Abstract
Live, attenuated vaccines remain the safest, most cost-effective intervention against viral infections. Because live vaccine strains are generated empirically and the basis for attenuation is usually ill defined, many important viruses lack an efficient live vaccine. Here, we present a general strategy for the rational design of safe and effective live vaccines that harnesses the microRNA-based gene silencing machinery to control viral replication. Using poliovirus as a model, we demonstrate that insertion of small miRNA homology sequences into a viral genome can restrict its tissue tropism, thereby preventing pathogenicity and yielding an attenuated viral strain. Poliovirus strains engineered to become targets of neuronal-specific miRNAs lost their ability to replicate in the central nervous system, leading to significant attenuation of neurovirulence in infected animals. Importantly, these viruses retained the ability to replicate in non-neuronal tissues. As a result, these engineered miRNA-regulated viruses elicited strong protective immunity in mice without producing disease.
Introduction
The use of vaccines to combat viral diseases is one of the greatest public health successes of the last century. Annual mortality rates in the United States attributed to RNA viruses including poliomyelitis, measles, mumps, and rubella have been reduced by more than 99.9% (CDC, 1999). A number of virus vaccine approaches have been developed, including live attenuated virus, inactivated virus, production of viral proteins, and gene delivery vehicles expressing viral antigens (Barney S. Graham and James E. Crowe, 2007). Live attenuated virus vaccines are the most effective method of immunization because they activate all components of the immune system, inducing both a balanced systemic and local immune response and a broad humoral and cellular response (Barney S. Graham and James E. Crowe, 2007; Zinkernagel, 2003). However, the potential risk of attenuated viruses reverting to the pathogenic phenotype is a major concern, along with production and distribution issues.
A major drawback in the development of live vaccines is the lack of rational approaches to attenuate virus pathogenicity. Ideally, a virus vaccine would replicate robustly in a limited number of tissues, so as to elicit a complex and long-lasting immune response, while at the same time not spreading to tissues where it may cause disease. For instance, poliovirus replication in the gut is not life threatening, while infection of the motor neurons in the spinal cord and brainstem leads to poliomyelitis (Andino et al., 1999; Wood and Macadam, 1997). The ability to rationally design viral strains that are selectively disabled in their replication in a given tissue could provide the basis for the development of vaccines. Altering cell tropism of a virus by passaging it in cell culture is the classic method for virus attenuation and vaccine strain development. In this method, attenuation of the pathogenic phenotype often results from the adaptation of the pathogenic virus to the particular cell line(s), thereby reducing virus fitness in specific organs and cell types within the vaccinee (Graff et al., 1997). This approach permitted the development of a number of virus vaccines, but the empirical nature of such passaging may alter the virus in unpredictable ways, and the number of new virus vaccines developed over the years remains limited.
A new approach for limiting the pathogenic potential of poliovirus by manipulating the error rate of the viral polymerase was recently described (Arnold et al., 2005; Pfeiffer and Kirkegaard, 2005; Vignuzzi et al., 2006; Vignuzzi et al., 2008). By increasing polymerase fidelity, the genetic diversity of the poliovirus population was restricted, and virulence was attenuated in an animal model of infection (Pfeiffer and Kirkegaard, 2005; Vignuzzi et al., 2006). This approach was subsequently used to engineer attenuated poliovirus vaccines with improved stability (Vignuzzi et al., 2008). However, the issue of limiting viral replication to specific tissues remains a key aspect of developing attenuated virus vaccines. In this report, we describe a rational strategy for controlling viral replication by exploiting the regulatory capacity of the RNA interference (RNAi) gene-silencing machinery.
RNAi functions as an adaptive antiviral immune response in plants and insects (Baulcombe, 2004; Sanchez-Vargas et al., 2004; Voinnet, 2005), and the importance of RNAi during viral infection in mammals is the focus of intense research. Evidence is emerging to support the interaction between the RNAi machinery and mammalian viruses. Viruses employ strategies to repress or usurp the RNAi machinery by encoding virus-derived siRNAs, microRNAs (miRNA), and viral suppressors that target components of the gene-silencing pathway (Cullen, 2006; van Rij and Andino, 2006). miRNAs are a family of RNA species that are structurally and functionally related to the short interfering RNAs (siRNA) that cause RNA silencing (Bartel, 2004). This family of non-coding genes functions as post-transcriptional regulators of gene expression (He and Hannon, 2004). Mature miRNAs are formed by two processing events; first, the nascent miRNA transcripts are processed into pre-miRNA precursors (~70 nucleotides in length) in the nucleus by the RNase-III enzyme, Drosha; next, the pre-miRNA is cleaved by a second RNase-III enzyme, Dicer, in the cytoplasm to generate the ~21–25-nucleotide mature miRNAs (Bernstein et al., 2001; Denli et al., 2004; Gregory et al., 2004; Grishok et al., 2001; Hutvagner et al., 2001; Lee et al., 2003; Lee et al., 2002; Lee et al., 2004). Current data suggests that miRNAs regulate gene expression through translational repression or mRNA degradation (He and Hannon, 2004). MiRNAs can also act as siRNAs to guide cleavage of a perfect complementary sequence (Gitlin et al., 2005; Hutvagner et al., 2001). This mRNA-cleavage step of RNAi is mediated by the endonucuclease, Argonaute2 (Ago2), within the context of the RNA-induced silencing complex (RISC) (Meister et al., 2004; Rand et al., 2005).
MiRNAs regulate numerous aspects of development and cellular physiology. Accordingly, miRNA expression patterns are tissue-specific and developmentally timed, such that each tissue is defined by a characterized set of specific miRNAs (Bartel, 2004; Lagos-Quintana et al., 2002). For example, let-7a, identified in worms, encodes a temporally regulated miRNA that controls the developmental transition from the L4 stage into the adult (Abrahante et al., 2003; Lin et al., 2003; Reinhart et al., 2000). The neuron-specific miRNA, miR-124, promotes mammalian neuronal differentiation by a number of mechanisms (Cao et al., 2007; Makeyev et al., 2007; Visvanathan et al., 2007). Interestingly, it has been shown that expression of transgenes encoded by gene therapy vectors can be regulated by endogenous miRNAs (Brown et al., 2007; Brown et al., 2006). We hypothesized that the diverse tissue-specific distribution of cellular miRNAs could also be harnessed to regulate the tissue tropism of pathogenic human viruses. Recently, interactions between endogenous miRNAs and viruses have been reported, providing evidence that miRNAs can effectively interact with viruses during their normal life cycle (Cullen, 2006; Jopling et al., 2005; Lecellier et al., 2005).
