Introduction
Assisted living (AL) facilities currently provide care to approximately 1 million people in the United States, and that number is expected to rise considerably over the coming decades.1 Although the provision of social and supportive services are central components of AL,2,3 the ability to safely manage the medical illnesses of residents has been identified as a priority.1,4 Indeed, “health care management and monitoring” is listed by the federal Administration on Aging as a core service offered by most ALs 5 and “increased medical needs” was the primary reason for moving into AL for 24% of residents in a previous report using this data.6 However, it has been suggested that some residents with multiple, complex medical conditions present a challenge that some ALs may not be prepared to manage.7 In addition, some authors have raised concerns about appropriate prescribing of medications for residents in AL.8–10 In response to these issues, both the American Geriatrics Society (AGS) and the American Medical Directors’ Association (AMDA) have issued position papers which emphasize the physician’s role in choosing a facility, as well as directing the care within AL.4, 11
In addition to concerns for patient safety, the scope and severity of medical illnesses and treatments may affect the resident’s ability to continue living in AL due to increased care needs. The ability to “age in place” has been emphasized as a priority to residents of assisted living.12, 13 The stability of medical illness over time, as well as the ability of staff to accommodate fluctuations in medical status, may play an important role in the ability to “age in place” 12, 14, 15 and it has been recommended that residents who require monitoring for “unstable medical conditions” are not appropriate for the AL setting.13 Furthermore, AL residents may have multiple chronic conditions which can be challenging to manage. Among community-dwelling older adults, the risk of hospitalization increases with the number of chronic conditions16 and the adherence to multiple Clinical Practice Guidelines within the same patient may have undesirable effects.17, 18 All of these factors underscore the potential challenge of providing appropriate care to AL residents with varying degrees of medical complexity.
Potential differences in the provision of care among AL facilities are an important consideration in the management of medical illnesses. ALs differ by size 3, 6, 19, 20, 21 staffing ratios,12 presence of staff with nursing degrees,15 “for profit” status,3 and age of facility.3 Each of these might affect the types of residents that are attracted to the facility, as well as the ability to provide the necessary care associated with chronic medical illness. The “mom-and-pop” atmosphere that has described many small facilities21 contrasts with larger, “purpose-built” facilities and could potentially affect the availability of ancillary medical services. While smaller facilities are more likely to serve residents with dementia and other mental health conditions compared to larger ones,22 it has not been well established whether or not the extent of chronic medical illness among the AL resident population differs according to size of facility or other provision of care measures. Because clinicians are potentially involved in the decision-making process in choosing an assisted living facility,23 it is important for them to understand the spectrum of medical care required by residents of different types of AL facilities.
The primary aim of this study was to categorize and quantify chronic medical conditions as well as treatments among AL residents, specifically identifying the coexistence of multiple conditions. A secondary aim was to identify any association between facility-level characteristics and those residents having a greater numbers of conditions. By evaluating a large number of residents from a range of facilities caring for a diverse population of older adults in central Maryland, these data will serve as an important source from which to propose quality of care evaluations and interventions for chronic medical illnesses in assisted living facilities.
Methods
Study Design
The Maryland Assisted Living Study, Phase I (MD-AL I) was designed primarily to evaluate the detection and management of dementia and non-dementia psychiatric illnesses; those particular results have been previously reported.22 Started in 2002, this study utilized a cross-sectional design to evaluate 198 residents from 22 randomly selected AL facilities in Central Maryland; 150 of these residents were selected from 10 “large” facilities (more than 15 beds) and 48 from 12 “small” facilities (15 or fewer beds). These residents were randomly selected from the participating facilities. Therefore, residents with dementia were not recruited preferentially and were included in this study at a rate that reflects the true prevalence of that condition in these randomly selected AL facilities.
For the study reported here, we analyzed resident and facility information that was collected during in-person interviews with residents, their family members and facility caregivers, as well as by AL chart review. Because we did not have access to records from physicians’ offices, hospitals or other types of facilities, we were not able to collect that information and it is not part of this data set. However, in Maryland, AL regulations require that all active medical conditions and treatments be confirmed and signed by a medical practitioner.
