Abstract
Background & Aims
TL1A is a tumor necrosis factor-like molecule that mediates a strong costimulation of T helper (TH) 1 cells. Expression of TL1A is increased in the mucosa of Crohn’s disease patients and murine models of ileitis. The aim was to determine the possible role of TL1A in chronic intestinal inflammation.
Methods
We used dextran sodium sulphate (DSS)-induced chronic colitis to investigate the effects of TL1A on the development of colitis. The cytokine profile in the gut-associated lymphoid tissue (GALT) was measured. Neutralizing anti-TL1A monoclonal antibodies (mAb) were injected intraperitoneally into DSS-induced chronic colitis and G protein αi2−/− T cell transfer colitis models. Severity of colitis was evaluated by body weight, colon length, histology, and cytokine production.
Results
DSS-induced chronic colitis was characterized by the infiltration of CD4+ T cells. TL1A, death receptor 3, interferon (IFN)-γ and interleukin (IL)-17 were significantly increased in GALT of DSS-treated mice. TL1A up-regulated both IFN-γ production from TH1 cells and IL-17 production from TH17 cells in GALT CD4+ T cells. Furthermore, IFN-γ and IL-17 production from CD4+ T cells, induced by IL-12 and IL-23 respectively, was synergistically enhanced by combination with TL1A. Anti-TL1A mAb prevented chronic colitis and attenuated established colitis by down-regulation of both TH1 and TH17 activation.
Conclusions
Our results reveal that TL1A is an important modulator in the development of chronic mucosal inflammation by enhancing TH1 and TH17 effector functions. The central role of TL1A represents an attractive, novel therapeutic target for the treatment of CD patients.
Introduction
The pathogenesis of Crohn’s disease and ulcerative colitis relates to an inappropriate and exaggerated mucosal immune response to constituents of the intestinal flora.1, 2 Antigen-presenting cells (APCs) such as dendritic cells (DCs) are likely to play a central role in the host response to intestinal flora, both in innate responses to bacteria and by shaping the character of the host’s adaptive immune response.3, 4 Furthermore, CD4+ T cells activated by APCs also have been shown to be involved in the pathogenesis of inflammatory bowel disease.3 A dysregulated T cell response leads to alterations in mucosal cytokine expression. CD has been characterized as having a T cell helper (TH) 1 and TH17 cytokine pattern, and antibodies to both interferon (IFN)-γ and p40 (interleukin (IL)-12/23) can treat subsets of CD patients.5, 6
TL1A, a recently identified tumor necrosis factor (TNF)-like factor, is the only known ligand for death receptor (DR) 3 which is primarily expressed on activated lymphocytes.7 TL1A is expressed on endothelial cells, lymphocytes, plasma cells, monocytes, and DCs.8, 9 TL1A can induce IFN-γ production of IL-12 and IL-18 primed gut-homing receptor CCR9+ T cells, but not CCR9− T cells, by the interaction of TL1A/DR3.10 In contrast, TL1A does not enhance IL-4 production from TH2 cells.11 Therefore, the interaction of TL1A and DR3 may be important in TH1-mediated responses of the intestine. In fact, we and others have shown up-regulation of both TL1A and DR3 in rheumatoid arthritis12 and IBD, particularly CD.11, 13, 14 Furthermore, recent genome-wide association studies revealed a highly significant association of single nucleotide polymorphism haplotypes of the TL1A gene with CD, in Japanese, European, and United States cohorts.15, 16 Bamias et al. showed an association of increased TL1A and DR3 in expression in ileitis models, but no direct proof of the role of TL1A in mucosal inflammation.9 Taken together, these results suggest that the interaction of increased APC-derived TL1A and lymphocytic DR3 is involved in TH1 mediated intestinal inflammation as is seen in human CD. The precise role of TL1A in IBD has not been elucidated.
In this study, we have investigated the function of TL1A in two different mouse models of chronic colitis resembling human CD. We show that TL1A enhances cytokine production from both TH1 and TH17 CD4+ T cells in intestinal mucosa and that neutralization of TL1A attenuates chronic colitis. These results suggest that TL1A is a central immune modulator in activation of mucosal TH1/ TH17 CD4+ T cells and that it is critical for the development of chronic colitis. Our findings suggest that neutralization of TL1A could be a novel, highly specific approach for therapeutic intervention in CD.
Materials and Methods
Mice
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions in the Animal Care Facility at Cedars-Sinai Medical Center. Gαi2−/− (129/Sv background) mice were housed at the University of California, Los Angeles (UCLA) Animal Care Facility. RAG2−/− mice (129/Sv background) were obtained from the UCLA Department of Radiation Oncology or purchased from Taconic Farms (Germantown, NY). Mice used in all experiments were handled according to the guidelines and approved protocols of the Cedars-Sinai Medical Center and UCLA Animal Care and Use Committees.
Induction and assessment of chronic colitis
Chronic colitis was induced by multiple-cycle administration of DSS drinking water.38 Female mice of 8 weeks of age received either regular drinking water (control) or 3% (w/v) DSS drinking water (40,000–50,000 MW) (MP Biomedicals, Irvine, CA) on days 1–5, 8–12, 15–19, and 22–26. Histology was used to evaluate inflammation, extent, regeneration, crypt damage, and percent involvement.
In the Gαi2−/− T cell transfer model, colitis was induced by colitigenic Gαi2−/− T cells.29 The histological scoring for colitis was based on an established scale using inflammatory and epithelial parameters, as previously reported.39 Disease severity was determined by a combination of macroscopic (Grade 0: normal, grade 1: mild, grade 2: moderate, grade 3: severe) and histological scores (Grade 0: histological score 0–1, grade 1: 2–4, grade 2: 5–7, grade 3: 8–10, grade 4: 11–14).
See the supplementary information.
Cell Isolation and Culture
Mononuclear cells from MLN and Lamina Propria were isolated. CD4+ T cells were isolated from MLN and LP by negative selection (StemCell Technologies Inc., Vancouver, BC). Cells were cultured under the conditions indicated in the Figure legends. In experiments, for IL-12 and IL-23 detection, cells were cultured with or without 1 µg/ml LPS.
See the supplementary information.
Flow Cytometry
Cells were stained with antibodies against murine CD4 (RM4-5), B220 (RA3-6B2), CD45RB (16A), CD11c (HL3), MHC class II (IA/IE, clone 114.15.2), CD16/CD32 (clone 2.4G2, to block nonspecific FcR binding) (BD Biosciences), TL1A (CoGenesys Inc., Rockville, MD). Intracellular staining was performed with antibodies against IFN-γ and IL-17 (BD Biosciences).
See the supplementary information.
Real-Time PCR
Total RNA was isolated from MLN cells and whole colons using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed into cDNA with Omniscript RT kit (Qiagen, Valencia, CA). cDNA was quantified by real-time PCR using iCycler Thermal cycler (Bio-Rad, Hercules, CA). TL1A, DR3, IFN-γ, IL-17, TNF-α and β-actin TaqMan probes and primers were designed using Beacon Design 4.0 (Premier Biosoft International, Palo Alto, CA).
See the supplementary information.
Monoclonal antibodies
Neutralizing hamster anti-mouse TL1A mAb (19E06A hydridoma) was obtained from CoGenesys Inc. (Rockville, MD). Neutralizing anti-mouse IL-23R mAb was a gift from Dr. Charles Elson (University of Alabama, Birmingham). For DSS-induced chronic colitis, 500 µg of anti-TL1A mAb or control IgG (Leico Technologies, Inc., St. Louis, MO) was administered intraperitoneally into mice once per week (days 1, 8, 15, and 22) or twice per week (days 15, 18, 22, and 25). 100 µg of anti-IL-23R mAb or control IgG was administered i.p. once per week (days 1, 8, 15, and 22) or twice per week (days 15, 18, 22, and 25). Mice were sacrificed at day 29 and colitis was evaluated by a trained pathologist blinded to the treatment of the mice. For Gαi2−/− T cell transferred colitis, 500 µg anti-TL1A mAb or control IgG was injected i.p. twice per week after transfer of colitigenic Gαi2−/− T cells.
Statistical Analysis
The Student t test was used for statistical analysis. Differences were considered significant at P < 0.05.