To test our hypothesis, we exploited the regulatory capacity of the miRNA machinery to control virus tropism, and show that this strategy reduces virus pathogenesis. We engineered poliovirus carrying endogenous miRNA-complementary target sequences. This virus is unable to replicate in cells expressing the corresponding miRNA, thus producing an attenuated phenotype. We further show that attenuation is due to the interaction between the cellular miRNA and the viral target, inducing miRNA repression. The restricted tissue tropism of these engineered viruses results in an attenuated neurovirulent phenotype in a mouse model of infection. Importantly, because the engineered viruses can replicate in certain tissues, they elicit protective immunity against challenge with a pathogenic strain of poliovirus. This approach provides a general mechanism for controlling viral replication in specific tissues to rationally design stable, attenuated vaccines that can elicit long-lasting immunity.
Results
A rational approach for engineering stably attenuated viruses
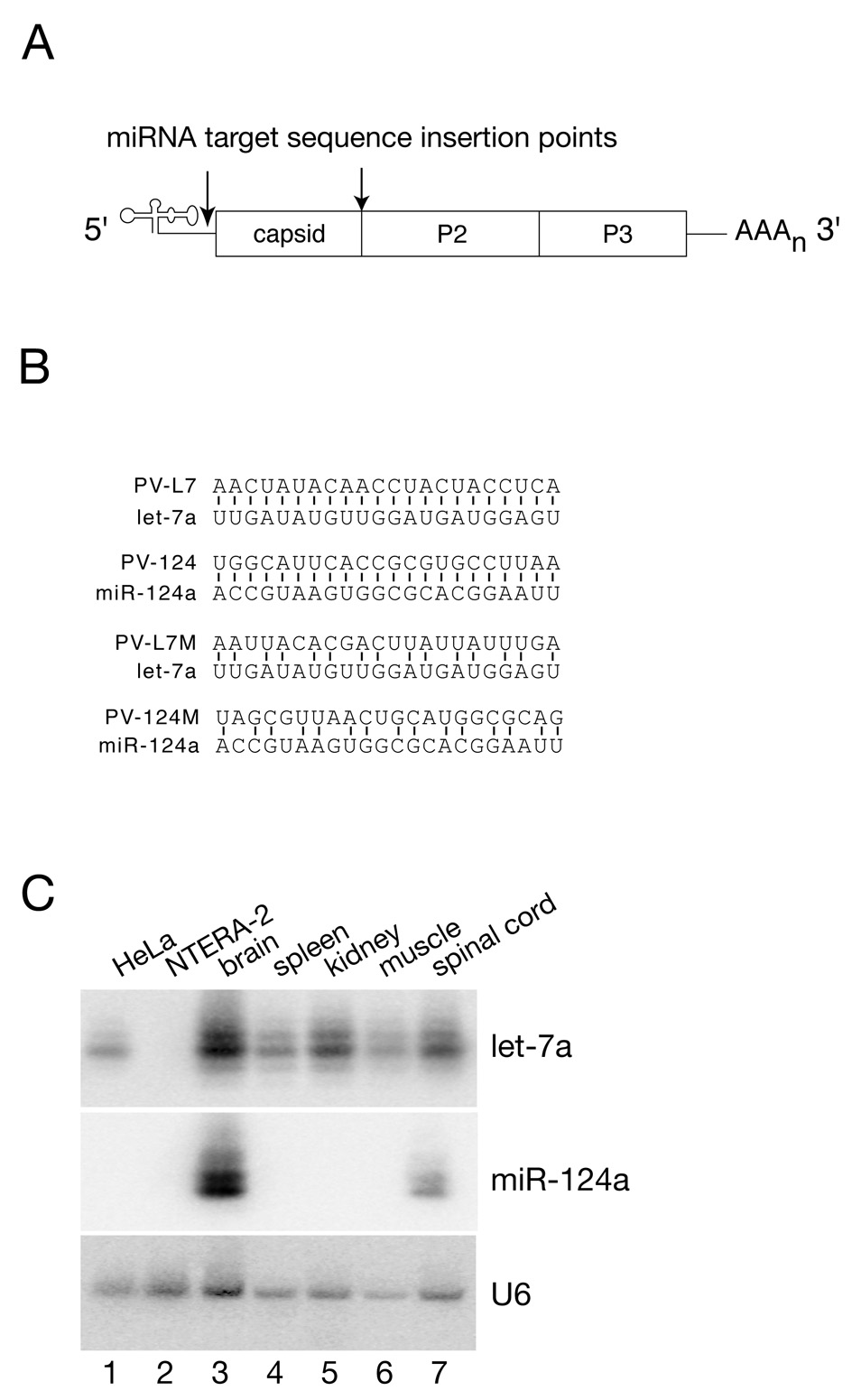
To determine whether the miRNA gene-silencing machinery could be used to rationally engineer viruses with tissue tropism determined by the miRNA expression profiles, polioviruses were constructed to contain two complementary miRNA target sequence insertions. The two insertions were introduced into the “variable” segment at the 3’ end of the 5’ untranslated region (UTR) and between the structural and nonstructural genes within the coding region (Figure 1A). These sites were previously shown to tolerate large insertions without adverse effects to virus replication (Tang et al., 1997). The miRNA target sequences chosen for this study are complementary to let-7a or miR-124a, and the viruses carrying these insertions were designed as PV-L7 and PV-124, respectively (Figure 1B). We also constructed control viruses with modified miRNA target sequences to prevent recognition and cleavage by these miRNAs, termed PV-L7M and PV-124M viruses, respectively (Figure 1B).
Figure 1. Engineering viruses with restricted tissue tropism.
(A) Target sequences complementary to two distinct miRNAs (let-7a and miR124a) were inserted into two locations within the poliovirus genome. The 5’ site is located in the “variable” segment between the poliovirus internal ribosome entry site (IRES) and the start codon. The 3’ site is located between the structural and nonstructural genes.
(B) Perfect sequence complementarity between the endogenous miRNA and the target sequence inserted into the poliovirus genome is illustrated. Silence mutations were engineered into the target sequences to create virus controls that disrupt base pairing with the endogenous miRNA, while conserving the wild type encoded amino acid sequence.
(C) Northern Blot analysis of let-7a and miR-124a miRNA expression levels observed in cell lines (HeLa and NTERA-2, lanes 1 and 2) and mouse tissues (lanes 3–7).