General Resident Information
From interviews with the residents, his/her family informants, and the residents’ AL charts, we extracted demographic information, including age, sex, marital status, race, Medicaid status, length of stay (L.O.S.) in AL, and “Do Not Resuscitate” (D.N.R.) status. Also, the ability to self-administer medications was recorded, as determined by the respective facility staff and documented in the AL record.
Facility-Level Information
To better evaluate certain aspects of providing care within AL, we identified the following characteristics of the facility for each resident: number of beds (capacity), staffing (number of residents at facility per staff member providing any form of care to residents), state-certified A.L.“level of care” status (1=lowest, 3=highest), cost per month (for that resident), locale of facility (urban, suburban, rural), years in operation, for-profit status, physician on-site visits (whether or not personal physician was noted to make visits to resident at facility).
Medical Illnesses
For each resident, we categorized all medical diagnoses that were listed in the AL medical chart according to 8 “disease groups”: 1) cardiovascular/hypertension (hypertension, CHF, coronary artery disease (CAD), arrhythmia, valvular disease, aortic aneurysm, peripheral vascular disease, 2) pulmonary (COPD), 3) central nervous system (CNS)/sensory (history of cerebrovascular accident (CVA), seizure disorder, Parkinson’s disease, normal pressure hydrocephalus), 4) endocrine (adrenal insufficiency, DM, hyper-and hypo-thyroidism, hyperlipidemia, hyperparathyroidism), 5) rheumatology/orthopedics (arthritis, osteoporosis, hip fracture, gout, spinal stenosis, osteomyelitis), 6) gastrointestinal (gastroesophageal reflux disease, peptic ulcer disease, irritable bowel syndrome, colon polyps), 7) hematology/oncology (anemia, breast, colon, lung, prostate and “other” cancers), 8) renal/urological (chronic renal failure, bladder dysfunction). We did not categorize dementia or other psychiatric illnesses, as those data have already been reported.22 We further characterized the complexity of disease management by quantifying the number of conditions from each disease group within each individual (none, 1, 2, 3 or more).
In addition to evaluations of these broader disease groups, we evaluated the specific prevalence rates of seven chronic medical conditions for which AMDA has created clinical practice guidelines (CPG) to be used in long-term care settings. These “CPG” conditions were specifically chosen and evaluated separately due to the complexity of their management. 24, 25 When evaluating the implementation of non-AMDA CPGs, it has been described as a particular concern when more than one of these diagnoses are present within one individual.17, 18 We further characterized the complexity of managing these conditions by quantifying the number of CPG conditions within each individual (0, 1, 2 or more).
Medications and Treatments
We categorized all medications that were “routinely” prescribed (i.e., not “as needed”), according to the same 8 “disease groups”, plus 2 additional groups: analgesics, vitamins.
The medications according to 8 “disease groups” included: 1) cardiovascular/hypertension (angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists, anticoagulants, anti-platelet agents, aspirin, digoxin, anti-arrhythmics, beta blockers, calcium channel blockers, diuretics, nitroglycerin, “other” anti-hypertensives), 2) pulmonary (COPD treatment), 3) central nervous system (CNS)/sensory (anti-Parkinson medications, anti-convulsants, glaucoma treatment), 4) endocrine (antidiabetic treatments, hormone treatment, lipid-lowering agents, calcium, vitamin D, prednisone, calcitonin, bisphosphonates), 5) rheumatology/orthopedics (non-steroidal anti-inflammatory agents), 6) gastrointestinal (histamine-2 antagonists, proton pump inhibitors), 7) hematology/oncology (cancer treatments), 8) renal/urological (bladder/incontinence medications), 9) analgesia (opiates, acetaminophen), 10) vitamins, Because it was difficult to determine the indication for prescribing in some situations, some medications were categorized in more than one group.
We created 2 special treatment categories to better describe the scope of medical care that can be required by AL residents: “Non-Oral Modes of Administration” (insulin injections, respiratory treatments, eye drops) and medications that “Require Additional Monitoring” (use of warfarin, digoxin, or prednisone; use of any diabetes treatment, any cancer treatment, any anti-seizure treatment, or any thyroid treatment).
Analysis
Descriptive statistics were used to describe the distribution of the sample characteristics. Logistic regression was performed to evaluate the odds that residents having 3 or more conditions from different organ system disease groups as well as the odds of having 2 or more CPG conditions, according to the specified facility-level provision of care measures These cut-points were chosen after analyzing the distribution of residents according to the number of conditions, representing the most complex half and quarter of the population, respectively. We utilized SPSS 14.0 software to perform analyses (SPSS Inc., Chicago, IL).
