Abstract
The basal ganglia portions of cortico-striato-thalamo-cortical (CSTC) circuits have consistently been implicated in the pathogenesis of Tourette syndrome, whereas motor and sensorimotor cortices in these circuits have been relatively overlooked. Using magnetic resonance imaging, we detected cortical thinning in frontal and parietal lobes in groups of Tourette syndrome children relative to controls. This thinning was most prominent in ventral portions of the sensory and motor homunculi that control the facial, orolingual and laryngeal musculature that is commonly involved in tic symptoms. Correlations of cortical thickness in sensorimotor regions with tic symptoms suggest that these brain regions are important in the pathogenesis of Tourette syndrome.
Tourette syndrome is a genetically based, childhood-onset neuro-developmental disorder that is defined by the presence of vocal and motor tics. Motor tics most commonly affect musculature of the face, neck and shoulders, and vocal tics affect skeletal muscles of the larynx, abdomen and upper respiratory system. Although the neural basis of Tourette syndrome is not yet fully understood, anatomical and functional disturbances in sensorimotor pathways in CSTC circuits are thought to be centrally involved in the pathogenesis of tics1. Neuroimaging studies have demonstrated reduced volumes of basal ganglia nuclei2,3 and enlarged volumes of dorsal prefrontal4 and parietal regions5. Finer-grained morphological analyses of the cortical portions of CSTC circuits have not yet been applied to the study of Tourette syndrome. We predicted that application of these techniques to the study of children with Tourette syndrome would reveal thinning in sensorimotor cortices, the portions of CSTC circuits that control movement and vocalization6, in direct proportion to the severity of tic symptoms.
We measured cortical thickness in high-resolution T1-weighted anatomical magnetic resonance images of the brain in 25 children with Tourette syndrome (7–18 years old) and 35 age- and sex-matched controls. Informed parental consent and subject assent was obtained from all participants, and the study had the approval of the local institutional review boards of Yale University School of Medicine and the New York State Psychiatric Institute. We used cortical pattern matching, which relies on manually defined sulcal and gyral landmarks as anchors, to drive a surface-warping algorithm to relate homologous features of cortical anatomy across subjects7. Cortical thickness (in millimeters) was measured at matched anatomical points using techniques described previously8. In all analyses, permutation methods were used to correct for multiple comparisons (see Supplementary Methods online for details of inclusion/exclusion criteria, demographic variables, image processing methods and permutation statistics).
Participants were assessed with tests of current and worst-ever tic symptom severity (Yale Global Tic Symptom Severity Scale, YGTSS)9. Simple facial and body tics were also evaluated separately using the YGTSS, given that a large proportion of the inferior sensorimotor strip is dedicated to controlling muscles of the face and neck (which are most commonly affected by tics in Tourette syndrome), whereas the dorsal portion of the sensorimotor strip controls the muscles of the body and extremities (which are less commonly affected by tics). Scores for simple facial tic symptoms were created by summing the number of items endorsed for each type of simple facial tic queried (for example, eye blinking and facial grimacing). Scores for simple tic symptoms of the body were created by summing the number of items endorsed for each type of body tic queried (for example, shoulder shrugs and hand movements). Each child’s score in each of these domains reflected the number of different tic symptoms present.