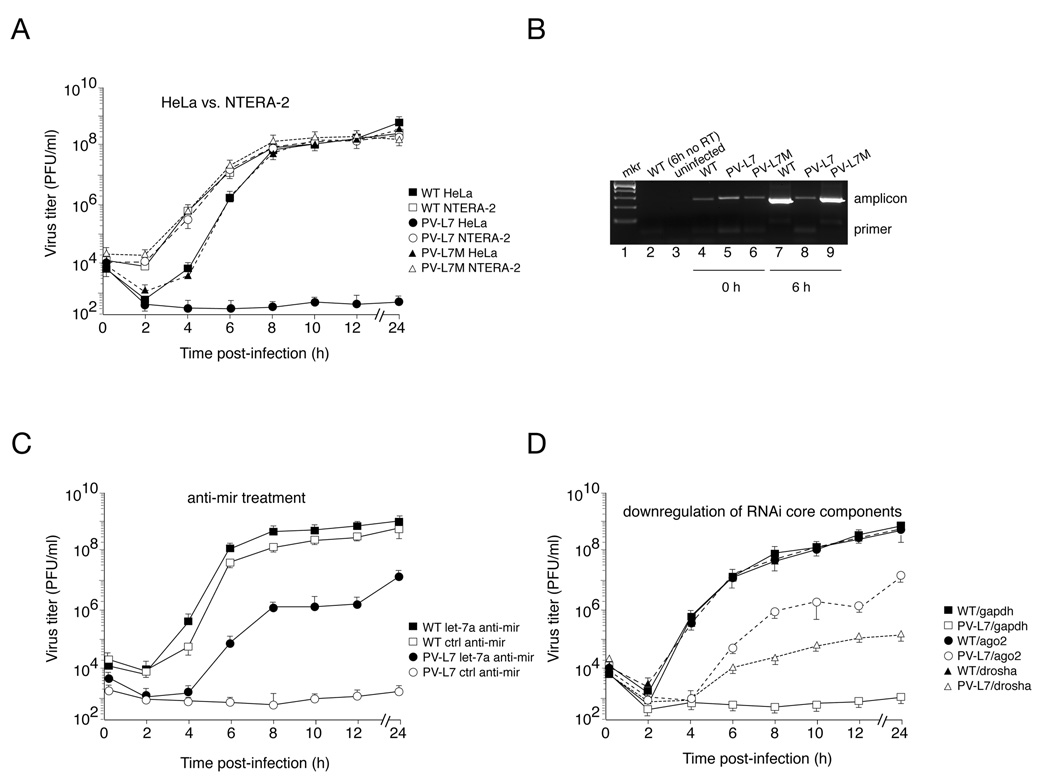
To evaluate the ability of virus containing these sequences to replicate in tissue culture, one-step growth curves were performed in HeLa cells which express let-7a but not miR-124a, and NTERA-2 cells which do not express either miRNA (Figure 1C, lanes 1 and 2). In NTERA-2 cells, wild type and engineered viruses replicated with identical kinetics (Figure 2A), indicating that the insertion of the miRNA target sequences did not impair virus replication. In contrast, replication of PV-L7 virus was severely compromised in HeLa cells, which express let-7a (Figure 2A). Control PV-L7M virus, carrying mutated let-7a target sequences, showed replication rates and final viral titers comparable to those observed for wild type virus (Figure 2A). PV-124 viral replication in HeLa was unaffected (data not shown), consistent with the fact that the neuronal specific miR-124a is not expressed in HeLa cells. To further characterize the replication of PV-L7 in HeLa cells, RNA extracted from infected cells was analyzed by semi-quantitative RT-PCR (Figure 2B). The amount of PCR product amplified from cells infected with wild type and PV-L7M was significantly higher at six hours (6 h) compared to the starting time of infection (0 h, Figure 2B, lanes 4 and 7, and 6 and 9). This result indicates, as expected, that wild type and PV-L7M virus RNA accumulates over the first hours of infection. In contrast, we observed a decrease in PV-L7 viral RNA over the same period of time (Figure 2B, compare lanes 5 and 8). We conclude that in HeLa cells, the pairing of cellular let-7a and the target sequences within PV-L7 leads to a decrease in viral RNA levels, thereby attenuating PV-L7 viral replication.
Figure 2. Engineered PV-L7 virus exhibits attenuated replication kinetics due to endogenous let-7a-mediated repression.
(A) Replication kinetics of wild type poliovirus (squares) and engineered viruses, PV-L7 (circles) and control PV-L7M (triangles), in the permissive (NTERA-2, white symbols) and non-permissive (HeLa, filled symbols) cell lines by virtue of endogenous let-7a expression. PV-L7 viral replication is attenuated in HeLa cells by six orders of magnitude, whereas the PV-L7M control virus replicates with wild type kinetics. Viral titer values represent the mean ± SD of three independent experiments. Error bars, SD.
(B) RT-PCR analysis of total RNA extracted from HeLa cells infected with wildtype, PV-L7, or PV-L7M virus at time zero (lanes 4–6) and six hours post infection (lanes 7–9). We amplified by RT-PCR a 269nt-long fragment (amplicon), spanning the 5’ target insertion site, from 200ng total RNA. The molecular marker is shown in lane 1. Lane 2 controls for the reverse transcription reaction, and lane 3 corresponds to RNA isolated from uninfected HeLa cells used as template for the RT-PCR reaction.
(C) Inhibition of endogenous let-7a using anti-mir technology rescues PV-L7 viral replication in the non-permissive HeLa cells. HeLa cells were transfected with small oligonucleotides corresponding to the let-7a complementary sequence (anti-mir let-7a, black symbols) or control nonspecific oligonucleotide (ctrl anti-mir, white symbols). HeLa cells were then infected with wild type poliovirus (squares) or engineered PV-L7 virus (circles). Viral titer values represent the mean ± SD of three independent experiments. Error bars, SD.
(D) Knocking-down core components of the RNAi machinery rescues PV-L7 replication. siRNAs targeting Ago2 (circles) and drosha (triangles) partially rescues replication of PV-L7 (white symbols) in the non-permissive HeLa cells, while a siRNA targeting GAPDH has no effect (squares). Transfecting siRNAs has not effect on wild type poliovirus replication (WT, black symbols). Viral titer values represent the mean ± SD of three independent experiments. Error bars, SD.
The miRNA machinery mediates attenuation of viral replication
To directly examine the effect of miRNA let-7a in PV-L7 viral growth, we measured viral replication in HeLa cells transfected with miRNA inhibitors (anti-miRs). Anti-miRs are chemically modified, single stranded nucleic acids that are designed to specifically bind and inhibit endogenous miRNA molecules. PV-L7 replication was partially rescued by transfection with a let-7a specific anti-miR, whereas PV-L7 growth remained inhibited in HeLa cells transfected with a control anti-miR (Figure 2C). The final virus titers (24 hour time point) for wild type poliovirus were 100-times greater than those levels observed for PV-L7 in cells transfected with let-7a anti-miR. It is likely that residual cellular let-7a, or newly processed mature miRNA, partially inhibit viral replication in anti-miR transfected HeLa cells. Similarly, transfection efficiency is usually not perfect, so viral growth remains inhibited in those untransfected cells. Nonetheless, these data indicate that the inhibition of PV-L7 replication depends on the availability of let-7a.