Human Subjects
Informed consent was obtained from all study participants in accordance with the study consent protocol and was reviewed and approved by the Johns Hopkins University School of Medicine Institutional Review Board. In cases of more severe cognitive impairment, either written or verbal assent was obtained from the participant and written consent was obtained from the participant’s legal representative.
Results
Table 1 describes the 198 AL residents (“Resident Characteristics”) and the 22 AL facilities (“Facility-level Characteristics”) included in this study. The average length of stay (L.O.S.) in AL was 2.1 years. Excluding those medications for dementia and psychiatric illnesses, residents were taking an average of 4.5 medications and these were administered by AL staff for most (87.4%) residents.
Table 1.
Resident and facility characteristics
| Variables | Mean (SD) or % of total |
|---|---|
| Resident Characteristics (n=198 residents) | |
| Age (years) | 85.7 (8.2) |
| Female | 78.8 |
| Widow | 70.7 |
| Caucasian | 82.8 |
| “Do Not Resucitate” status | 32 |
| Length of Stay at AL (years) | 2.1 (1.9) |
| Medicaid recipient | 4.5 |
| Facility-level Characteristics (n=22 facilities) | |
| Number of residents (bed capacity) | 29.1 (31.5) |
| Number residents/staff (day shift)a | 5.84 (4.52) |
| Number resident/staff (night shift)b,c | 12.10 (11.0) |
| R.N. or L.P.N. on regular staff | 54.5 |
| Locale | |
| Urban | 45.5 |
| Suburban | 45.5 |
| Rural | 9 |
| Years in operation | 10.98 (8.16) |
| For-profit | 72.7 |
| Physician on-site visit capability | 45.4 |
| Monthly cost ($) | 2883 (1357) |
| Certification level (1-lowest, 3-highest) | 1.46 (0.61) |
Abbreviations: R.N.= Registered Nurse; L.P.N.= Licensed Practical Nurse
Day shift defined as 7 am – 3 pm
Night shift defined as 11 pm – 7 am
2 small facilities did not have awake night staff and were excluded
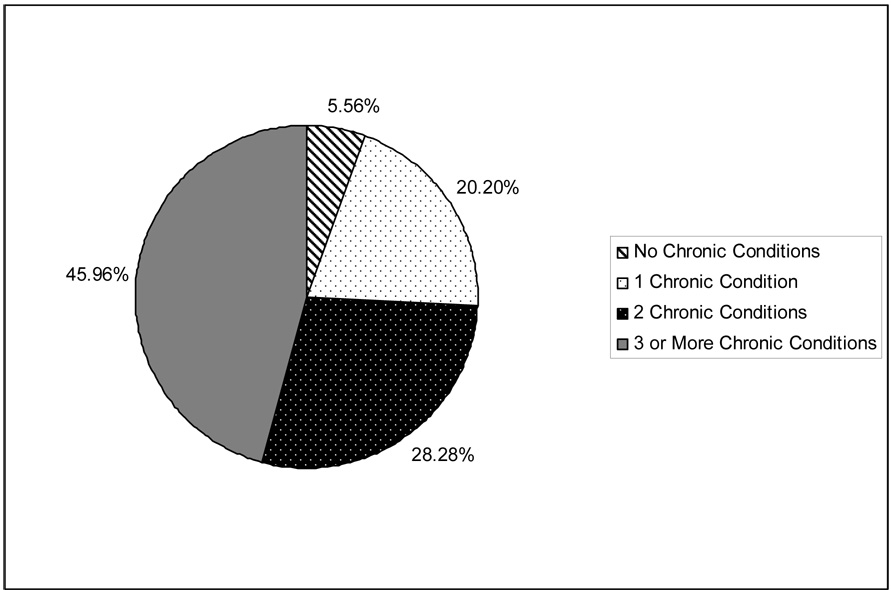
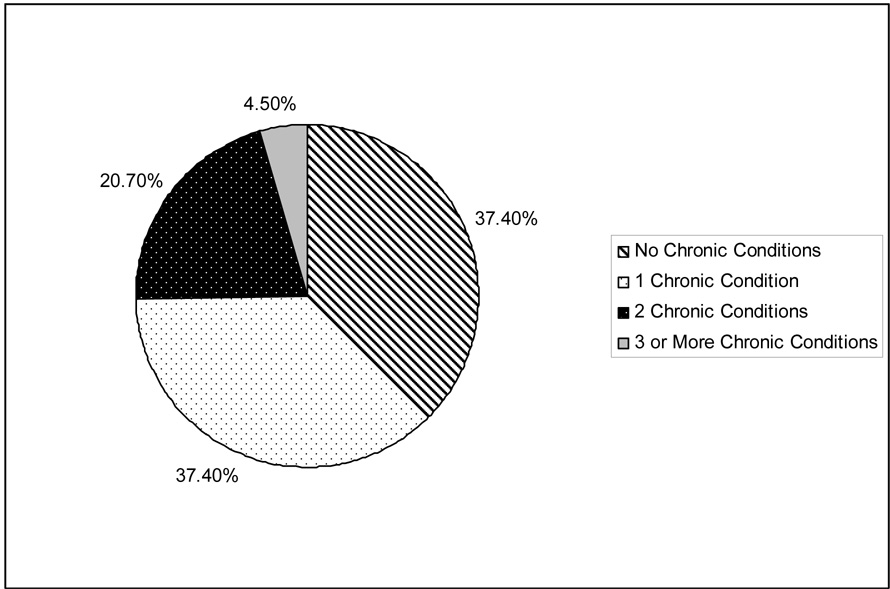
Table 2 summarizes the prevalence rates for having a condition in the 8 general disease categories, as well as each specific CPG condition. Figure 1 shows the distribution of residents according to the number of different general disease categories from within which the resident had at least one condition. Figure 2 shows the distribution of residents according to the number of specific CPG conditions.
