self-efficacy during A-CT, particularly in the early sessions, were large, clinically significant, and durable. Changes in negative cognitive content were accounted for by changes in depressive symptoms rather than vice versa. We look forward to future studies of the relations between early A-CT changes in cognitive content and later relapse, recurrence, and recovery. We are optimistic that emerging and future methodologies will allow researchers and cognitive therapists to describe relations between changes in cognitive content and process and depression with corresponding changes in basic behavior and biology.
Acknowledgments
We thank our colleagues for contributing to this research. Dolores Kraft coordinated the trial and provided clinical support. Jeanette Doyle, Greg Eaves, Paul Silver, Marjorie Woodruff, Bethany Hampton, Catherine Judd, Douglas Lisle, Regina Kinney, Maria Marwill-Magee, Andrew Clifford, Martin Schaffer, and Rodger Kobes also provided clinical support. Research support was provided by Barbara Foster, Michelle White, Edna Christian, Joseph Begue, Julie Lowe, Daisha Cipher, Patricia Green, Demetria Clinton, and Paula Reese. Brian F. Shaw rated the cognitive therapists. We are also grateful to Amy McSpadden for her assistance in manuscript preparation. We appreciate the administrative support of Eric J. Nestler.
Footnotes
The results of this article were presented in part at the annual meeting of the Association for Behavioral and Cognitive Therapies, Chicago, November 2006. The clinical trial was supported in part by National Institute of Mental Health Grants MH-38238 and MH-01571 to Robin B. Jarrett and was conducted at the Psychosocial Research and Depression Clinic, Department of Psychiatry, University of Texas Southwestern Medical Center at Dallas.
Contributor Information
Robin B. Jarrett, Department of Psychiatry, University of Texas Southwestern Medical Center at Dallas
Jeffrey R. Vittengl, Division of Social Science, Truman State University
Kimberly Doyle, Department of Psychiatry, University of Texas Southwestern Medical Center at Dallas.
Lee Anna Clark, Department of Psychology, University of Iowa.
References
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, et al. The Temple–Wisconsin Cognitive Vulnerability to Depression Project: Lifetime history of Axis I psychopathology in individuals at high and low cognitive vulnerability to depression. Journal of Abnormal Psychology. 2000;109:403–418. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Barber JP, DeRubeis RJ. Change in compensatory skills in cognitive therapy for depression. Journal of Psychotherapy Practice and Research. 2001;10:8–13. [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New York: International University Press; 1976. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Keitner GI, Ryan CE, Miller IW. Cognitive predictors of symptoms return following depression treatment. Journal of Abnormal Psychology. 2003;112:488–496. doi: 10.1037/0021-843x.112.3.488. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM. A critique of the theoretical bases of cognitive–behavioral theories and therapy. Clinical Psychology Review. 1986;6:177–197. [Google Scholar]
- Beutler LE, Guest PD. The role of cognitive change in psychotherapy. In: Freeman A, editor. Comprehensive handbook of cognitive therapy. New York: Plenum Press; 1989. pp. 123–143. [Google Scholar]
- Bowers WA. Treatment of depressed in-patients: Cognitive therapy plus medication, relaxation plus medication, and medication alone. British Journal of Psychiatry. 1990;156:73–78. doi: 10.1192/bjp.156.1.73. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory and therapy of depression. New York: Wiley; 1999. [Google Scholar]
- Cohen J. Statistical power analyses for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- DeRubeis RJ, Evans MD, Hollon SD, Garvey M, Grove WM, Tuason VB. How does cognitive therapy work? Cognitive change and symptom change in cognitive therapy and pharmacotherapy for depression. Journal of Consulting and Clinical Psychology. 1990;58:862–869. doi: 10.1037//0022-006x.58.6.862. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Breiter HJ. Cognitive assessment of depression: Reliability and validity of three measures. Journal of Abnormal Psychology. 1983;92:107–109. doi: 10.1037//0021-843x.92.1.107. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Covin R, Brinker JK. Normative data on cognitive measures of depression. Journal of Consulting and Clinical Psychology. 2003;71:71–80. [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS. Depression. In: Antony MM, Barlow DH, editors. Handbook of assessment and treatment planning for psychological disorders. New York: Guilford Press; 2002. pp. 259–299. [Google Scholar]
- Eaves G, Rush AJ. Cognitive patterns in symptomatic and remitted unipolar major depression. Journal of Abnormal Psychology. 1984;93:31–40. doi: 10.1037//0021-843x.93.1.31. [DOI] [PubMed] [Google Scholar]
- Fear C, Sharp H, Healy D. Cognitive processes in delusional disorders. British Journal of Psychiatry. 1996;168:61–67. doi: 10.1192/bjp.168.1.61. [DOI] [PubMed] [Google Scholar]