We next determined whether core components of the miRNA machinery are required for PV-L7 replication control. To this end, we transfected HeLa cells with siRNAs that specifically deplete two core components of the miRNA machinery, Drosha and Ago2 (human homologue, eIF2C2). Downregulation of both Drosha and Ago2 resulted in a significant increase in PV-L7 replication in HeLa cells (Figure 2D). Targeting Drosha and Ago2 increased PV-L7 viral replication by 100- to 105-fold as compared to control cells transfected with a siRNA targeting GAPDH (Figure 2D). Together, these data establish that an interaction between the cellular let-7a and the target sequences within the PV-L7 virus, by triggering the “slicing” activity of Ago2 in RISC, account for the potent inhibition of viral replication.
Controlling viral replication by endogenous miRNAs strongly attenuates poliovirus pathogenesis
The strong inhibition of PV-L7 replication by the miRNA machinery in tissue culture cells prompted us to evaluate the effect of miRNAs on poliovirus pathogenesis in an animal model of infection. First, we determined the 50% lethal dose (LD50) for wild type, PV-L7, PV-L7M, PV-124, and PV-124M viruses in transgenic mice expressing the human poliovirus receptor (cPVR, Table 1). The LD50 of wild-type poliovirus is 2.2 × 106 infectious particles (PFU). In comparison, the LD50 of PV-L7 and PV-124 viruses was greater than the highest dose administered, 1 × 108 PFU. Control viruses PV-L7M and PV-124M displayed LD50 values similar to wild type, as expected. Intramuscular injection of 108 PFU of wild type virus into cPVR mice resulted in a rapid invasion of the central nervous system, causing paralysis and death within six days (Figure 3A). A similar dose of 108 PFU PV-L7 and PV-124 viruses resulted in no symptoms of paralysis or death among twenty injected mice (Figure 3A). In contrast, control PV-L7M and PV-124M viruses carrying mutated miRNA target sites were fully pathogenic (Figure 3A). Thus, insertion of miRNA target sites strongly attenuates poliovirus in this murine model of infection.
Table 1.
Engineered PV-L7 and PV-124 viruses are less virulent than wild type poliovirus. LD50 values were calculated for each virus in both cPVR and IFNAR mice using the Reed and Muench method.
| LD50(PFU) | ||
|---|---|---|
| PVR+ mice | INFR−/−, PVR+ | |
| Wildtype | 2.2 × 106 | 37 |
| PV-L7 | >1 × 109 | 2.0 × 108 |
| PV-L7M | 6.4 × 106 | 290 |
| PV-124 | >1 × 109 | 1.3 × 104 |
| PV-124M | 1.5 × 107 | 1400 |
Figure 3. Engineered viruses, PV-L7 and PV-124, show attenuated neuropathogenicity in transgenic mice.
Percentage of (A) cPVR mice and (B, C) IFNAR mice surviving intramuscular injections of different doses (108 - 103 PFU); n=20 mice per group. (A) cPVR mice infected with 108 PFU PV-L7 (filled squares) and PV-124 (filled squares) viruses are unaffected, whereas mice infected with control viruses, PV-L7M (filled triangles) and PV-124M (filled circles), and wild type (white squares) poliovirus do not survive past day six.
B) IFNAR mice infected with 107 PFU of PV-L7 (filled squares) virus all survive, whereas most mice infected with 103 PFU wild type poliovirus (white squares) and the PV-L7M control virus (filled circles) were paralyzed by day 11.
C) PV-124 virus is attenuated in IFNAR mice, albeit less dramatically than PV-L7 infected IFNAR mice. The white and black squares indicate wild type and PV-124, respectively.
In order to further characterize these viruses, similar experiments were performed in PVR-transgenic mice defective in αβ-Interferon (αβ-IFN) signaling due to deletion of the αβ-IFN receptor (IFNAR mice) (Ida-Hosonuma et al., 2005). These mice are significantly more susceptible to poliovirus infection, affording a larger dynamic range for measuring viral pathogenicity, and providing a very sensitive host to investigate pathogenic properties of the modified polioviruses PV-L7 and PV-124. Indeed, the LD50 value of wild type virus in this mouse background was 37 PFU, five orders of magnitude lower than the LD50 observed in cPVR mice (Table 1). Strikingly, PV-L7 viral pathogenesis was completely attenuated in IFNAR mice (Figure 3B), and the measured LD50 value for PV-L7 was greater than 108 PFU, the highest dose used. As expected, the control PV-L7M virus displayed a wild type pathogenic phenotype with an LD50 value of 290 PFU (Figure 3B, table 1). PV-124 virus was also attenuated in IFNAR mice (Table 1, Figure 3C), albeit to a lesser degree than PV-L7. These findings suggest that the ubiquitous expression of let-7a is able to suppress viral replication more completely than miR-124a, which is only expressed in the central nerve tissues.
miRNAs control poliovirus tissue tropism
We hypothesized that let-7a and miR-124a miRNAs would control the replication of viruses encoding complementary sequences selectively in tissues where these miRNAs are expressed. Furthermore, we expected that this tissue selectivity would account for the observed differences in virulence of the engineered viruses. To test these hypotheses, we examined viral replication in various tissues obtained six days after infection of mice inoculated intravenously with sub lethal doses (0.1LD50) of wild type or engineered viruses (Figure 4). Both control viruses, PV-L7M and PV-124M, accumulated to wild type levels in the brain and spinal cord of the infected mice (Figure 4A and 4B). In contrast, PV-L7 and PV-124 virus failed to, or only barely accumulated, in these tissues. We did not detect PV-L7 in the brain or spinal cord of injected mice. Low levels (102 PFU) of PV-124 could be isolated from brains of three of the five infected mice measured, and one of these mice showed a similarly low titer in the spinal cord six days after injection. However, these mice did not show any neuropathogenic side effects associated with poliovirus infection. Notably, in the spleen, wild type levels of replication were observed for all viruses except PV-L7, which was found at a lower level by one order of magnitude (Figure 4C).
Figure 4. Tissue tropism is controlled by the miRNA machinery.