Table 2.
Prevalence Rates of Chronic Medical Conditions Among AL Residents
| General Disease Categories | Frequency (%) |
|---|---|
| Cardiovascular/HTN | 143 (72.2%) |
| Pulmonary | 16 (8.1%) |
| CNS/Sensory | 74 (37.4%) |
| Rheumatologic/Orthopedic | 103 (52.0%) |
| Endocrine | 76 (38.4%) |
| Gastrointestinal | 25 (12.6%) |
| Hematologic/Oncologic | 15 (7.6%) |
| Renal/Urologic | 10 (5.1%) |
| Specific Clinical Practice Guidelines (CPG) conditions | |
| Anemia | 7 (3.5%) |
| History of CVA | 36 (18.2%) |
| Congestive Heart Failure | 30 (15.2%) |
| Chronic Obstructive Pulmonary Disease | 16 (8.1%) |
| Diabetes | 36 (18.2%) |
| Osteoporosis | 51 (25.8%) |
| Parkinson’s Disease | 10 (5.1%) |
Figure 1.
Number of Different General Disease Categories for which Resident Has At Least One Condition
Figure 2.
Number of Specific Clinical Practice Guideline (CPG) Diagnoses
Residents with conditions in 3 or more different disease categories were more likely to be residing in larger facilities than residents with conditions in 2 or fewer disease categories (O.R. 1.01 per 1 bed increase in facility size, C.I. 1.00–1.20). However, there were no differences between the 2 groups with regard to: number of residents per day staff, having a licensed nurse on staff, facility level-of-care certification, resident cost per month, age of facility, need for help with medications, or having a physician who made visits to the facility. When comparing residents with 2 or more CPG conditions to those residents with none or one CPG condition, no differences in any of these measures were noted, including the size of facility (number of beds).
Table 3 summarizes the prevalence rates of medications use within the 8 disease categories, as well as vitamins and analgesics. In addition to these routinely administered oral medications, the treatments that were administered by other routes (e.g., injections) or that typically require additional monitoring (e.g., blood tests) are shown in Table 4. One-fourth (25.3%) of residents were receiving one of the medications by “non-oral” modes of administration, and one-half (49.5%) were receiving at least one medication that typically requires additional monitoring.
Table 3.