Results
Characterization of dextran sodium sulphate (DSS) induced chronic colitis
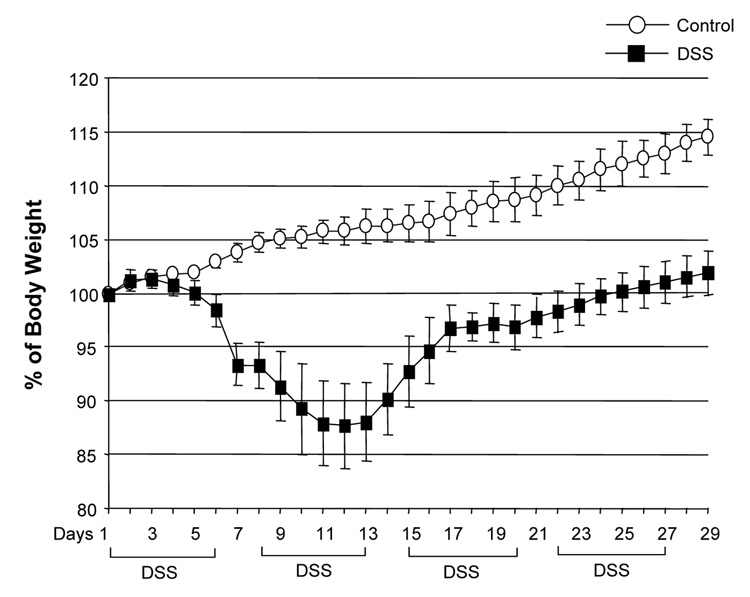
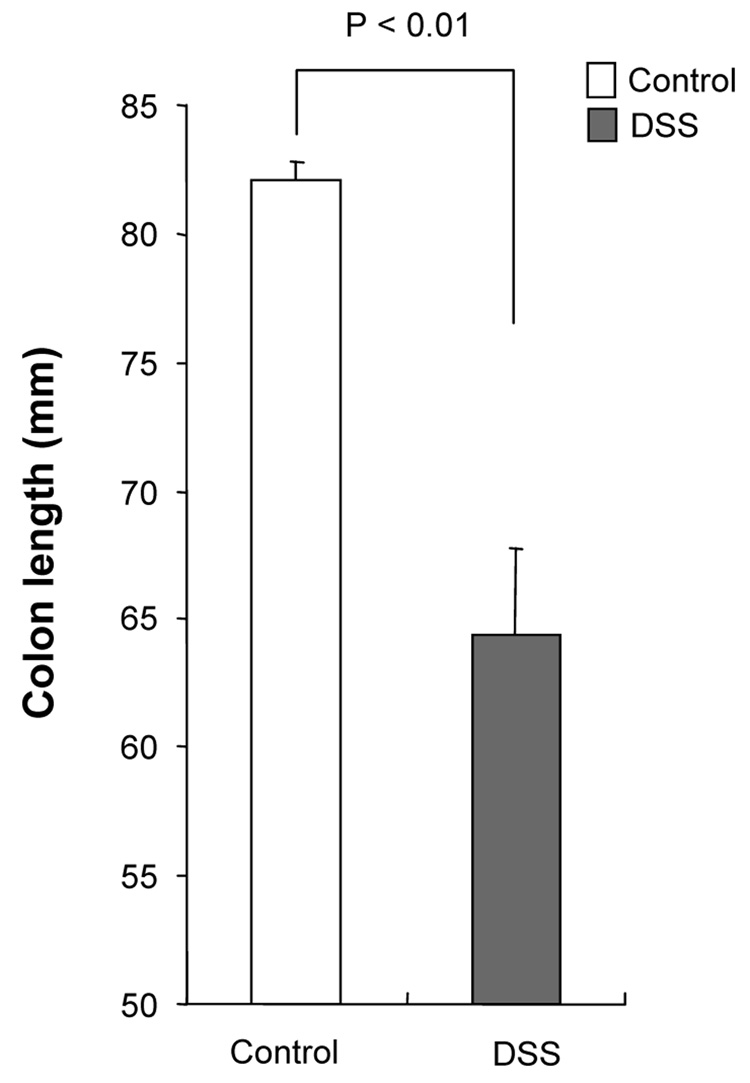
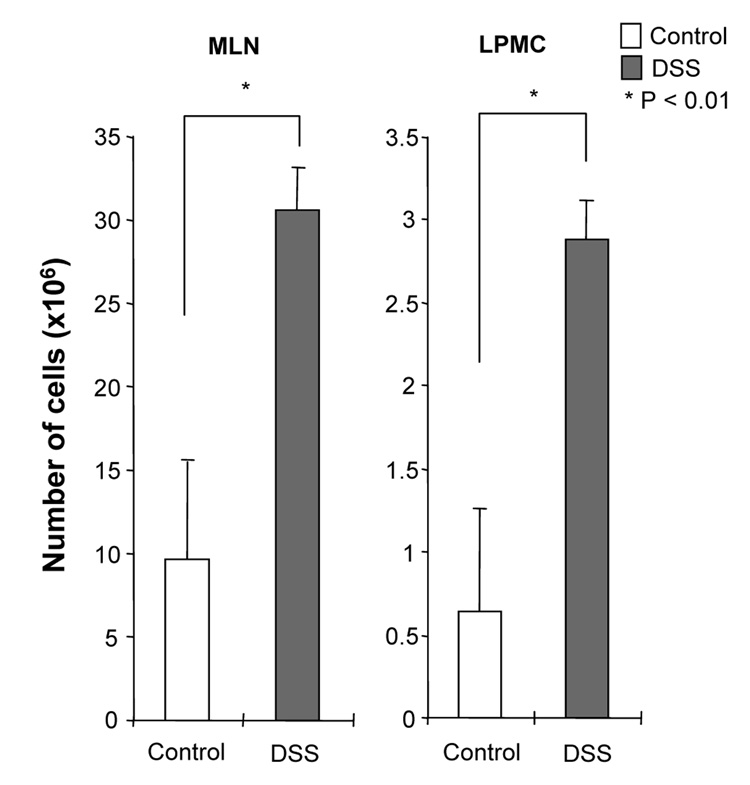
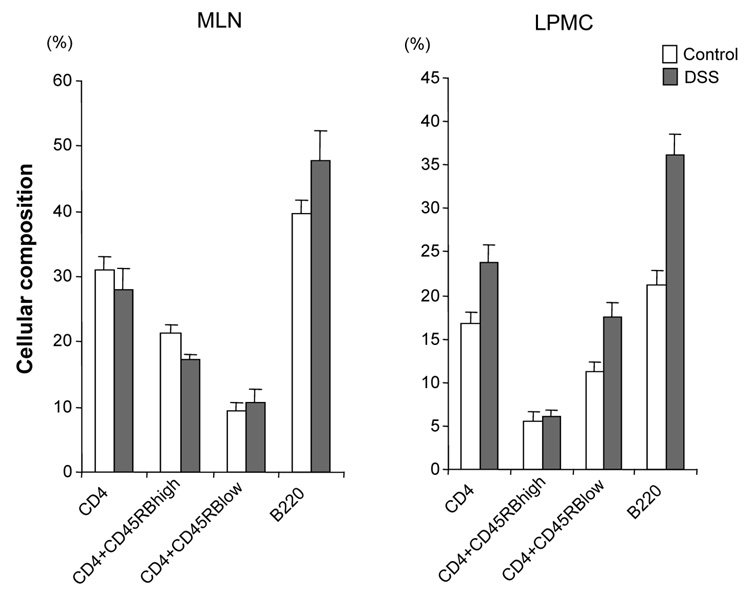
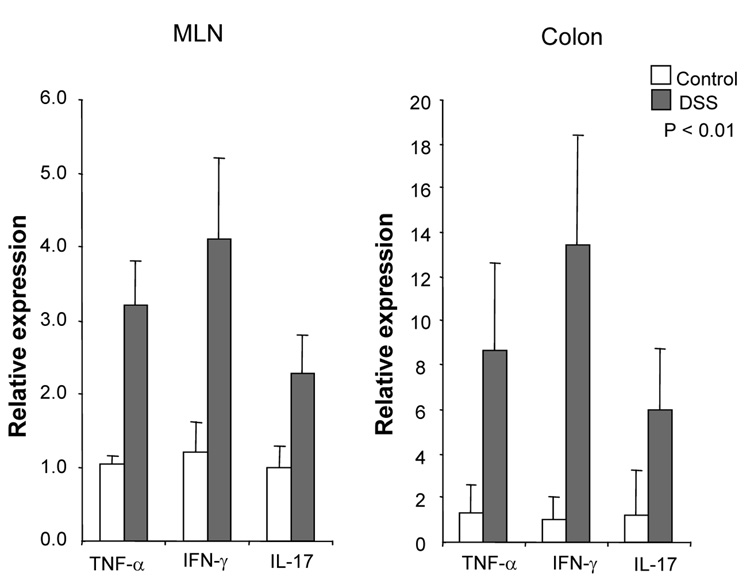
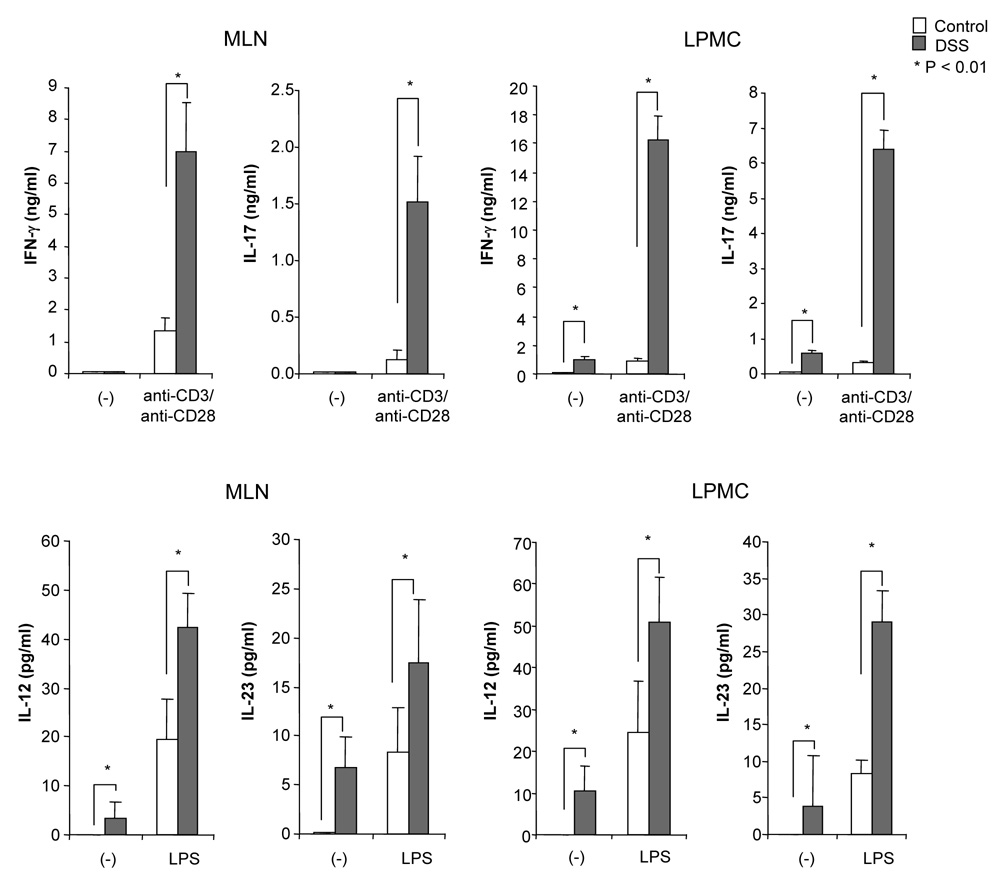
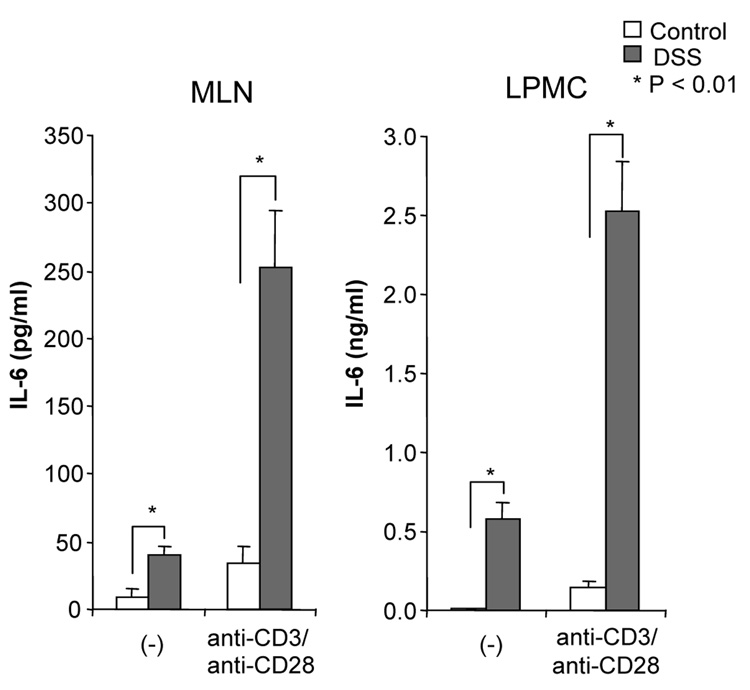
DSS-induced acute colitis has been known as a T cell independent model.17 However, in chronic colitis induced by multiple cycles or in the recovery phase of DSS, adaptive immunity plays an important role in the disease process.18, 19 Chronic colitis was induced by administration of four cycles of 3 % DSS drinking water to C57BL/6 mice. Loss of body weight was first observed at day 5. Maximum weight loss was seen at day 12 and mice regained body weight after 2 cycles of DSS (Figure 1A). Mice were sacrificed at day 29. Cecum and colon of DSS-treated mice showed signs of severe colitis including significantly shortened colon length Figure 1B. Mononuclear cell numbers from MLN and LP were increased due to infiltration of inflammatory cells in the DSS-treated mice (Figure 1C). CD4+CD45RBlow memory T ceslls and B cells were significantly increased in LPMCs of DSS-treated mice (Figure 1D). In MLN, CD4+ T cells, especially CD4+CD45RBlow T cells were also increased however the percentage of CD4+CD45RBlow cells in total CD4+ cells in MLN was less than in LP. IFN-γ from TH1 cells and IL-17 from TH17 cells are both key mediators in several autoimmune diseases and IBD.5, 6, 20, 21 In DSS-treated mice, IFN-γ, IL-17, and TNF-α mRNA (Figure 1E) and protein (Figure 1F) expressions were significantly higher than in untreated mice. However, IL-4 produced by TH2 cells was rarely detectable in this model (data not shown). In addition to production of IFN-γ and IL-17, IL-12 and IL-23 production were also increased in DSS induced chronic colitis (Figure 1F).