A set of five cPVR mice was infected intravenously with each engineered virus. Virus isolated from the brain (A), spinal cord (B), and spleen (C) was analyzed by a standard plaque assay six days post infection. Viral titers (PFU/ml), normalized by tissue mass, observed in the three tissues are plotted for five mice infected with each virus. Black squares designate wild type, white squares PV-L7, black circles PV-L7M, white circles PV-124, and black triangles PV-124M. In the brain and spinal cord, PV-L7 and PV-124 infected mice exhibit viral titers that are four to six orders of magnitude lower than viral titers observed in mice infected with wild type poliovirus and the control viruses, PV-L7M and PV-124M. PV-L7 viral replication is measurable in the spleen, but is still an order of magnitude lower than viral titers observed in mice infected with the other viruses. In the spleen, wild type levels are observed for PV-124 virus.
Northern blot analysis revealed ubiquitous let-7a RNA accumulation in all tissues examined, whereas miR-124 RNA was found solely in the central nervous system (Figure 1C, lanes 3 to 7). These data correlate with the observations that PV-L7 failed to replicate or replicated only at barely detectable levels in all tissues, whereas PV-124 viral replication was permitted in non-neuronal tissues. Thus, PV-124 replication in peripheral tissues might allow the accumulation of mutations within the viral target sequence to escape endogenous miR-124a RNA recognition in the central nervous system. In fact, sequence analysis of PV-124 virus isolated from the spinal cord of the infected mouse contained deletions of the miRNA targets at both loci (data not shown). Of note, the live attenuated Sabin strains of poliovirus currently used as a vaccine exhibit similar LD50 values in transgenic mice (Vignuzzi et al., 2008) as the engineered viruses characterized in this study. We conclude that poliovirus recombinants carrying miRNA target sites are highly attenuated in an experimental model of infection.
miRNA-restricted polioviruses are promising vaccine candidates
Next, we examined the immunogenic potential of PV-L7 and PV-124 viruses in mice inoculated intraperitoneally with engineered viruses. Neutralizing antibodies are considered a key correlate of poliovirus vaccine efficacy. Sera collected from immunized mice four weeks after injection revealed that PV-124 virus induced high levels of poliovirus-specific neutralizing antibodies (Figure 5A). Mice immunized with UV-inactivated wild type poliovirus elicited a similar response to PV-L7-immunized mice, and titers for mock-immunized mice (PBS) were below the detection level of the assay. The neutralizing antibody response elicited by PV-124 was three-fold higher than in G64S-immunized mice (Figure 5A). It was previously shown that the high-fidelity replication variant, G64S, was a superior immunogen compared to the current Sabin type 1 vaccine strain (Vignuzzi et al., 2008). These observations indicated that the immunogenic potential of PV-124 is comparable or better than that of currently used vaccines, thus strongly supporting further use of this technology for engineering virus vaccines.
Figure 5. Infection with PV-L7 or PV-124 virus induces high levels of neutralizing antibody and confers immunity against a lethal challenge with wild type poliovirus.
(A) Neutralizing antibody titers (reciprocal of the serum dilution able to neutralize 100 TCID50 of wild-type poliovirus) were determined for serum collected from immunized mice. Data for individual mice (black squares specify UV-inactivated wild type virus, white squares PV-L7, black circles PV-124, white circles G64S, and black triangles for PBS) and the group average (bar) are shown. Titers for PBS-immunized mice were below the detection level of the assay, indicated by the dashed line.
(B) cPVR and IFNAR mice were immunized with either phosphate buffer saline (PBS) or 1×107 PFU (0.1LD50) of wild type irradiated virus, PV-L7 virus, or PV-124 virus. Four weeks after immunization, serum was collected. Mice were subsequently challenged with either 10LD50 or 104LD50 of wild-type poliovirus via intramuscular injection. The numbers of mice surviving are indicated. PV-124 immunized cPVR mice are protected against a lethal challenge of wild-type poliovirus, whereas cPVR mice immunized with PV-L7 and UV-inactivated wild-type virus are much less protected. PV-L7 immunized IFNAR mice were protected against a lethal challenge with 10LD50 of wild-type poliovirus, and seventy percent of the immunized IFNAR mice even survived a challenge dose of 104LD50.
The high neutralizing antibody titers identified in the vaccinated animals suggested that the immunized mice would be protected from a lethal challenge of wild type pathogenic virus. To examine this possibility, four weeks after intraperitoneal immunizations, twenty mice from each set were challenged with a lethal amount (10LD50) of wild type poliovirus via an intramuscular injection. Eighteen out of twenty mice immunized with the engineered poliovirus PV-124 immunized mice were completely protected against a lethal challenge of wild type poliovirus (Figure 5B). In comparison, 50% of the animals immunized with PV-L7 were protected. Mice receiving UV-inactivated wild type poliovirus vaccinations were only partially protected compared to PBS-treated control mice. These results indicated that PV-124 and PV-L7 induced neutralizing antibody responses that conferred protective immunity to a very stringent test of viral infection. The production of neutralizing antibodies in immunized mice correlated well with the percent of mice surviving a lethal dose of pathogenic virus.
To further examine the protection afforded by PV-L7 immunizations, we conducted similar experiments in IFNAR mice. One month after intraperitoneal injections, mice immunized with 107 PFU PV-L7 were challenged with either 10LD50 or 104LD50 via intramuscular injection. Seventy percent of mice survived the severely lethal 104LD50 dose, and all the immunized mice were protected against 10LD50 injection of wild type poliovirus (Figure 5B). In comparison, mice treated with UV-inactivated virus and PBS were not protected at all (Figure 5B). These data demonstrate the efficacy of these viruses as vaccine candidates, and validate this approach as a effective strategy for rationally engineering live attenuated virus vaccines.
Discussion
Here, we describe a strategy for rationally engineering live attenuated virus vaccines. Our approach takes advantage of the tissue-specific expression patterns of miRNAs, and employs the nucleic acid-based RNAi response, to substantially control virus replication in tissues where the virus causes disease. The miRNA complementary sequences engineered into the virus genome are targeted by the miRNA/RNAi machinery, thus inhibiting virus replication in a tissue-specific fashion. Importantly, these engineered viruses retained their replicative capacity while exhibiting attenuated neurovirulence, thus permitting the development of an effective immune response. Production of high levels of neutralizing antibodies established the immunogenicity of these test strains. Mice vaccinated with these vaccine candidates induced comparable neutralizing antibody titers to those observed for the Sabin type 1 poliovirus vaccine strain (Vignuzzi et al., 2008). Importantly, immunized mice were protected against lethal challenges of pathogenic wild type virus. We conclude that this strategy can be used to specifically reduce viral pathogenicity and create viable vaccine candidates.