Prevalence Rates of Medications by Category
| Frequency (%) | |
|---|---|
| Cardiovascular/HTN | 149 (75.3%) |
| Pulmonary | 20 (10.1%) |
| CNS/Sensory | 58 (29.3%) |
| Rheumatologic/Orthopedic | 25 (12.6%) |
| Endocrine | 111 (56.1%) |
| Gastrointestinal | 47 (23.7%) |
| Hematologic/Oncologic | 7 (3.5%) |
| Renal/Urologic | 16 (8.1%) |
| Analgesia | 27 (13.6%) |
| Vitamins | 66 (33.3%) |
Table 4.
Prevalence Rates of Medications with Non-Oral Modes of Administration or that Require Additional Monitoring
| Non-Oral Modes of Administration | Frequency (%) |
|---|---|
| Insulin Injections | 11 (5.6%) |
| Eye Drops | 26 (13.1%) |
| Inhaler/Nebulizer Therapy | 19 (9.6%) |
| Any of the above | 50 (25.3%) |
| Additional Monitoring Required | |
| Diabetes therapy | 29 (14.6%) |
| Cancer therapy | 7 (3.5%) |
| Prednisone | 3 (1.5%) |
| Seizure Disorder | 24 (12.1%) |
| Digoxin | 27 (13.6%) |
| Thyroid | 32 (16.2%) |
| Warfarin Therapy | 15 (7.6%) |
| Any of the above | 98 (49.5%) |
Table 5 reveals the percentage of AL residents that were receiving daily pharmacological treatment and had a chart diagnosis that was categorized into the corresponding general medical disease category. Overall, this ranged from 10% of AL residents with a diagnosis of a renal/urologic condition to 89.5% of AL residents with a diagnosis of a cardiovascular/HTN condition who were receiving a treatment from within that respective disease treatment category.
Table 5.
Prevalence Rates of Residents that Were Receiving Daily Pharmacological Treatment who also had a Chart Diagnosis that was Categorized into the Corresponding Medical Disease Category
| Frequency (%) | |
|---|---|
| Cardiovascular/HTN (n=143) | 128 (89.5 %) |
| Pulmonary (n=16) | 9 (56.3%) |
| CNS/Sensory (n=74) | 51 (68.9%) |
| Rheumatologic/Orthopedic (n=103) | 20 (19.4%) |
| Endocrine (n=76) | 66 (86.8%) |
| Gastrointestinal (n=25) | 14 (56.0%) |
| Hematologic/Oncologic (n=15) | 5 (33.3%) |
| Renal/Urologic (n=10) | 1 (10.0%) |
Discussion
This study reveals that AL residents often have multiple chronic medical conditions, including many for which long-term care CPGs have been created. In addition to the expectation that AL staff play a role in monitoring the stability of these conditions and appropriately communicating with medical providers, AL staff also administers the various forms of treatment prescribed for these conditions to the vast majority of residents. One-half of residents were receiving treatments that typically require additional monitoring to ensure safety and one-fourth of residents were receiving respiratory inhalers, injections and/or eye drops in addition to oral medications. All of these factors reveal the heightened responsibility that is placed on AL staff with regard to the medical care and oversight required by many residents.
Concerns about the implementation of multiple Clinical Practice Guidelines in older adult have been raised.17, 18 In a study evaluating the impact of adhering to non-AMDA CPGs among a nationally representative sample of Medicare beneficiaries, Boyd et al. showed that a considerable burden of increased medication quantity and cost, as well as non-pharmacological care may result when multiple CPG conditions are present.17 Of note, a similar study evaluating the impact of adhering to AMDA CPGs has not been done, and it is not known whether this would yield different results. It has also been noted that medication under-treatment within AL is prevalent for conditions including CHF, CAD, history of CVA and osteoporosis.10 Noting that one-fourth of residents in our study had 2 or more CPG conditions, these studies underscore the challenge facing AL staff and medical providers as to the best approach regarding management of medically complex residents.