Figure 1. Development of DSS-induced chronic colitis.
Chronic colitis induced by four cycles of DSS was evaluated and compared with untreated mice (n=7 per group). All data represent the mean ± SD. (A) Body weight as a percentage of the initial weight at day 1 are shown. (B) Colon length from cecum to rectum. (C) Tsotal numbers of mononuclear cells in GALT. (D) Cellular composition of MLN and LP cells was analyzed by flow cytometry and expressed as percentage of the whole cell population. (E) Cytokine mRNA expression was determined in MLN and colon by real-time PCR. Data were normalized to the expression of β-actin mRNA. (F) Mononuclear cells from GALT were cultured with or without anti-CD3ε and anti-CD28 Abs for IFN-γ and IL-17 and with or without LPS for IL-12 and IL-23. Cytokine productions were measured by ELISA.
Increased TL1A and DR3 expression in DSS-induced chronic colitis
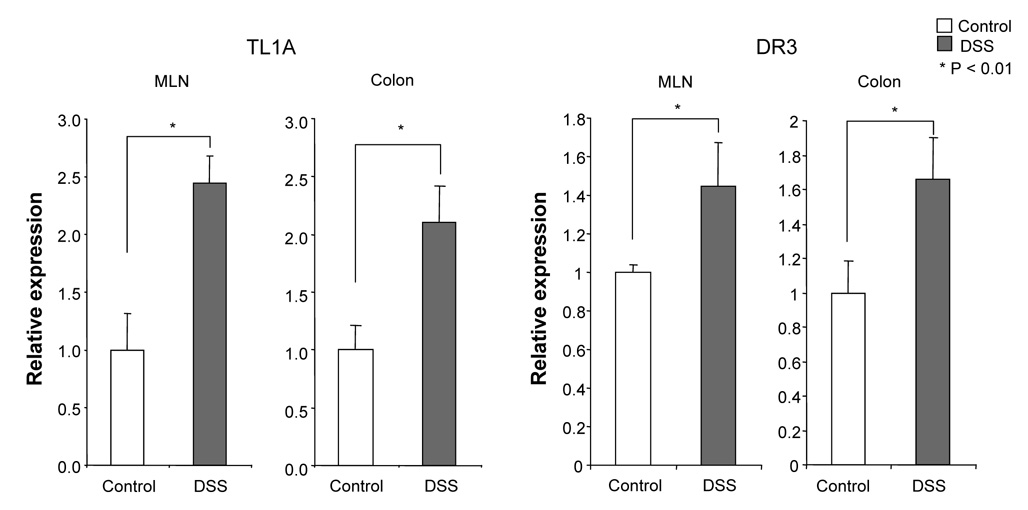
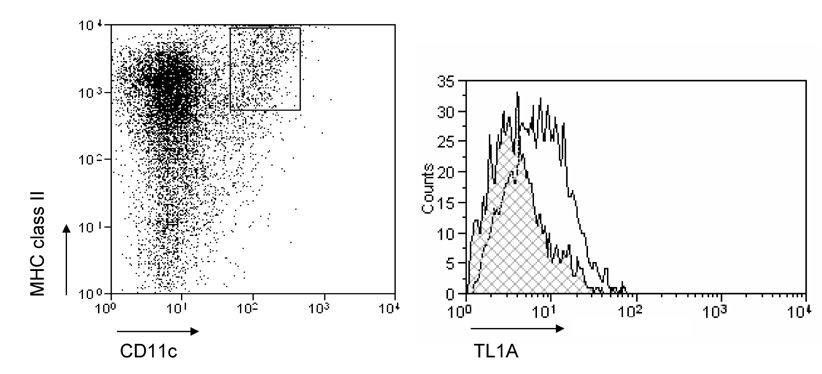
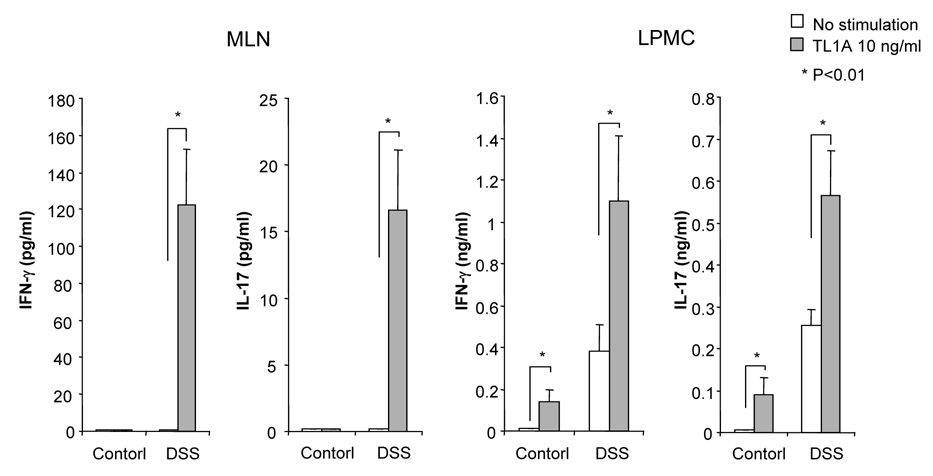
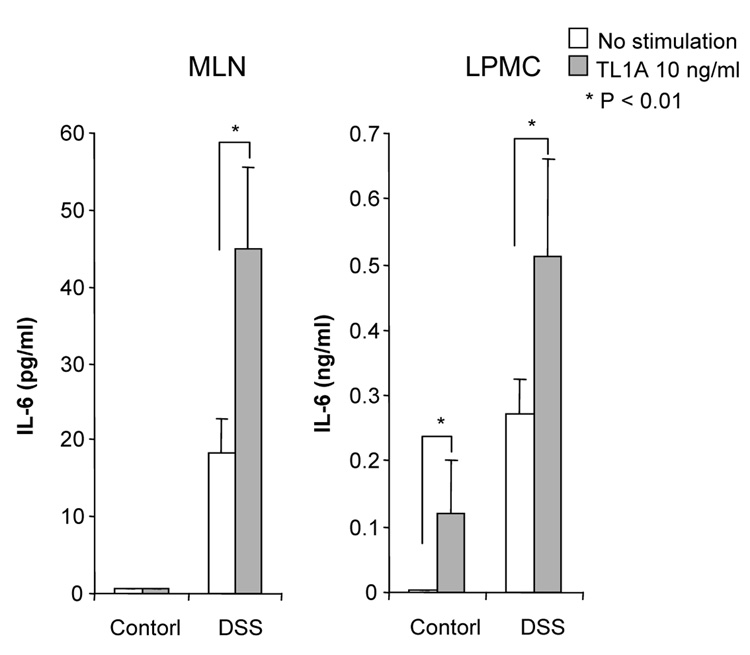
To evaluate the expression of TL1A and its receptor DR3 in DSS-induced chronic colitis, we performed Real-Time PCR in MLN and colon. TL1A mRNA and DR3 mRNA were significantly increased in DSS-treated mice (Figure 2A). FACS analysis revealed that MLN CD11chigh MHC class II+ DCs derived from DSS-treated mice expressed TL1A (Figure 2B), as Bamias et al. have demonstrated previously.9 Since the interaction of APC derived TL1A and DR3, mainly expressed on CD4+CD45RBlow memory T cells,9 was shown to induce T cell activation in the mucosa, we investigated the effect of TL1A on GALT CD4+ cells. IFN-γ and IL-17 produced in DSS-treated mice was greatly enhanced by the stimulation with TL1A (Figure 2C). These results reveal that the interaction of TL1A with DR3 leads to the activation of both TH1 and TH17 cells on CD4+ memory T cells in the GALT of chronic colitis.