A unique and exciting advantage of our new approach to rationally engineer live vaccines is its versatility, which may be applicable to a wide variety of viruses. Cellular miRNAs have been implicated in fundamental biological processes, and each cell type displays a particular miRNA repertoire (Bartel, 2004). These cellular miRNAs could be used to effectively control viral replication of a wide variety of viruses with different pathogenic profiles in a tissue-specific manner. For example, miRNA expression profiles have been effectively exploited to regulate transgene expression from lentiviral vectors according to tissue, lineage, and differentiation state (Brown et al., 2007). Another advantage of this approach for vaccine production is that viral replication remains robust in tissues without the targeting miRNA, thus allowing activation of multiple components of the immune system to elicit a more complete and long-lasting protective immunity. Importantly, this technology may also be applicable to DNA viruses and negative strand RNA viruses (Edge et al., 2008). We feel that this strategy may pave the way for the development of safe and efficacious vaccines to target a wide range of emerging and established viral diseases that have been refractory to vaccine development.
Our strategy to engineer polioviruses that are responsive to miRNA regulation was successful at precluding viral replication in cells expressing the target miRNA. PV-L7 replication was attenuated in HeLa cells, and that attenuation correlated with a decrease in viral RNA levels six hours after infection. However, knockdown of Ago2 rescued viral replication in tissue culture (Figure 2D), implicating the catalytic “slicer” component of the RNAi machinery as a key factor of the virus control mechanism. Previous data in our lab demonstrated that let-7a could act as a siRNA to direct the cleavage of a perfect complementary viral target RNA (Gitlin et al., 2002; Gitlin et al., 2005), and only Ago2 can catalyze cleavage of complementary target RNAs (Forstemann et al., 2007; Meister et al., 2004). Based on these data, we propose that Ago2 can use endogenous miRNAs to catalyze the cleavage of viral RNA containing perfect complementary target sites to control virus replication in specific tissues and attenuate virulence.
One concern with attenuated vaccines is reversion to a virulent phenotype. Viral escape from RNAi suppression has been described (Boden et al., 2003; Das et al., 2004; Gitlin et al., 2002; Gitlin et al., 2005; Lecellier et al., 2005; Wilson and Richardson, 2005). In these reports, single mismatches within the targeted region, or entire deletions, generated RNAi escape mutants. To prevent viral escape, multiple miRNA complementary target sequences were introduced within the viral genome. Indeed, we did not observe let-7a RNA escape mutants in experiments investigating PV-L7 viral replication. On the other hand, virus isolated from the spinal cord of mice infected with PV-124 virus did contain partial deletions of both miR-124a target sequences. However, even though these mutations disrupt the cellular miRNA/viral target hybrid, disease symptoms were not observed in these mice. It is possible, that the remaining partial complementarity to miR-124a resulted in a repression of PV-124 translation. Mice vaccinated with PV-124 show a higher neutralizing antibody response and complete protection from lethal challenge as compared to PV-L7 immunized animals. However, the increased efficacy observed for PV-124 correlates with a decrease in safety. Clearly, these results demonstrate that although PV-L7 is safer than the PV-124 vaccine strain, the latter is as efficacious as the currently used sabin vaccine.
Viruses could also be engineered with a combination of miRNA target sequences to increase cell selectivity, eliminate the potential for cytopathic side effects, and limit escape from replication-silencing activity. To further prevent potential problems associated with viral escape, a rational combinatorial approach could be utilized, incorporating polymerase fidelity into viruses carrying multiple miRNA target sequences. Thus, our approach may be complementary to a recently described method for vaccine design that exploits the observation that increasing replication fidelity attenuates pathogenicity (Vignuzzi et al., 2008). Together, these strategies could effectively increase genetic stability without compromising replication robustness, and bestow additional safeguards upon these virus vaccines to improve current vaccines. However, obtaining high-fidelity variants may not be straightforward for all viruses. Therefore, our new miRNA-based approach provides a more flexible, general alternative for rationally designing attenuated live vaccines, which may allow the rapid production of vaccines in case of emerging viral threat.
While our work has focused on miRNA-mediated attenuation of live vaccines, this technology is likely to be applicable to other viral-based therapeutics. For example, the insertion of a broadly expressed target sequence, such as let-7a, into a pathogenic virus may provide an additional safeguard against accidental infection when large amounts of virus are produced for inactivated vaccines. Similarly, incorporating miRNA target sequences into gene therapy viral vectors or oncolytic viruses may improve their safety and minimize off-target effects. Therefore, further development of this technology may have broad implication for the fine-tuning of cancer, gene, and vaccine therapies.
Methods
Molecular biology and viruses
Plasmid prib(+)XpAlong (Herold and Andino, 2001), wild type poliovirus type 1 Mahoney cDNA, was engineered to contain unique restriction sites in the 5’-UTR (BssHII and SacI) and 2A site (EcoRI and XhoI) to facilitate cloning the miRNA target sequences into the plasmid. Complementary oligos (Elim Biopharmaceuticals, Hayward, CA) were annealed in 40mM Tris pH 7.5, 20mM MgCl2, and 50mM NaCl for two minutes at 90 °C and allowed to slowly cool to room temperature. To construct the viruses, we inserted EcoRI and XhoI restriction site-flanked fragments into the cDNA of the poliovirus genome at the 2A position. Positive clones were subsequently used to insert a second BssHII and SacI restriction site-flanked fragment into the cDNA in the 5’-UTR. The DNA sequence for the PV-L7 oligos at the 2A and 5’-UTR sites are 5’-AATTCAACTATACAACCTACTACCTCAGTCGAC-3’ and 5’-TCGAGTCGACTGAGGTAGTAGGTTGTATAGTTG-3’, 5’- CGCGCGGCCGAACTATACAACCTACTACCTCAGAGCT-3’ and 5’- CTGAGGTAGTAGGTTGTATAGTTCGGCCG-3’, respectively. The DNA sequence for the PV-L7M oligos at the 2A and 5’-UTR sites are 5’-AATTCAATTACACGACTTATTATTTGAGTCGAC-3’ and 5’-TCGAGTCGACTCAAATAATAAGTCGTGTAATTG-3’, 5’-CGCGCGGCCGAATTACACGACTTATTATTTGAGAGCT-3’ and 5’- CTCAAATAATAAGTCGTGTAATTCGGCCG-3’, respectively. The DNA sequence for the PV-124 oligos at the 2A and 5’-UTR sites are 5’-AATTCGTCGACAGTGGCATTCACCGCGTGCCTTAAC-3’ and 5’-TCGAGTTAAGGCACGCGGTGAATGCCACTGTCGACG-3’, 5’-CGCGCCGGCCGAGTGGCATTCACCGCGTGCCTTAAGAGCT-3’ and 5’-CTTAAGGCACGCGGTGAATGCCACTCGGCCGG-3’, respectively. The DNA sequence for the PV-124M oligos at the 2A and 5’-UTR sites are 5’-AATTCGTCGACTGTAGCGTTAACTGCATGGCGCAGC-3’ and 5’-TCGAGCTGCGCCATGCAGTTAACGCTACAGTCGACG-3’, 5’-CGCGCCGGCCGTGTAGCGTTAACTGCATGGCGCAGGAGCT-3’ and 5’-CCTGCGCCATGCAGTTAACGCTACACGGCCGG-3’, respectively. The DNA sequence for the control viruses (PV-L7M and PV-124M) were generated by altering the codon sequence according to the poliovirus amino acid usage table to conserve the correct amino acid. We produced virus stocks by electroporation of 20 µg in vitro transcribed RNA, generated from the engineered plasmids above, into 800 µl NTERA-2 cells (ATCC HTB-106, 5×106 cells/ml) in a 4 mm cuvette with the following pulse: 300 V, 24 Ω, and 500 µF. The progeny were passaged twice at a high multiplicity of infection (MOI=10) at 37 °C in NTERA-2 cells and this latter passage virus was used for the described experiments. RT-PCR of viral RNA as previously described (Vignuzzi et al., 2006) was performed to confirm that the insertion sequences were unaltered in the viral stocks.