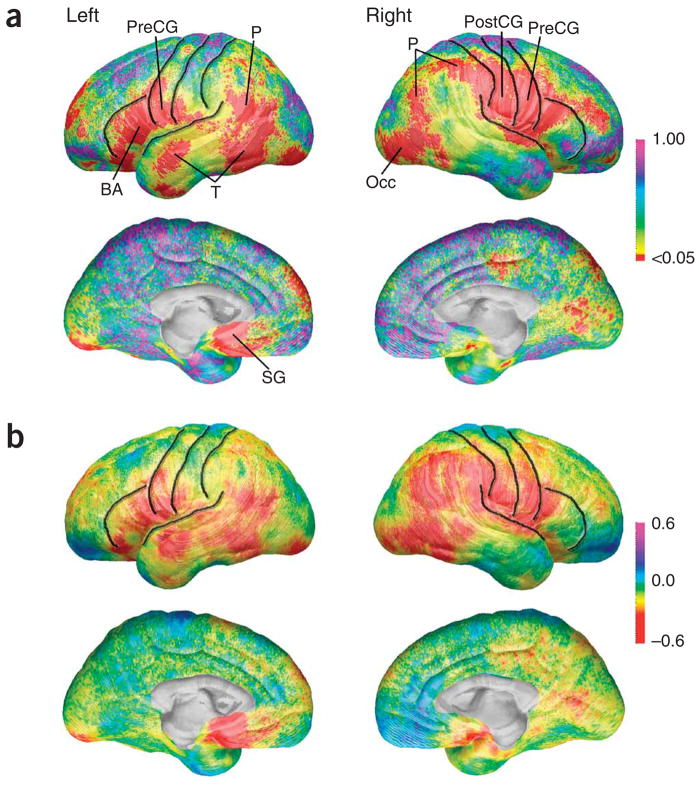
We created statistical maps of differences in cortical thickness between children with Tourette syndrome and controls (Fig. 1a). All group effects reflected decreased cortical thickness in the children with Tourette syndrome, as we detected no regions with statistically significant increases in cortical thickness (on permutation correction) in the Tourette syndrome group relative to the controls. The maps revealed that the Tourette syndrome group had significantly thinner cortices in ventral frontal regions bilaterally (permutation P = 0.0039, left; P = 0.0082, right) including ventral portions of the precentral and postcentral gyri, and more anteriorly in the inferior frontal gyrus, including Broca’s Area. We also detected thinning in the Tourette syndrome group more posteriorly, in the right dorsal parietal cortex (Fig. 1a). Permutation testing (Table 1) confirmed the significance of these findings (permutation P = 0.0291), in which the average cortex in the Tourette syndrome group was up to 0.45 mm thinner than the average cortex in the controls (Fig. 1b). Analyses of the 16 children with ‘pure’ Tourette syndrome and without comorbid diagnoses of attention deficit hyperactivity disorder or obsessive compulsive disorder compared with 19 age- and gender-matched control subjects yielded results similar to those of the entire group (Supplementary Fig. 1 and Table 1 online). All effects were independent of group differences in overall intellectual functioning (Tourette syndrome mean intelligence quotient = 108, control mean intelligence quotient = 119), whether statistically controlled in multiple regression analyses (Fig. 1a) or controlled using intelligence quotient–matching techniques in which we removed the ten controls with the highest scores to eliminate the group intelligence quotient difference (data not shown). Thinner cortices were also detected in older children and adolescents with Tourette syndrome, but not in the younger children of that cohort (producing a diagnosis-by-age interaction), in the lateral temporal and inferior parietal cortices, including the inferior primary sensory cortex, of the left hemisphere (Supplementary Fig. 2 and Table 1 online). Although cortical thickness in these regions was similar in younger children with Tourette syndrome and younger controls, cortices thickened with increasing age in the control children, but not in those with Tourette syndrome.
Figure 1.
Cortical thickness group differences. (a) Statistical P maps of the effect of diagnosis on cortical thickness with intelligence quotient statistically controlled. P maps are color-coded as indicated (also applies to Fig. 2a,b). Regions in red are statistically significant (uncorrected, P < 0.05). Regions in purple or pink do not approach significance (P approaching 1.0). Central, precentral and postcentral sulci, which bound the precentral and postcentral gyri, and the inferior frontal sulcus and Sylvian fissure are superimposed in black. Broca’s area, BA; occipital cortex, Occ; parietal cortex, P; postcentral gyrus, PostCG; precentral gyrus,PreCG; subgenual, SG; temporal cortex, T. (b) Cortical thickness differences in millimeters. Maps of differences between the entire group of Tourette syndrome and control subjects in thickness of gray matter (Tourette syndrome coded 1, controls coded 0 for all maps shown) showing differences in gray matter (in millimeters) between the Tourette syndrome and control subjects, as indicated by the color bar. Warmer colors (<0 on the color bar) indicate regions where the gray matter thickness is less in the Tourette syndrome subjects than in the control subjects, and cooler colors (>0) indicate regions where individuals with Tourette syndrome have greater gray matter thickness than the control subjects. Note that cortical thickness in Tourette syndrome subjects is reduced bilaterally in ventral frontal and parieto-occipital regions by approximately 0.45 mm. These maps are constructed without any brain scaling and represent decreases in absolute thickness of the cortex in the Tourette syndrome group.