Although there is a considerable literature on the characteristics of AL residents (including cognitive and functional impairment, as well as specific care requirements),2, 3, 15, 26 information on the prevalence rates of specific chronic medical conditions among AL residents is limited. A recent Veterans Affairs (VA) study evaluating the characteristics of AL residents found the prevalence of DM to be 26.4% (higher than the 18.2% reported in our study).27 It is possible that the male predominance (98.1%) and other health characteristics explain part of that difference. In another study of AL residents, Zimmerman, et al. found that between 38 and 49% had a “heart condition” (which did not include HTN) - lower than the rate of cardiovascular disease among our population (72%), which included HTN.3
In a study of the nursing home population, similar rates were seen of the following conditions (compared to the AL residents in this study): diabetes (17.7% vs. 18.2%) and history of CVA (18.8% vs. 18.2%).28 However, NH residents had lower rates of osteoporosis (7.8% vs. 25.8%) and higher rates of COPD (13.9% vs. 8.1%).28 On the other hand, reports describing community-dwelling older adults have revealed lower rates of the following conditions (compared to the AL residents in this study): CHF (8% vs.15.2%)29 and DM (13% vs. 18.2%);30 and higher rates of COPD (10% vs. 8.1%).31 Among home health care patients (all ages) in 2000, the primary diagnosis was listed as diabetes in 7.9%, CVA in 7.3% and CHF in 3.9%.32
Certain chronic conditions pose particular management challenges within AL. The leading cause for hospitalization among all older adults in 2004 was CHF, with COPD the 8th leading cause.33 Among our sample of AL residents, these 2 conditions were relatively common; CHF was present in 15.2% and COPD 8.1%. The risk of hospitalization with these conditions reflects the complexity and potential instability of these residents. Furthermore, hospitalization also potentially threatens the ability of these residents to “age-in-place”. One study revealed that discharge from AL to NH is more likely to occur in residents that were hospitalized in the prior 6 months14 and, in another study, “requiring a hospital stay” was the “destination” for 18% of residents leaving AL.15
The average number of medicines for AL residents has been reported between 5.8 9 and 6.8.8 Because our study was focusing on medical illness and treatment, we excluded medications for dementia and psychiatric illnesses in our analysis which likely explains the lower number of medications reported. Most (87.4%) residents received help with their medication and other treatments from AL staff, which is similar to the rate noted by Hedrick, et al. (84%).27 In addition to the number of medications received, we specifically evaluated aspects of medication delivery that could be burdensome to AL staff. These included care beyond dispensing medications orally. One-fourth of residents required these types of treatments, which adds to the complexity of the treatment routine. We also discovered that 50% of AL residents were taking medications that typically require additional monitoring to ensure safety. In particular, warfarin was prescribed for 7.6% of all residents in this study and has been associated with significant side effects. An A.M.D.A. survey identified warfarin as being involved in 5 of the 10 most concerning drug interactions in long-term care.34 This information underscores the importance of having reliable and accessible laboratory services within AL, as well as a system to communicate results to the physician.1, 23 The challenges of medication management and the risk of medication errors in long-term care settings (including assisted living) have been recognized by A.M.D.A., which led to its development of a multi-disciplinary medication management tool.35
Certain facility-level characteristics may potentially affect the ability to manage the medical illnesses of some residents. Among these, we noted that residents with more complex medical issues (conditions in 3 or more different disease categories) were more likely to live in larger facilities. It is not clear if this trend was driven by recruitment and the willingness to accept more complex residents, the preferences of older adults with such needs to live in larger facilities or the ability of such facilities to support these residents and allow for aging-in-place. Our study found that direct care staff ranged from an average of 5.8 residents per staff member during the day shift and an average of 12.l residents per staff member during night shift, and that just over half of facilities had a licensed nurse on staff. Other studies have found that 60% of ALs that are free standing and 85% that are part of multilevel facilities have a licensed nurse on staff.2 Of note, in our study sample, residents with more chronic conditions were no more likely than residents with fewer conditions to be living in facilities with more licensed nurses, lower resident-to-staff ratios, or in facilities with higher state-regulated “level of care” certification. Although a preliminary finding, it is possible that this lack of association (with the exception of facility size) represents a disconnect between the medical complexity of residents and some aspects of AL care. Because many (24%) residents enter AL for “increasing medical needs”,6 these expectations of medical management capability must be addressed by facility management with regard to resident recruitment and ongoing care.