Figure 2. TL1A and DR3 expression are up-regulated in MLN and colon during chronic DSS-induced colitis.
(A) TL1A and DR3 mRNA expression were determined in MLN and colon by real-time PCR (n=5 per group). Data were normalized to the expression of β-actin mRNA. (B) Left panel: Dot plot of FACS staining for CD11c and MHC class II of MLN of DSS-treated mice. Right panel: Histogram plot shows TL1A expression of CD11chigh MHC class II+ gated cells derived from DSS-treated mice (bold line) or untreated mice (shaded area). (C) CD4+ T cells from GALT were cultured with or without TL1A. IFN-γ and IL-17 production were measured by ELISA (n=5 per group).
TL1A synergistically enhances IL-12-induced IFN-γ, and IL-23-induced IL-17 and IL-6 secretion from mucosal CD4+ T cells
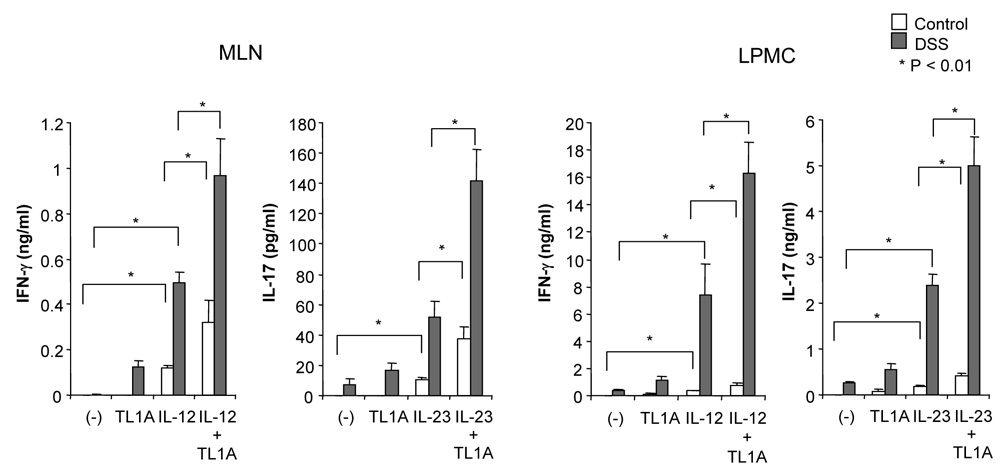
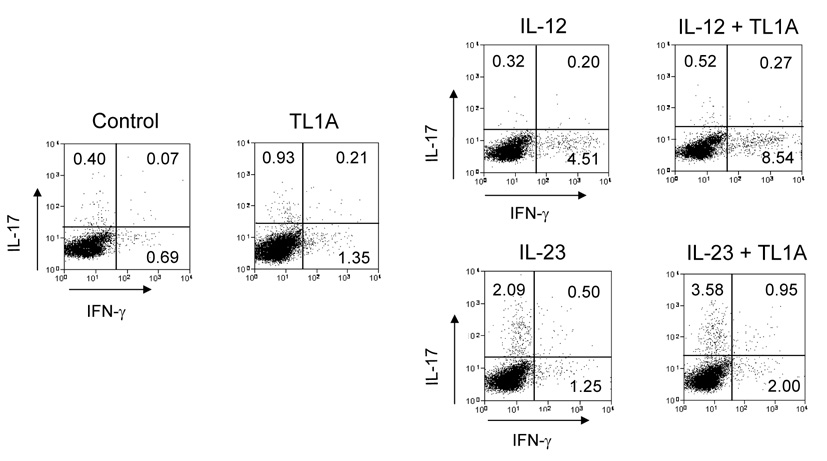
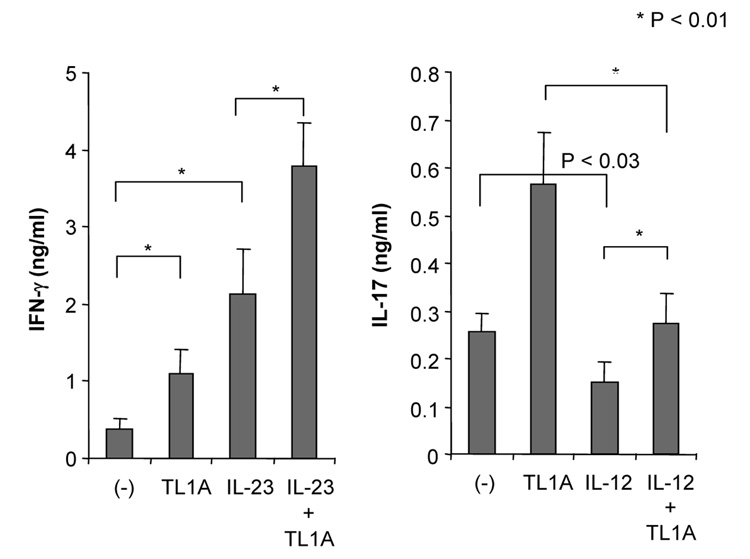
To determine the role that TL1A might play in both IFN-γ and IL-17 production in the mucosa of DSS-treated mice, we examined the synergistic effect of TL1A with IL-12 or IL-23 in GALT on the production of IFN-γ and IL-17. CD4+ T cells were stimulated with TL1A, IL-12, IL-23, IL-12 plus TL1A, or IL-23 plus TL1A. Stimulation with IL-12 plus TL1A enhanced IFN-γ production (Figure 3A). Interestingly, IL-23 plus TL1A could significantly up-regulate IL-17 production compared to IL-23 alone. The enhancing effects of TL1A on the production of IFN-γ or IL-17 were much more pronounced in the LPMC (Figure 3A). To confirm the effect of TL1A on CD4+ T cells, we performed intracellular staining of LPMC under conditions activating either TH1 or TH17 cells. TL1A increased the numbers of both IFN-γ-producing cells and IL-17-producing cells (Figure 3B). This result reveals that TL1A itself could up-regulate IFN-γ and IL-17 production in both TH1 and TH17 cells even in the absence of cytokines known to activate these T cell populations. Moreover, IL-12 plus TL1A, and IL-23 plus TL1A further increased numbers of IFN-γ- and IL-17-producing cells, respectively (Figure 3B). To further investigate this finding, we examined the effect of IL-23 and IL-23 plus TL1A on IFN-γ production, and the effect of IL-12 and IL-12 plus TL1A on IL-17 production. Our data showed that IL-23 and IL-23 plus TL1A could up-regulate IFN-γ production, however, IL-12 slightly decreased IL-17 production compared to control and IL-12 plus TL1A significantly inhibited IL-17 production compared to TL1A (Figure 3C). Consistent with these findings, IL-17 production was reduced when stimulated with IL-23 plus IL-12, and TL1A could not neutralize the inhibitory effect IL-12 has on IL-17 production (Supplementary Figure 3). These findings suggest that IL-23 and IL-23 plus TL1A can work synergistically to activate both TH1 and TH17 cells, and to enhance both IFN-γ and IL-17 production from TH17 cells, as shown in Figures 3B and C. TL1A alone is sufficient to induces IL-17 production from LPMC but exerts its maximal synergistic effect in the presence of IL-23 (Supplementary Figure 4).