Northern Blot analysis
Total RNA from cells (HeLa and NTERA-2) and tissues (brain, spleen, kidney, muscle, and spinal cord), from 8-week-old cPVR mice, was isolated using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s suggested protocol. Electrophoresis of twenty micrograms total RNA was resolved in 12% acrylamide-urea denaturing gels at 400 V for 1 hour, followed by 500 V until the bromophenol blue dye reached the bottom of the gel. RNA was subsequently transferred onto Hybond N+ membranes (Amersham) for 2 hours at 225 milliamps using a Semi-Dry Transfer Cell (Bio-Rad). The membranes were cross-linked at 1,200 × 100 µJoules and dried overnight. DNA oligos (ELIM Biopharmaceuticals) complementary to let-7a (AACTATACAACCTACTACCTCA), miR-124a (TGGCATTCACCGCGTGCCTTAA), and U6 (TGGAACGCTTCACGATTTTG) were end-labeled with γ-32P by T4 kinase (NEB) and g-25 spin columns (Amersham) were used to remove unincorporated nucleotides. The probes were hybridized to membranes in ULTRAhyb-oligo buffer (Ambion) overnight at 37 °C. The membranes were washed twice with 2x SSC/0.1% SDS at 37 °C for 30 minutes. Images were obtained using a Typhoon Variable Mode Imager (GE Healthcare).
One-step growth curve studies
These assays characterizing virus growth in tissue culture have been previously described (Vignuzzi et al., 2006). The data presented here represents three independent experiments for each virus at each time point (error bars are included). Cell lines used in these studies include HeLa S3 (ATCC CCL-2.2), grown in suspension at 37 °C in SMEM (Lonza) media supplemented with 10% fetal bovine serum (Sigma), and NTERA-2 (ATCC HTB-106), grown adherently at 37 °C in DMEM/High glucose media supplemented with 10% fetal bovine serum (Sigma). Standard plaque assays to measure PV-L7 viral replication were performed on NTERA-2 monolayers, and all other viruses were titered on HeLa cells. Slight modifications were made to the protocol when studying viral growth in cells that were transfected with Ambion (Foster City, CA) anti-miR miRNA inhibitors or Dharmacon (Lafayette, CO) siRNAs. In both cases, 2.5×105 cells were seeded in wells of a 12-well plate and transfected with 30 pmol anti-miR inhibitor specific for let-7a, 30 pmol anti-miR control inhibitor, or 20 pmol Dharmacon siRNA according to the Lipofectamine 2000 (Invitrogen, Carlsbad, CA) manufacturer’s suggested protocol for siRNA transfections. The media was changed after 12 hours and viral infections were carried out 24 hours later. The siRNA sense and antisense sequences used to target the human Ago2 homologue, eIF2C2, are GGAAAAUGAUGCUGAAUAUUU and AUAUUCAGCAUCAUUUUCCUU, respectively. The siRNA sense and antisense sequences used to target the dsRNA-specific RNase-III-type endonuclease, Drosha, are GAGUAGGCUUCGUGACUUAUU and UAAGUCACGAAGCCUACUCUU, respectively. A GAPDH siRNA from Dharmacon was used as a control in these experiments. The oligonucleotides were converted to the 2’-hydroxyl, annealed, and desalted prior to receiving the duplex siRNAs.
RT-PCR of viral RNA from infected cells
The experiments described here are slightly modified from previously published data (Vignuzzi et al., 2006). Each well of a 6-well plate was seeded with 1 × 106 HeLa cells and infected the following day with wildtype, PV-L7, or PV-L7M virus at a multiplicity of infection of 10 (MOI=10). The infections were performed similar to the one-step growth curves described above with a slight modification. Virus absorption was carried out at 4 °C to allow receptor binding while preventing cellular internalization. The cells were washed twice with cold PBS, and warm DMEM/High glucose media supplemented with 10% fetal bovine serum (Sigma) was subsequently added. Trizol (Invitrogen, Ca) extraction of total RNA from the adherent cells was carried out according to the manufacturer’s suggested protocol at various times following infection. A Thermoscript™ RT-PCR kit (Invitrogen, Ca) was used to amplify a 269nt fragment, spanning the 5’ target insertion site, from 200ng total RNA. The forward and reverse primer sequences for the PCR reaction are GGCTGCTTATGGTGACAATCACAG and GTGGTGTAATTAATGGTAGAACCACC, respectively. The PCR reaction was cycled twenty-five times and the fragments were resolved on a 2% agarose gel.