Limitations of this study include the relatively small number of subjects as well as its cross-sectional design, which limits the ability to describe any dynamic component of medical treatment within AL. However, we will be able to evaluate this important issue longitudinally in the future, as MD-AL Phase II is an ongoing, prospective study. Our ability to identify all possible chronic medical illnesses was limited to chart review for diagnosis extraction. Therefore, the prevalence values reported here might be underestimations, implying that the actual burden of medical illness may be even more substantial than reported here. In addition, we were not able to determine or record the graded severity of the specific medical illnesses. We also could not determine the specific condition for which a particular medication was prescribed in some cases. Lastly, this study was conducted within central Maryland only, which could affect interpretation of results. Of note, there were 1,248 Maryland AL facilities serving 17,148 residents in 2004 (6th greatest AL bed capacity in the U.S.).36
In conclusion, the prevalence rates of chronic medical illnesses and treatments among AL residents are high. In fact, challenging medical illnesses such as DM and cerebrovascular disease were similar to the NH population. Safe and competent monitoring of medically complex residents with various forms of treatment requirements an immediate and imposing challenge to AL providers. Longitudinal studies of medical illness management within AL should be performed to evaluate which facility and resident characteristics are associated with better outcomes.
Acknowledgments
This research was supported by the National Institutes of Mental Health Grant # R01 MH060626. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stefanacci RG, Podrazik PM. Assisted living facilities: Optimizing outcomes. J Am Geriatr Soc. 2005;53:538–540. doi: 10.1111/j.1532-5415.2005.53189_2.x. [DOI] [PubMed] [Google Scholar]
- 2.Hawes C, Phillips CD, Rose M, Holan S, Sherman M. A national survey of assisted living facilities. Gerontologist. 2003;43:875–882. doi: 10.1093/geront/43.6.875. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman S, Gruber-Baldini AL, Sloane PD, et al. Assisted living and nursing homes: Apples and oranges? Gerontologist. 2003;43(Spec No 2):107–117. doi: 10.1093/geront/43.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 4.AGS Health Care Systems Committee. Assisted living facilities: American geriatrics society position paper. J Am Geriatr Soc. 2005;53:536–537. doi: 10.1111/j.1532-5415.2005.53189_1.x. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services, Administration on Aging. [Accessed October 31, 2007];Assisted living. 2007 Available at www.aoa.gov.
- 6.Leroi I, Samus QM, Rosenblatt A, et al. A comparison of small and large assisted living facilities for the diagnosis and care of dementia: The maryland assisted living study. Int J Geriatr Psychiatry. 2007;22:224–232. doi: 10.1002/gps.1665. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SL, Zimmerman S, Eckert JK, Zimmerman S, Sloane PD, Eckert JK, editors. Assisted Living : Needs, Practices, and Policies in Residential Care for the Elderly. Vol 1. Baltimore, MD: The Johns Hopkins University Press; 2001. pp. 224–225.pp. 241 [Google Scholar]
- 8.Gray SL, Hedrick SC, Rhinard EE, et al. Potentially inappropriate medication use in community residential care facilities. Ann Pharmacother. 2003;37:988–993. doi: 10.1345/aph.1C365. [DOI] [PubMed] [Google Scholar]
- 9.Sloane PD, Zimmerman S, Brown LC, Ives TJ, Walsh JF. Inappropriate medication prescribing in residential care/assisted living facilities. J Am Geriatr Soc. 2002;50:1001–1011. doi: 10.1046/j.1532-5415.2002.50253.x. [DOI] [PubMed] [Google Scholar]
- 10.Sloane PD, Gruber-Baldini AL, Zimmerman S, et al. Medication undertreatment in assisted living settings. Arch Intern Med. 2004;164:2031–2037. doi: 10.1001/archinte.164.18.2031. [DOI] [PubMed] [Google Scholar]
- 11.American Medical Directors Association. [Accessed May 11, 2007];Position statement on assisted living. Available at http://www.amda.com/governance/resolutions/d04.cfm.