Figure 3. TL1A synergizes with IL-12 or IL-23 in the development of a TH1 or TH17 response during chronic colitis.
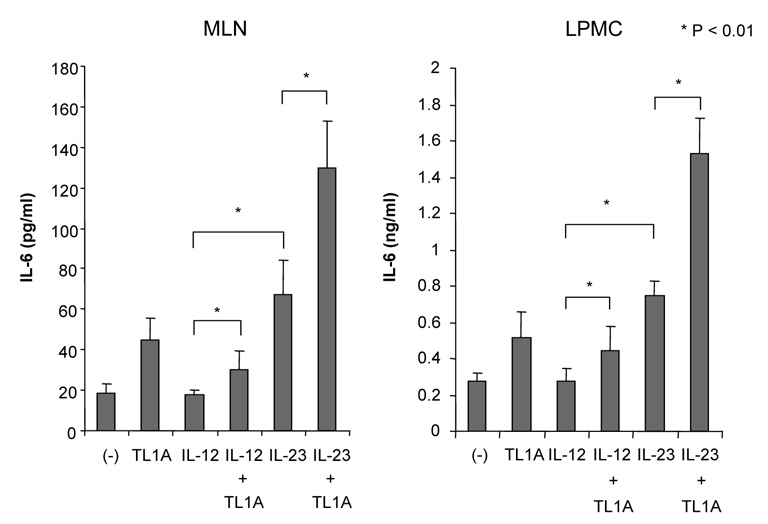
CD4+ T cells from GALT of untreated or DSS-treated mice were cultured with IL-12 or IL-23 with or without the additsion of TL1A (10 ng/ml) for 72 h. (A) IFN-γ and IL-17 production were measured by ELISA (n=5 per group). Data presented are means ± SD. (B) FACS analysis of intracellular IFN-γ and IL-17 production by CD4+ T cell isolated from the LP of DSS-treated mice. One representative experiment out of 3 independent experiments is shown. (C) IFN-γ and IL-17 production in LP of DSS-treated mice was measured by ELISA (n=5 per group). (D) IL-6 production from GALT stimulated with or without anti-CD3ε and anti-CD28 was measured by ELISA. (E) IL-6 production from GALT CD4+ T cells stimulated with or without TL1A. (F) IL-6 production from GALT CD4+ T cells of DSS-treated mice stimulated with IL-12 or IL-23 with or without the addition of TL1A.
IL-6 has been shown to play an important role in the pathogenesis of chronic IBD.22, 23 Recent studies demonstrated that IL-23-dependent TH17 CD4+ T cells produced both IL-17 and IL-6.24, 25 IL-6 production from T cells was significantly increased in DSS-treated mice (Figure 3D). TL1A itself up-regulated IL-6 production in the GALT (Figure 3E). Furthermore, IL-23, but not IL-12, induced IL-6 production and TL1A significantly enhanced IL-23-induced IL-6 production in DSS-treated mice (Figure 3F). In addition, IL-6 