Table 1.
Permutation test results for cortical thickness analyses
| All Tourette syndrome versus control, intelligence quotient corrected (n = 70) | Symptom severity within Tourette syndrome group (n = 24) | Simple facial tics within Tourette syndrome group (n = 21) | ||||
|---|---|---|---|---|---|---|
| ROI | ||||||
| Lateral | Left | Right | Left | Right | Left | Right |
| Dorsal Frontal | ns | 0.0800 | 0.0130 | 0.0210 | ns | ns |
| Ventral Frontal | 0.0039 | 0.0082 | 0.0635 | ns | ns | 0.0304 |
| Parietal | 0.0739 | 0.0291 | 0.0320 | ns | 0.0777 | 0.0096 |
| Occipital | ns | 0.0688 | 0.0132 | 0.0658 | ns | ns |
| Temporal | 0.0953 | ns | 0.0872 | ns | 0.0558 | 0.0360 |
Permutation test results are shown for all lateral regions of interest that were significant for decreased cortical thickness in the Tourette syndrome group relative to the control group, or at trend-level significance. Also shown are permutation test results for correlations between cortical thickness and overall symptom severity, and facial tics. The P values shown reflect the likelihood of observing the number of significant (at P = 0.05) surface points in each region of interest (ROI) by chance on 10,000 randomizations. Permutation results for medial ROIs were significant only for the left medial dorsal frontal region in the symptom severity versus thickness analyses (P = 0.0117) and are thus not shown for the other nonsignificant medial ROIs. ns, nonsignificant.
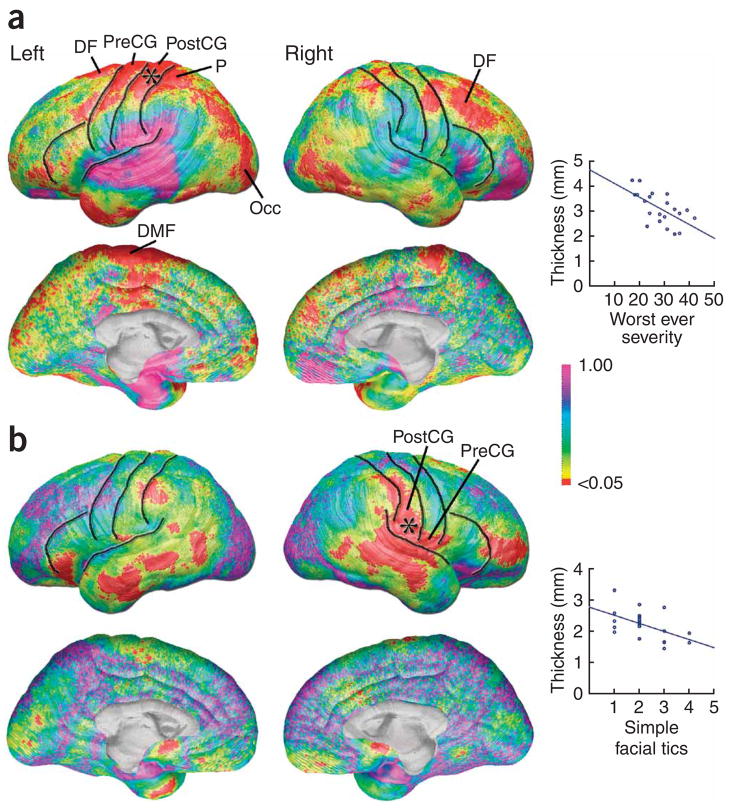
Maps correlating worst-ever tic severity on the YGTSS with cortical thickness in the Tourette syndrome group (Fig. 2a) were statistically significant in large areas of the left and right dorsal frontal (permutation P = 0.0130, left; P = 0.0210, right) and left dorsal parietal lobes (permutation P = 0.0320), including the dorsal portions of the pre-and postcentral gyri, and in the left dorsal medial frontal and left occipital cortices. In each of these regions, thinner cortices were accompanied by more severe tic symptoms (permutation results in Table 1). As predicted, maps correlating the number of simple facial tics with cortical thickness were significant in the more ventral portions of the sensory and motor strips (permutation P = 0.0096, right parietal; Fig. 2b), partially overlapping with regions of the brain where cortex was too thin in the Tourette syndrome subjects. Correlations were also significant in right ventral frontal (permutation P = 0.0304) and right lateral temporal regions (permutation P = 0.0360). Correlations using the scores for trunk and extremity were not significant (P >.05) in any brain region on permutation correction for multiple comparisons.