- Free ML, Oei TP, Appleton C. Biological and psychological processes in recovery from depression during cognitive therapy. Journal of Behavior Therapy and Experimental Psychiatry. 1998;29:213–226. doi: 10.1016/s0005-7916(98)00016-0. [DOI] [PubMed] [Google Scholar]
- Furlong M, Oei TPS. Changes to automatic thoughts and dysfunctional attitudes in group CBT for depression. Behavioural and Cognitive Psychotherapy. 2002;30:351–360. [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive–behavioral treatment for depression: Relapse prevention. Journal of Clinical and Consulting Psychology. 1998;66:377–384. doi: 10.1037//0022-006x.66.2.377. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Abramson LY, Voelz ZR, Metalsky GI, Halberstadt L, Dykman BM, et al. Negative cognitive styles, dysfunctional attitudes, and the remitted depression paradigm: A search for the elusive cognitive vulnerability to depression factor among remitted depressives. Emotion. 2005;5:343–348. doi: 10.1037/1528-3542.5.3.343. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin BL, Fraley RC, Abela JRZ. Daily depression and cognitions about stress: Evidence for a trait-like depressogenic cognitive style and the prediction of depressive symptoms in a prospective daily diary study. Journal of Personality and Social Psychology. 2005;88:673–685. doi: 10.1037/0022-3514.88.4.673. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the national epidemiological survey on alcoholism and related conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Klosko JS, Dodge CS, Shadick R, Becker RE, Barlow DH. Anxiety disorders, depression, and attributional style: A further test of the specificity of depressive attributions. Cognitive Therapy and Research. 1989;13:21–36. [Google Scholar]
- Hollon S, Beck AT. Research on cognitive therapy. In: Garfield SL, Bergen AE, editors. Handbook of psychotherapy and behavior change. New York: Wiley; 1986. pp. 259–281. [Google Scholar]
- Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM, Tuason VB. Cognitive therapy and pharmacotherapy for depression: Singly and in combination. Archives of General Psychiatry. 1992;49:774–781. doi: 10.1001/archpsyc.1992.01820100018004. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush A. Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? Journal of Clinical Psychiatry. 2005;66:455–468. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Kendall PC. Cognitive self-statements in depression: Development of an automatic thoughts questionnaire. Cognitive Therapy and Research. 1980;4:383–395. [Google Scholar]
- Ilardi SS, Craighead WE. The relationship between personality pathology and dysfunctional cognitions in previously depressed adults. Journal of Abnormal Psychology. 1999;108:51–57. doi: 10.1037//0021-843x.108.1.51. [DOI] [PubMed] [Google Scholar]
- Imber SD, Pilkonis PA, Sotsky SM, Elkin I, Watkins JT, Collins JF, et al. Mode-specific effects among three treatments for depression. Journal of Consulting and Clinical Psychology. 1990;58:352–359. doi: 10.1037//0022-006x.58.3.352. [DOI] [PubMed] [Google Scholar]
- Jack RL, Williams JMG. The role of attributions in self-poisoning. British Journal of Clinical Psychology. 1991;30:25–35. doi: 10.1111/j.2044-8260.1991.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollon JK, et al. A component analysis of cognitive–behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64:295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jamison C, Scogin F. The outcome of cognitive bibliotherapy with depressed adults. Journal of Consulting and Clinical Psychology. 1995;63:644–650. doi: 10.1037//0022-006x.63.4.644. [DOI] [PubMed] [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar depressive disorder: The continuation/maintenance phase. 1989 Unpublished manuscript. [Google Scholar]
- Jarrett RB, Basco MR, Risser R, Ramanan J, Marwill M, Kraft D, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? Journal of Consulting and Clinical Psychology. 1998;66:1036–1040. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D. Prophylactic cognitive therapy for major depressive disorder. In Session: Psychotherapy in Practice. 1997;3:65–79. [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: A randomized clinical trial. Archives of General Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Schaffer M, McIntire D, Witt-Browder A, Kraft D, Risser RC. Treatment of atypical depression with cognitive therapy or phenelzine: A double-blind placebo controlled trial. Archives of General Psychiatry. 1999;56:431–437. doi: 10.1001/archpsyc.56.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Sheldrick RC. Normative data for normative comparisons. Journal of Consulting and Clinical Psychology. 2000;68:767–773. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Replication Study (NCS-R) Journal of the American Medical Association. 2003;23:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kolko DJ, Brent DA, Baugher M, Bridge M, Birmaher B. Cognitive and family therapies for adolescent depression: Treatment specificity, mediation, and moderation. Journal of Consulting and Clinical Psychology. 2000;68:603–614. [PubMed] [Google Scholar]
- Kuiper NA, MacDonald MR. Reason, emotion, and cognitive therapy. Clinical Psychology Review. 1983;3:297–316. [Google Scholar]