production was increased in IL-12 plus TL1A conditions, yet IL-12 itself did not enhance IL-6 production, confirming that TL1A could enhance IL-6 production. These findings indicated that TH17 cells produced both IL-17 and IL-6 in response to IL-23 and that TL1A enhanced IL-6 production from IL-23 primed TH17 cells. It has been reported that IL-6 plays a crucial role in the differentiation of TH17 cells from naïve T cells.26, 27 To exclude the possibility that the increase of IL-17 was induced indirectly via the up-regulation of IL-6 secretion by TL1A, we neutralized endogenous IL-6 by anti-IL-6Rα chain (CD126) antibodies. Endogenous IL-6 did not directly affect IL-17 production by TH17 cells in our experiments (data not shown).
Neutralization of TL1A attenuates DSS-induced chronic colitis and T cell mediated colitis in the Gαi2−/− T cell transfer model
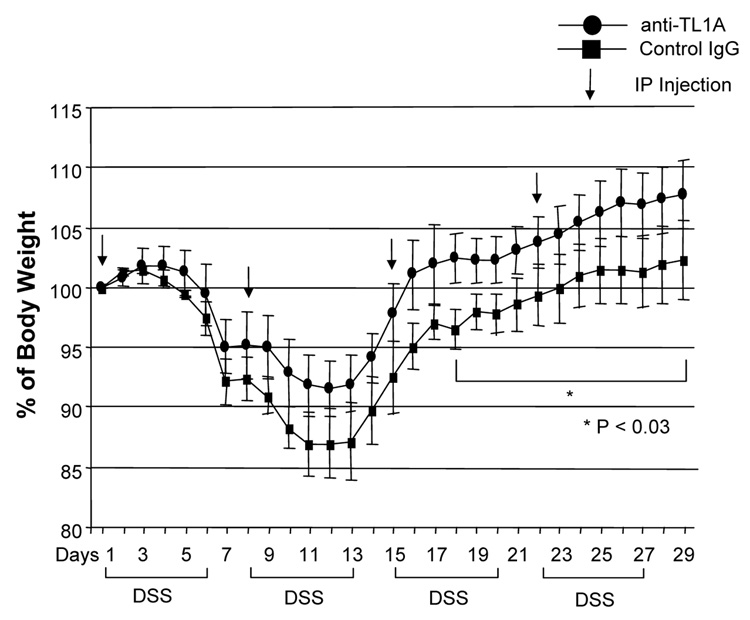
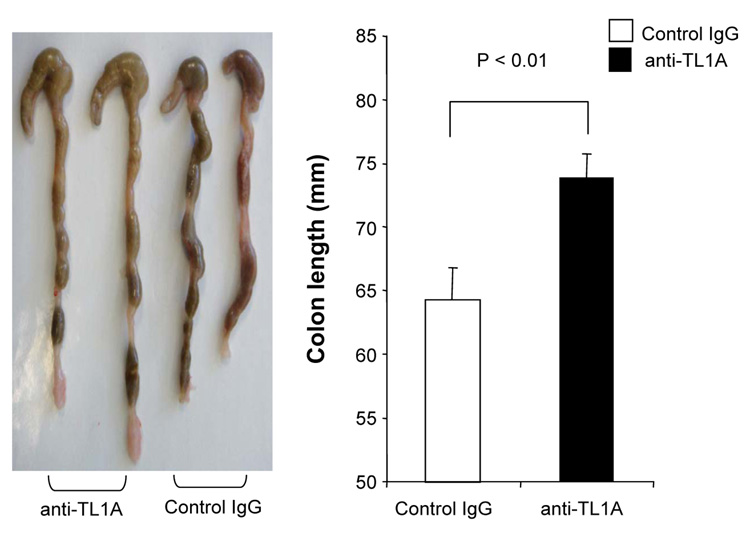
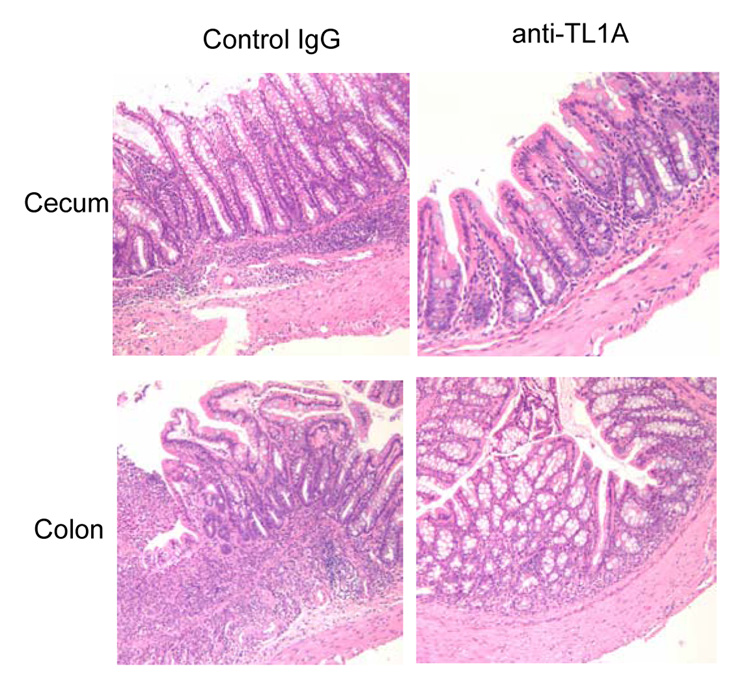
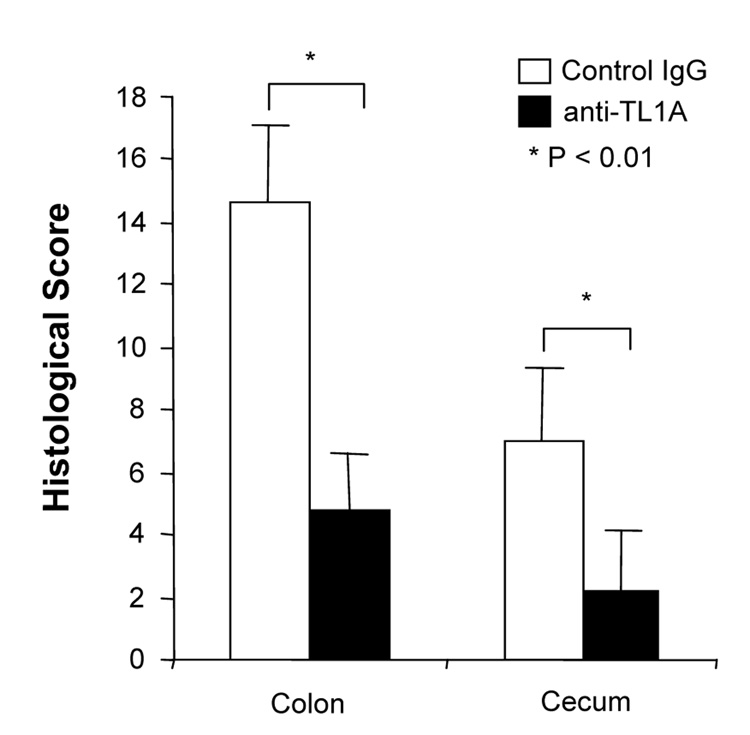
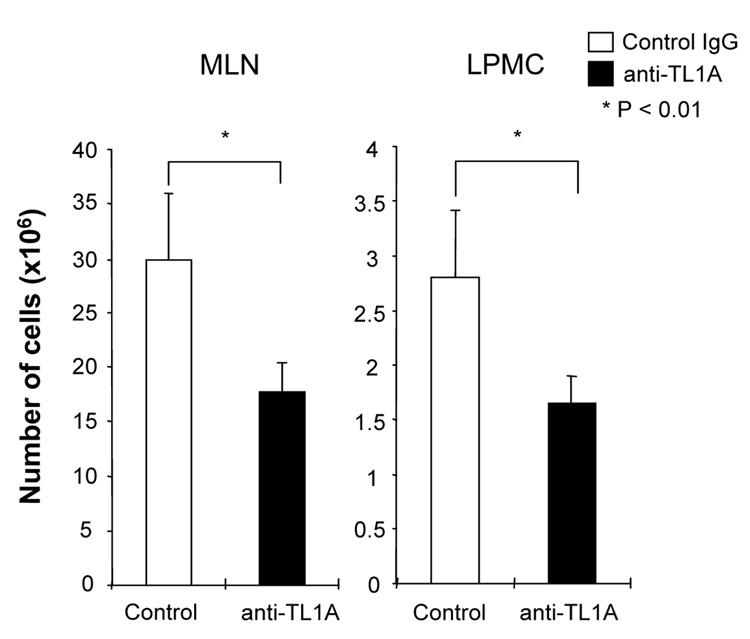
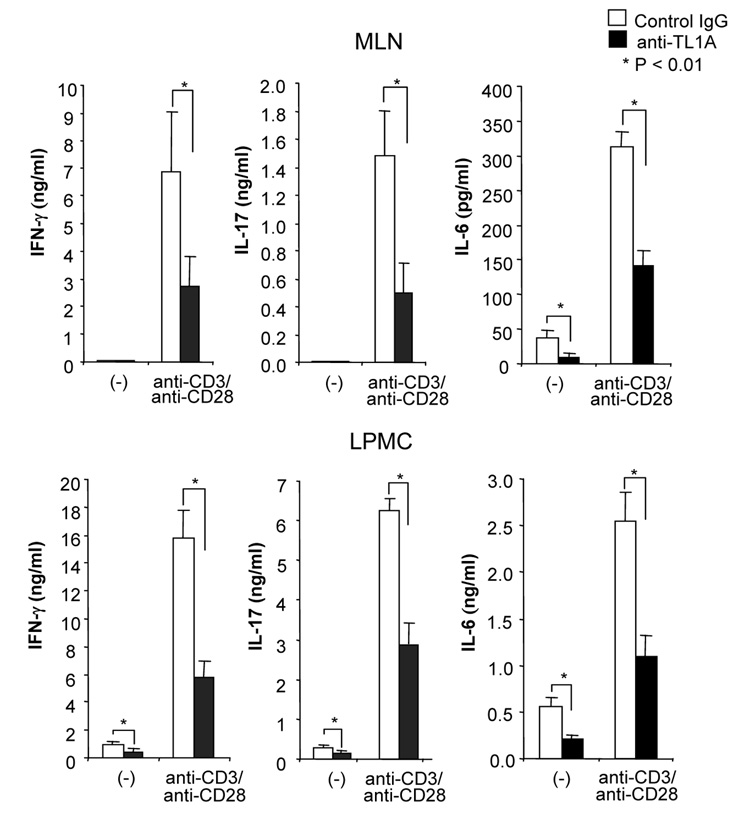
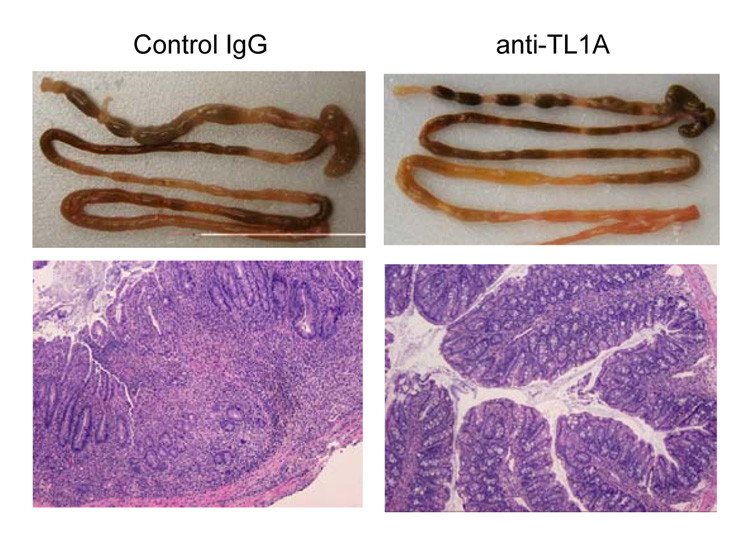
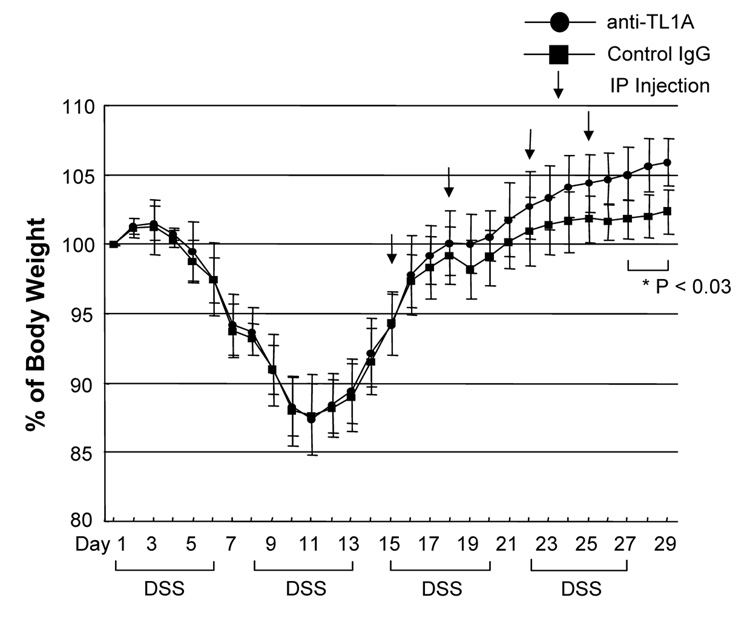
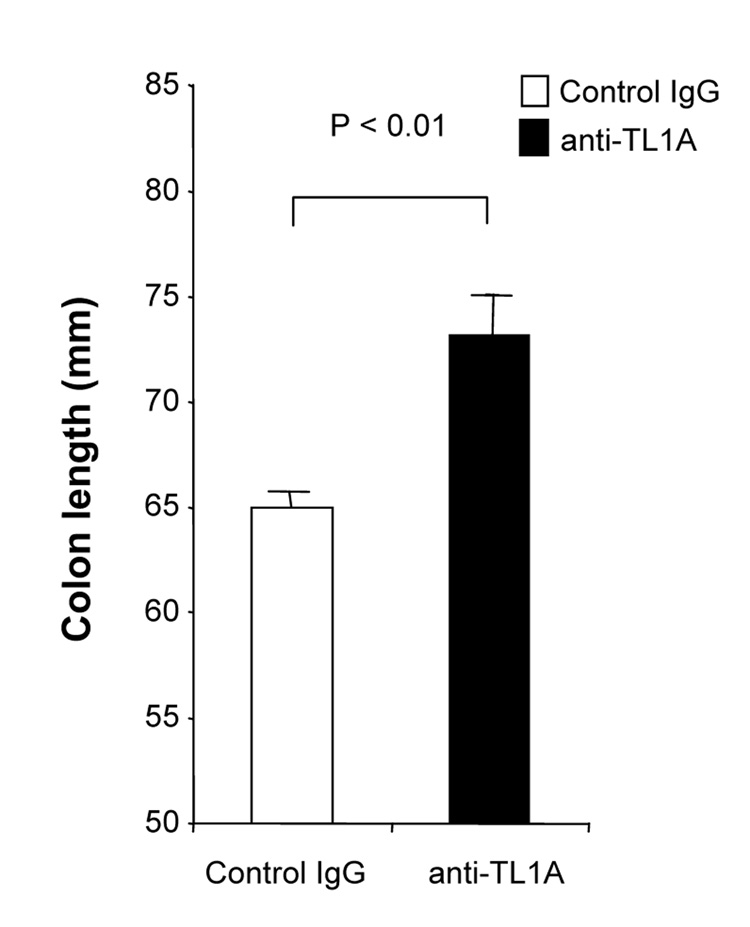
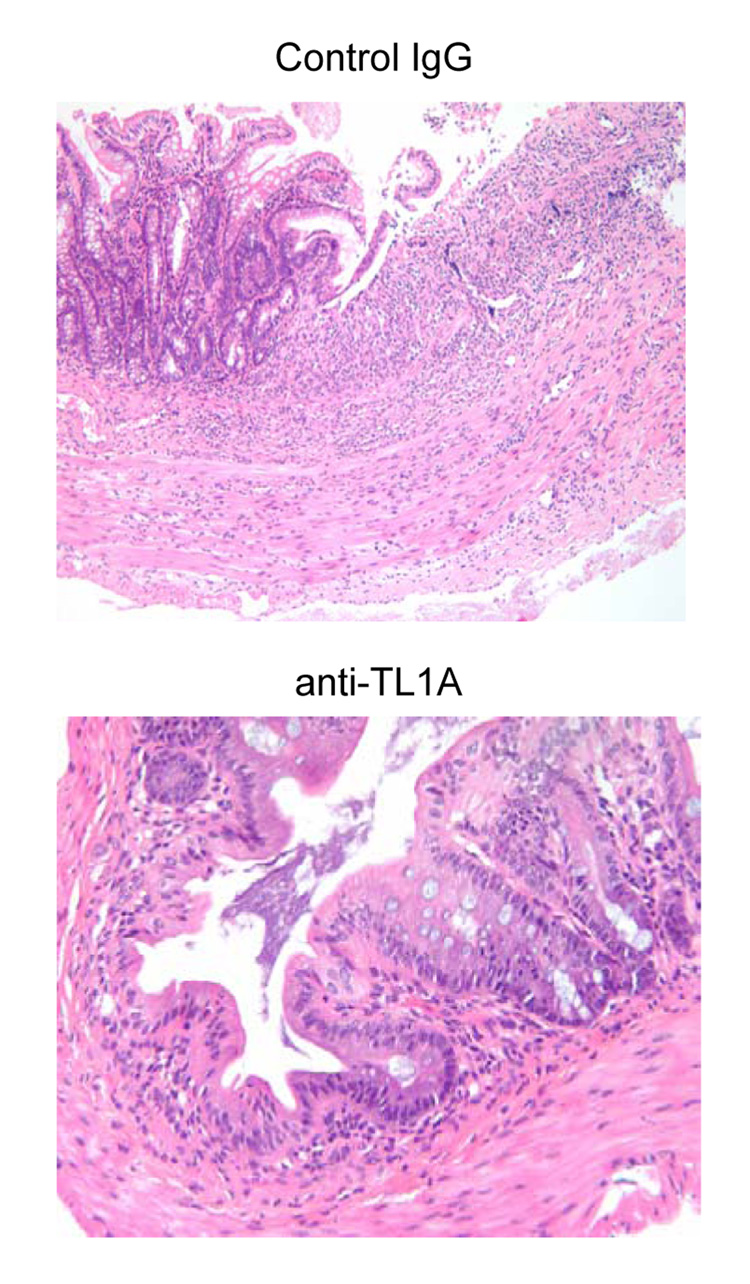
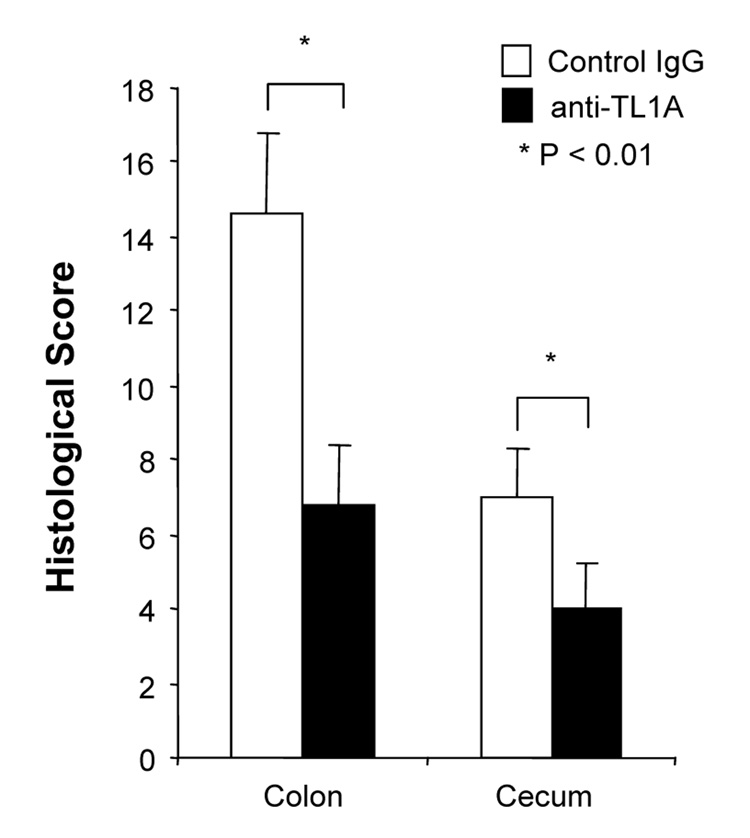
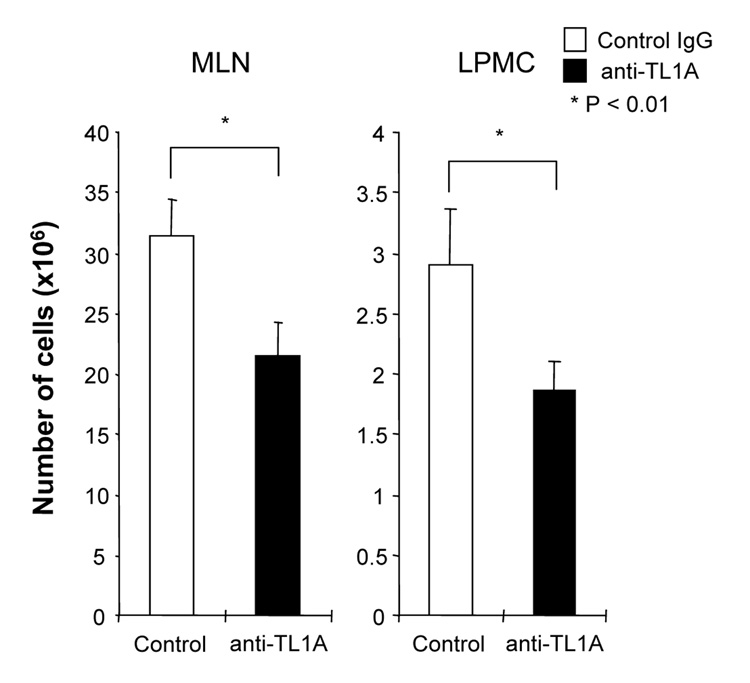
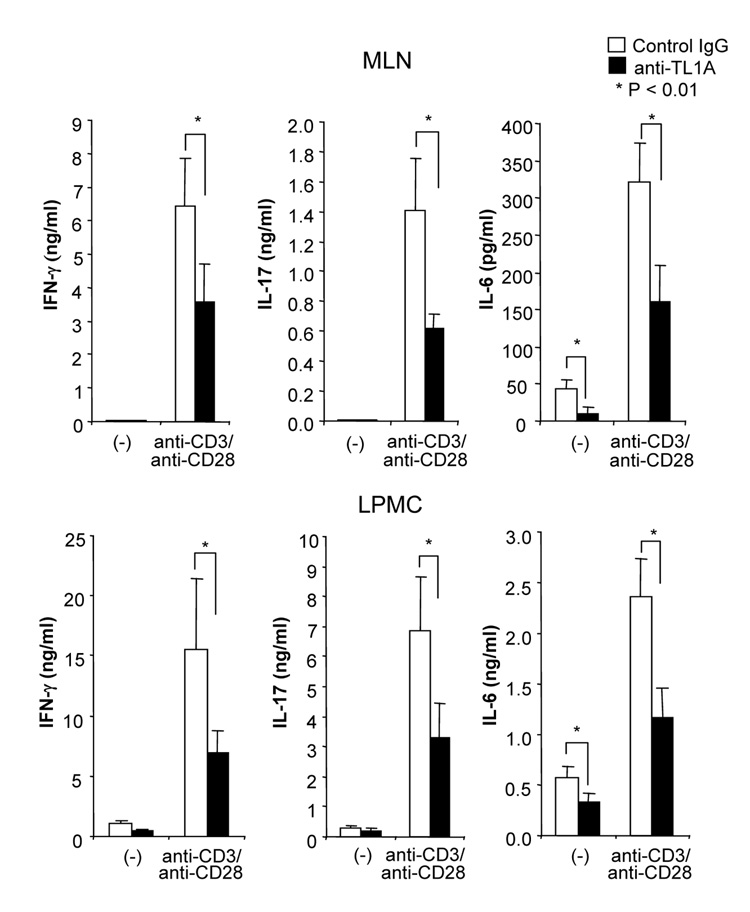