Figure 2.
Symptom severity and cortical thickness. (a) Statistical P maps of the correlation between the worst-ever severity of tic symptoms and cortical thickness in a subsample of 24 individuals in the Tourette syndrome group for whom YGTSS data were available. The graph illustrates the negative correlation between symptom severity and cortical thickness (thinner cortex associated with higher severity scores) at one brain surface point in the superior portion of the sensory strip, indicated by the asterisk on the brain map. dorsal frontal, DF; dorsal medial frontal, DMF. (b) Simple facial tics and cortical thickness. Statistical P maps of the correlation between the number of different types of facial tic symptoms and cortical thickness in a sub-sample of 21 individuals in the Tourette syndrome group for whom detailed YGTSS data were available. The graph illustrates the negative correlation between facial tics and cortical thickness (thinner cortex associated with more types of facial tics) at one brain surface point in the inferior portion of the sensory strip, indicated by the asterisk on the brain map.
As predicted, we detected cortical thinning in sensorimotor portions of CSTC circuits in children with Tourette syndrome. Areas of thinning also extended anteriorly into the frontal cortex and posteriorly into the parietal cortex. Greater overall tic severity was accompanied by greater degrees of cortical thinning in the sensorimotor cortices, but in their dorsal regions, rather than in their ventral portions, where group differences were most prominent, and more in the left than in the right hemisphere. These dorsal portions of the primary motor cortex control muscles of the trunk and extremities. Ventral portions of the sensorimotor cortex control muscles of the face, mouth and larynx, and, as expected, thinner cortices in this region were accompanied by a larger number of facial tics in the Tourette syndrome group. The additional association of thinner dorsolateral prefrontal cortices with more severe tic symptoms is consistent with the previously hypothesized role of this portion of the cortex in the control or modulation of the severity of tic symptoms10.
Motor portions of CSTC circuits have long been postulated to be involved in the pathogenesis of Tourette syndrome11. Until now, the involvement of cortical portions of those circuits has not been widely suspected, and instead the motor portions of the basal ganglia nuclei have been assumed more important in etiology. Similarly, the participation of sensory portions of CSTC pathways in Tourette syndrome has not been strongly suspected. The sensory urges that precede and initiate tic behaviors have been generally thought to originate in the basal ganglia or thalamic portions of these circuits1. Previous reports of reduced volumes of the basal ganglia3,12 and the thinning of motor cortices reported here suggest a wider circuit-based dysfunction in both motor and sensory pathways in individuals with Tourette syndrome. The presence of cortical thinning in children with Tourette syndrome early in the course of illness, before compensatory and epiphenomenal effects are likely to dominate the morphological landscape, confirmation of these findings in children without co-occurring obsessive compulsive disorder or attention deficit hyperactivity disorder, and the association of greater thinning with more severe tic symptoms suggest that sensorimotor cortices, together with the basal ganglia portions of CSTC circuits, are important in the pathogenesis of Tourette syndrome.