Infection of susceptible mice
The University of California, San Francisco Institutional Animal Care and Use Committee (IACUC) approved the protocols for the mouse studies described here. In these experiments, we used 6–8-week-old cPVR and IFNAR transgenic mice expressing the poliovirus receptor. The following inoculations were performed while the mice were under anesthesia: intramuscular (2 × 50 µl, one in each quadriceps), intraperitoneal (250 µl), and intravenous (100 µl, tail vein). Mice were monitored daily for the onset of paralysis and euthanized when total paralysis was imminent. In order to determine the LD50 values according to the Reed and Muench method, 20 mice were infected intramuscularly with serial dilutions of virus. To analyze viral tissue tropism, we harvested whole organs from five mice in each group that had been infected intravenously, and homogenized the organs in 2mls PBS with a T1 Basic S1 disperser from Ika Works, Inc. (Wilmington, NC). The tissue homogenates were clarified and titered on HeLa cells by standard plaque assay. Neutralization assays were performed with sera collected from five mice (from a group of 20 cPVR mice) one month after receiving intraperitoneal immunizations with PBS, or 1×107 PFU UV-inactivated wild type, PV-L7, or PV-124 virus. One hundred times the 50% tissue culture infectious dose (TCID50) of wild type poliovirus was treated for 2 hours with serial dilutions of sera (performed in octuplicate). The serial dilution was determined that completely neutralized 100TCID50 by incubating samples on 1×104 HeLa cells (DMEM, 2% final FBS concentration) in 96-well plates for one week. Using the Reed and Muench method, the neutralizing antibody dilution was determined. The reciprocal of this dilution is the neutralizing antibody titer that is represented in the graph. Following sera collection, the groups of twenty mice (or group of 10 IFNAR mice) were challenged intramuscularly with 10LD50 (and 104LD50 in immunized IFNAR mice) wild type poliovirus and subsequently monitored for paralysis.
Acknowledgments
We thank members of the Andino Laboratory for advice, discussion and technical support for this work and Judith Frydman, Michel Tassetto, Michelle Flenniken, Jeremy Jones, and Adam Lauring for discussions and comments on the manuscript. D.B. is an Abbott Fellow of the Life Sciences Research Foundation. This work was financially supported by NIH grants AI36178 and AI064738 awarded to R.A. and grants from the Academy of Finland, the Sigrid Juselius Foundation, and the Helsinki University Central Hospital to K.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We describe a general strategy for rationally engineering live attenuated virus vaccines. Our approach takes advantage of the tissue-specific expression of miRNAs to control virus replication in tissues where the virus causes disease. Using poliovirus as a model, we demonstrate that insertion of small miRNA homology sequences into a viral genome elicits an RNAi-based response against the virus that restricts its tissue tropism, thereby preventing pathogenicity and yielding an attenuated viral strain. A unique and exciting advantage of our method to rationally engineer live vaccines is its versatility, which may be applicable to a wide variety of viruses. The ability to generate replication-competent recombinant viruses with this technology may have widespread implications in cancer, gene, and vaccine therapies.
References
- Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev Cell. 2003;4:625–637. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Andino R, Boddeker N, Silvera D, Gamarnik AV. Intracellular determinants of picornavirus replication. Trends Microbiol. 1999;7:76–82. doi: 10.1016/s0966-842x(98)01446-2. [DOI] [PubMed] [Google Scholar]
- Arnold JJ, Vignuzzi M, Stone JK, Andino R, Cameron CE. Remote site control of an active site fidelity checkpoint in a viral RNA-dependent RNA polymerase. J Biol Chem. 2005;280:25706–25716. doi: 10.1074/jbc.M503444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham Barney S, James E Crowe J. Immunization against viral diseases. Vol Volume 1. Philadelphia: Lippincott Williams and Wilkins, a Wolters Kluwer Business; 2007. [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Human immunodeficiency virus type 1 escape from RNA interference. J Virol. 2003;77:11531–11535. doi: 10.1128/JVI.77.21.11531-11535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, Baccarini A, Lazzari G, Galli C, Naldini L. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol. 2007;25:1457–1467. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, U. S. Impact of vaccines universally recommended for children--United States, 1900–1998. Jama. 1999;281:1482–1483. [PubMed] [Google Scholar]
- Cullen BR. Viruses and microRNAs. Nat Genet. 2006;38 Suppl:S25–S30. doi: 10.1038/ng1793. [DOI] [PubMed] [Google Scholar]
- Das AT, Brummelkamp TR, Westerhout EM, Vink M, Madiredjo M, Bernards R, Berkhout B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J Virol. 2004;78:2601–2605. doi: 10.1128/JVI.78.5.2601-2605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC. A let-7 MicroRNA-sensitive Vesicular Stomatitis Virus Demonstrates Tumor-specific Replication. Mol Ther. 2008 doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- Forstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287–297. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L, Karelsky S, Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature. 2002;418:430–434. doi: 10.1038/nature00873. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Stone JK, Andino R. Poliovirus escape from RNA interference: short interfering RNA-target recognition and implications for therapeutic approaches. J Virol. 2005;79:1027–1035. doi: 10.1128/JVI.79.2.1027-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Normann A, Flehmig B. Influence of the 5' noncoding region of hepatitis A virus strain GBM on its growth in different cell lines. J Gen Virol. 1997;78(Pt 8):1841–1849. doi: 10.1099/0022-1317-78-8-1841. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Ida-Hosonuma M, Iwasaki T, Yoshikawa T, Nagata N, Sato Y, Sata T, Yoneyama M, Fujita T, Taya C, Yonekawa H, Koike S. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol. 2005;79:4460–4469. doi: 10.1128/JVI.79.7.4460-4469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. Embo J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev Cell. 2003;4:639–650. doi: 10.1016/s1534-5807(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pfeiffer JK, Kirkegaard K. Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS Pathog. 2005;1:e11. doi: 10.1371/journal.ppat.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vargas I, Travanty EA, Keene KM, Franz AW, Beaty BJ, Blair CD, Olson KE. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 2004;102:65–74. doi: 10.1016/j.virusres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Tang S, van Rij R, Silvera D, Andino R. Toward a poliovirus-based simian immunodeficiency virus vaccine: correlation between genetic stability and immunogenicity. J Virol. 1997;71:7841–7850. doi: 10.1128/jvi.71.10.7841-7850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij RP, Andino R. The silent treatment: RNAi as a defense against virus infection in mammals. Trends Biotechnol. 2006;24:186–193. doi: 10.1016/j.tibtech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignuzzi M, Wendt E, Andino R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med. 2008;14:154–161. doi: 10.1038/nm1726. [DOI] [PubMed] [Google Scholar]
- Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet. 2005;6:206–220. doi: 10.1038/nrg1555. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Richardson CD. Hepatitis C virus replicons escape RNA interference induced by a short interfering RNA directed against the NS5b coding region. J Virol. 2005;79:7050–7058. doi: 10.1128/JVI.79.11.7050-7058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DJ, Macadam AJ. Laboratory tests for live attenuated poliovirus vaccines. Biologicals. 1997;25:3–15. doi: 10.1006/biol.1997.0055. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]