To investigate the role of TL1A in this TH1/ TH17 mediated chronic colitis, we utilized anti-mouse TL1A mAb (provided by CoGenesys Inc.) in our mouse model. The neutralizing effect of anti-TL1A mAb was assessed in vitro using MLN and co-culturing cells with IL-12 or IL-23 plus TL1A with increasing concentrations of neutralizing anti-TL1A mAb. Ten µg/ml of anti-TL1A mAb completely neutralized the enhancing effect of TL1A on IFN-γ and IL-17 production (Supplementary figure 1). Because our data showed that TL1A enhanced mucosal T cell activation and IFN-γ, IL-17, and IL-6 production in DSS-induced chronic colitis, neutralizing anti-TL1A mAb were administered once per week in DSS-treated mice starting at day 1 (Figure 4A). Five hundred µg of mAb were administered by repeated intraperitoneal injection on days 1, 8, 15, and 22. Disease severity and cytokine production were evaluated at day 29. Administration of anti-TL1A mAb led to a significant protection as indicated by significant attenuation of weight loss and colon shortening (Figure 4A, B). Histological sections of the cecum and colon revealed reduced mucosal inflammation and injury in anti-TL1A mAb treated mice (Figure 4C). The histological scores and cell numbers in GALT as well as memory CD4+ T cells (data not shown) were significantly reduced in the anti-TL1A mAb treatment group (Figure 4D, E). IFN-γ, IL-17, and IL-6 produced from GALT, were significantly decreased by anti-TL1A mAb treatment (Figure 4F). Thus, in addition to a significant modulation of chronic colitis, anti-TL1A mAb suppressed the production of IFN-γ (TH1) and IL-17/IL-6 (TH17) cytokines from mucosal T cells.