- Kwon SM, Oei TPS. Cognitive change processes in a group cognitive behavior therapy of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:73–85. doi: 10.1016/s0005-7916(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Antonucci TC, Akiyama H, Takahashi K. A quantitative and qualitative approach to social relationships and well-being in the United States and Japan. Journal of Comparative Family Studies. 2005;36:1–22. [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic– cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Peselow ED, Fieve RR. Depressive attributional style and the dexamethasone suppression test: Relationship to the endogenous/ melancholic distinction and to each other. Psychopathology. 1992;25:173–182. doi: 10.1159/000284769. [DOI] [PubMed] [Google Scholar]
- Peterson C, Seligman ME. Causal explanations as a risk factor for depression: Theory and evidence. Psychological Review. 1984;91:347–374. [PubMed] [Google Scholar]
- Peterson C, Semmel A, von Baeyer C, Abramson LY, Metalsky GI, Seligman MEP. The Attributional Style Questionnaire. Cognitive Therapy and Research. 1982;6:287–300. [Google Scholar]
- Riskind JH. The status of cognitive change in cognitive therapy and other therapies. Journal of Cognitive Psychotherapy. 1995;9:195–204. [Google Scholar]
- Rush AJ, Beck AT, Kovacs M, Hollon SD. Comparative efficacy of cognitive therapy and pharmacotherapy in the treatment of depressed outpatients. Cognitive Therapy and Research. 1977;1:17–37. [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory for Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Research. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kovacs M, Beck AT, Weissenburger J, Hollon SD. Differential effects of cognitive therapy and pharmacotherapy on depressive symptoms. Journal of Affective Disorders. 1981;3:221–229. doi: 10.1016/0165-0327(81)90024-0. [DOI] [PubMed] [Google Scholar]
- Schwab JJ, Bialow MR, Clemmons RS, Holzer CE. Hamilton Rating Scale for Depression with medical inpatients. British Journal of Psychiatry. 1967;113:83–88. doi: 10.1192/bjp.113.494.83. [DOI] [PubMed] [Google Scholar]
- Scogin F, Hamblin D, Beutler L. Bibliotherapy for depressed older adults: A self-help alternative. Gerontologist. 1987;27:383–387. doi: 10.1093/geront/27.3.383. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Abramson LY, Semmel A, von Bayer C. Journal of Abnormal Psychology. 1979;88:242–248. doi: 10.1037//0021-843x.88.3.242. [DOI] [PubMed] [Google Scholar]
- Sherer M, Adams CH. Construct validation of the Self-Efficacy Scale. Psychological Reports. 1983;53:899–902. [Google Scholar]
- Sherer M, Maddux J, Mercandante B, Prentice-Dunn S, Jacobs B, Rogers R. The Self-Efficacy Scale: Construction and validation. Psychological Reports. 1982;51:663–671. [Google Scholar]
- Siegle G, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Simons AD, Garfield SL, Murphy GE. The process of change in cognitive therapy and pharmacotherapy for depression. Archives of General Psychiatry. 1984;41:45–51. doi: 10.1001/archpsyc.1984.01790120049007. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM–III–R. New York: New York State Psychiatric Institute; 1989. [Google Scholar]
- Tang TZ, DeRubeis RJ. Sudden gains and critical sessions in cognitive– behavioral therapy for depression. Journal of Consulting and Clinical Psychology. 1999;67:894–904. doi: 10.1037//0022-006x.67.6.894. [DOI] [PubMed] [Google Scholar]
- Tang TZ, DeRubeis RJ, Beberman R, Pham T. Cognitive changes, critical sessions, and sudden gains in cognitive–behavioral therapy for depression. Journal of Consulting and Clinical Psychology. 2005;73:168–172. doi: 10.1037/0022-006X.73.1.168. [DOI] [PubMed] [Google Scholar]
- Tennen H, Herzberger S, Nelson HF. Depressive attributional style: The role of self-esteem. Journal of Personality. 1987;55:631–660. doi: 10.1111/j.1467-6494.1987.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychological Medicine. 2004a;34:643–658. doi: 10.1017/S0033291703001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Self-directed affiliation and autonomy across acute and continuation phase cognitive therapy for recurrent depression. Journal of Personality Assessment. 2004b;83:235–247. doi: 10.1207/s15327752jpa8303_07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, Jarrett RB. Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute phase cognitive therapy. Psychological Medicine. 2005;35:693–704. doi: 10.1017/s0033291704004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM. The Dysfunctional Attitudes Scale: A validation study. Dissertation Abstracts International. 1979;40:1389B–1390B. [Google Scholar]
- Whisman MA, Miller IW, Norman WH, Keitner GI. Cognitive therapy with depressed inpatients: Specific effects on dysfunctional cognitions. Journal of Consulting and Clinical Psychology. 1991;59:282–288. doi: 10.1037//0022-006x.59.2.282. [DOI] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating manual. Philadelphia: Center for Cognitive Therapy; 1980. [Google Scholar]
- Zimmerman M, Coryell W, Corenthal C, Wilson S. Dysfunctional attitudes and attributional style in healthy controls and patients with schizophrenia, psychotic depression, and nonpsychotic depression. Journal of Abnormal Psychology. 1986;95:403–405. doi: 10.1037//0021-843x.95.4.403. [DOI] [PubMed] [Google Scholar]