- 12.Ball MM, Perkins MM, Whittington FJ, et al. Managing decline in assisted living: The key to aging in place. J Gerontol B Psychol Sci Soc Sci. 2004;59:S202–S212. doi: 10.1093/geronb/59.4.s202. [DOI] [PubMed] [Google Scholar]
- 13.Kissam S, Gifford DR, Mor V, Patry G. Admission and continued-stay criteria for assisted living facilities. J Am Geriatr Soc. 2003;51:1651–1654. doi: 10.1046/j.1532-5415.2003.51519.x. [DOI] [PubMed] [Google Scholar]
- 14.Dobbs D, Hayes J, Chapin R, Oslund P. The relationship between psychiatric disorders and the ability to age in place in assisted living. Am J Geriatr Psychiatry. 2006;14:613–620. doi: 10.1097/01.JGP.0000209268.37426.69. [DOI] [PubMed] [Google Scholar]
- 15.Golant SM. Do impaired older persons with health care needs occupy U.S. assisted living facilities? an analysis of six national studies. J Gerontol B Psychol Sci Soc Sci. 2004;59:S68–S79. doi: 10.1093/geronb/59.2.s68. [DOI] [PubMed] [Google Scholar]
- 16.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 17.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 18.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman S, Sloane PD, Eckert JK, et al. How good is assisted living? findings and implications from an outcomes study. J Gerontol B Psychol Sci Soc Sci. 2005;60:S195–S204. doi: 10.1093/geronb/60.4.s195. [DOI] [PubMed] [Google Scholar]
- 20.Park NS, Zimmerman S, Sloane PD, Gruber-Baldini AL, Eckert JK. An empirical typology of residential care/assisted living based on a four-state study. Gerontologist. 2006;46:238–248. doi: 10.1093/geront/46.2.238. [DOI] [PubMed] [Google Scholar]
- 21.Morgan LA, Eckert JK, Gruber-Baldini AL, Zimmerman S. Policy and research issues for small assisted living facilities. J Aging Soc Policy. 2004;16:1–16. doi: 10.1300/J031v16n04_01. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt A, Samus QM, Steele CD, et al. The maryland assisted living study: Prevalence, recognition, and treatment of dementia and other psychiatric disorders in the assisted living population of central maryland. J Am Geriatr Soc. 2004;52:1618–1625. doi: 10.1111/j.1532-5415.2004.52452.x. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher JG. Examining the physician's role with assisted living residents. J Am Med Dir Assoc. 2006;7:377–382. doi: 10.1016/j.jamda.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.American Geriatrics Society Clinical Practice Committee. [Accessed May 14, 2007];AGS practice guidelines. Available at http://www.americangeriatrics.org/education/cp_index.shtml.
- 25.American Medical Directors Association. [Accessed May 14, 2007];Clinical practice guidelines. Available at http://www.amda.com/tools/guidelines.cfm.
- 26.Fonda SJ, Clipp EC, Maddox GL. Patterns in functioning among residents of an affordable assisted living housing facility. Gerontologist. 2002;42:178–187. doi: 10.1093/geront/42.2.178. [DOI] [PubMed] [Google Scholar]
- 27.Hedrick S, Guihan M, Chapko M, et al. Characteristics of residents and providers in the assisted living pilot program. Gerontologist. 2007;47:365–377. doi: 10.1093/geront/47.3.365. [DOI] [PubMed] [Google Scholar]
- 28.Doshi JA, Shaffer T, Briesacher BA. National estimates of medication use in nursing homes: Findings from the 1997 medicare current beneficiary survey and the 1996 medical expenditure survey. J Am Geriatr Soc. 2005;53:438–443. doi: 10.1111/j.1532-5415.2005.53161.x. [DOI] [PubMed] [Google Scholar]
- 29.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the united states. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 30.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. the third national health and nutrition examination survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 31.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--united states, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 32.National Center for Health Statistics. [Accessed November 4, 2007];National home and hospice care data. 2007 Available at http://www.cdc.gov/nchs/data/nhhcsd/curhomecare00.pdf.
- 33.Nagamine M, Jiang JH, Merrill CT. Trends in elderly hospitalizations, 1997–2004. Rockville, MD: Agency for Healthcare Research and Quality; 2006. Statistical Brief #14:1-2-8. [PubMed] [Google Scholar]
- 34.American Medical Directors Association. [Accessed October 31, 2007];Top 10 particularly dangerous drug interactions in long term care. 2007 Available at http://www.amda.com/tools/clinical/m3/topten.cfm.
- 35.American Medical Directors Association. [Accessed October 31, 2007];Clinical corner: Medication management. 2007 Available at http://www.amda.com/tools/clinical/m3/background.cfm.
- 36.Mollica R, Johnson-LaMarche H, editors. State Residential Care and Assisted Living Policy: 2004. 2005. [Google Scholar]