The cellular determinants of the thinner cortices that we detected in children with Tourette syndrome are unknown. Increased white-matter volumes have been reported in the frontal cortices of children with Tourette syndrome4, however, and cortical thinning could therefore be a consequence of increased myelination between gray and white matter in the periphery of the cerebrum. Furthermore, age-by-diagnosis interactions suggest an absence of thickening of the lateral temporal, inferior parietal and inferior sensory cortices in the left hemisphere of younger children with Tourette syndrome, and cortical thinning in older children could be a marker of persistent illness, given that symptoms typically attenuate through adolescence. Cortical thinning could also be a consequence of reduced numbers of GABAergic interneurons in sensorimotor cortices, a possibility that was previously suggested by transcranial magnetic stimulation study that found reduced intracortical inhibition in the motor cortices of children with Tourette syndrome, particularly in those who have tics affecting the muscle groups stimulated by transcranial magnetic stimulation13. Moreover, reduced densities of GABAergic inhibitory interneurons have been reported in the striatum of individuals with Tourette syndrome14, and GABAergic interneurons in the cortex and striatum are known to have a single common embryological origin15.
The cortical mantle contains the cell bodies, neuropil and synapses of neurons, any of which, in isolation or in combination, could create the cortical thinning that we detected in children with Tourette syndrome. Postmortem studies are needed to assess the ultrastructural features and functional properties of all cell types, including GABAergic interneuorons that could produce thinning of sensorimotor cortices. In addition, other in vivo imaging experiments should assess the anatomical, functional and neurochemical features of the axonal pathways and synaptic connections between the sensorimotor cortices and basal ganglia in children with Tourette syndrome. Longitudinal studies are needed to assess whether any of these anatomical features are markers for the persistence of symptoms into adulthood and to determine whether medications have long-term influences on the structure and function of these circuits.
Acknowledgments
The authors thank J. Leckman, R. King and L. Scahill for helping to refer participants to the study. Funding support for this work was provided by a US National Institute of Mental Health grant (K01 MH01733) to E.R.S., a US National Institutes of Health (NIH)/National Center for Research Resources resource grant (P41 RR013642), NIH Roadmap for Medical Research grant (U54 RR021813) and US National Institute of Neurological Disorders and Stroke grant (NS3753) to A.W.T., NIH grants (AG016570, LM05639, EB01651 and RR019771) to P.M.T., and National Institute of Mental Health grants (MHK02-74677, MH59139 and MH068318) to B.S.P.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
AUTHOR CONTRIBUTIONS
The study was conceived and designed by E.R.S. and B.S.P., who also carried out the analysis and interpretation of the data and wrote the manuscript. A.W.T. and P.M.T. reviewed the manuscript and obtained funding for the study, along with E.R.S. and B.S.P. E.K., J.Y., D.X. and R.B. provided administrative, technical and material support. E.R.S. and B.S.P. had access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Albin RL, Mink JW. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Hyde TM, et al. Neurology. 1995;45:1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BS, et al. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 4.Fredericksen KA, et al. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- 5.Peterson BS, et al. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 6.Schieber MH. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PM, et al. Neuroimage. 2004;23(Suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 8.Sowell ER, et al. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leckman JF, et al. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Spessot A, Peterson BS. In: Manual of Developmental Psychopathology. Cichetti D, Cohen DJ, editors. John Wiley; Hoboken, NJ: 2006. pp. 436–469. [Google Scholar]
- 11.Singer HS, Minzer K. Brain Dev. 2003;25(Suppl 1):S70–S84. doi: 10.1016/s0387-7604(03)90012-x. [DOI] [PubMed] [Google Scholar]
- 12.Singer HS, et al. Neurology. 1993;43:950–956. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- 13.Ziemann U, Paulus W, Rothenberger A. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 14.Kalanithi PS, et al. Proc Natl Acad Sci USA. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wonders CP, Anderson SA. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]