Figure 4. Attenuation of TH1 and TH17 responses during DSS-induced chronic colitis by the administration of neutralizing TL1A mAb.
500 µg anti- TL1A mAb or control IgG as control were injected i.p. once per week into mice receiving DSS (n=10 per group). (A) Body weights as percentage of initial weight at day 1 were shown. (B) Left panel: Photographs of representative colons from DSS-treated mice receiving anti-TL1A mAb or control are shown. Right panel: Colon length from cecum to rectum. (C) H&E staining of cecum and colon (Original magnification: x 100). (D) Evaluation of histological score. (E) Mononuclear cell numbers of GALT. (F) IFN-γ, L-17, and IL-6 productions from GALT.
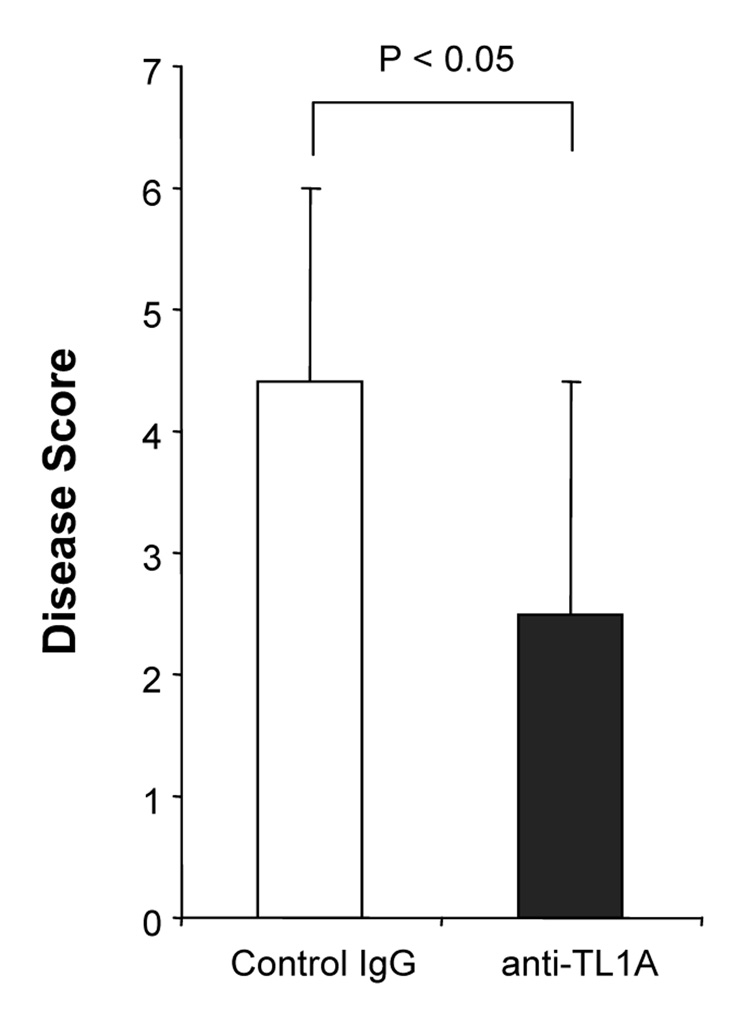
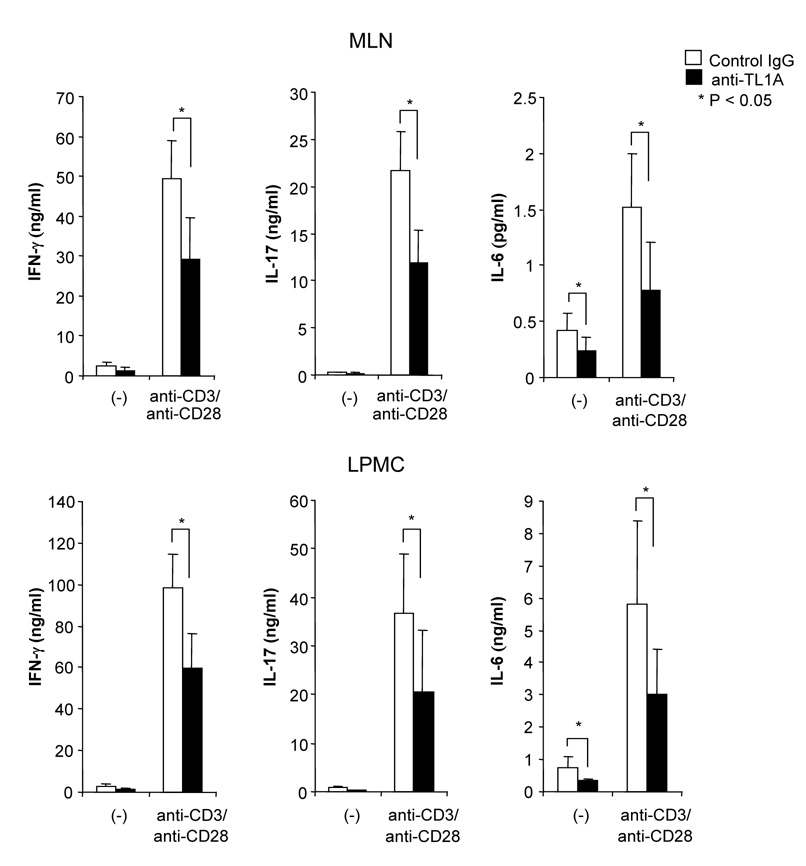
Next, we evaluated whether anti-TL1A treatment prevents the development of intestinal inflammation in a second model of T cell-mediated chronic colitis, namely the G protein αi2−/− T cell transfer model (Gαi2−/−). Gαi2−/− mice are well known as a spontaneous colitis model.28 Mice intravenously receiving Gαi2−/− T cells, cells that produce large amounts of both IFN-γ and IL-17 (data not shown), developed severe colitis within 3 weeks.29 Injections of 500 µg anti-TL1A mAb (twice per week) significantly improved thickness of colon and infiltration of inflammatory cells into the mucosa (Figure 5A). Disease scores were also improved by anti-TL1A mAb treatment (Figure 5B). Cytokine productions from GALT were decreased in the anti-TL1A mAb treatment group. (Figure 5C) These results demonstrated that anti-TL1A mAb is also effective in a second T cell-mediated model of colitis by down-regulating T cell activation.
Figure 5. Attenuation of T cell-mediated colitis in Gαi2−/− T cells transferred model by anti-TL1A mAb.
(A) Severe T cell-mediated colitis was induced by Gαi2−/− T cells transferred into RAG−/− mice. 500 µg anti-TL1A mAb or control IgG i.p. injections twice per week were started at day 1 (n=10 per group). Mice were sacrificed 4 weeks after cell transfer. Upper panel: Photographs of representative ileum and colons from Gαi2−/− T cells transfer induced colitis receiving anti-TL1A mAb or control IgG are shown. Lower panel: H&E staining of proximal colon (Original magnification: x 100). (B) Disease scores (n=9 and 10 for control IgG and anti-TL1A, respectively). (C) Cytokine productions from GALT were measured by ELISA (n=6 mice per group).
Anti-TL1A mAb treatment improves clinical symptoms of established chronic colitis
Next, we investigated whether anti-TL1A mAb attenuated established chronic intestinal inflammation. Anti-TL1A mAb or control IgG were injected twice per week into mice that had received 2 cycles of DSS administration (Figure 6A). The anti-TL1A mAb treatment group regained body weight faster than the control group (Figure 6A). The colon shortening and the histological scores of cecum and colon were significantly improved by anti-TL1A mAb treatment (Figure 6B, C, D). Cell numbers in GALT and production of IFN-γ, IL-17, and IL-6 were significantly decreased by anti-TL1A mAb treatment (Figure 6E, F). Therefore, these results clearly revealed that in addition to preventing colitis, anti-TL1A mAb administration efficiently improves established chronic colitis.
Figure 6. Effect of neutralizing TL1A mAb after the development of DSS induced chronic colitis.
Treatment with mAb was started after 2 cycles of DSS (n=5 per group). (A) 500 µg anti-TL1A mAb or control IgG i.p. injections twice per week were started at day 15. Body weights as percentage of initial weight at day 1 were shown. (B) Colon length from cecum to rectum. (C) H&E staining of representative colons from DSS-treated mice receiving control IgG (upper panel; Original magnification: x 100) or anti- TL1A mAb (lower panel; x 200) or are shown. (D) Evaluation of histological score. (E) Mononuclear cell numbers (F) Cytokine production from GALT were measured by ELISA.
Anti-IL-23 receptor mAb attenuates DSS-induced chronic colitis
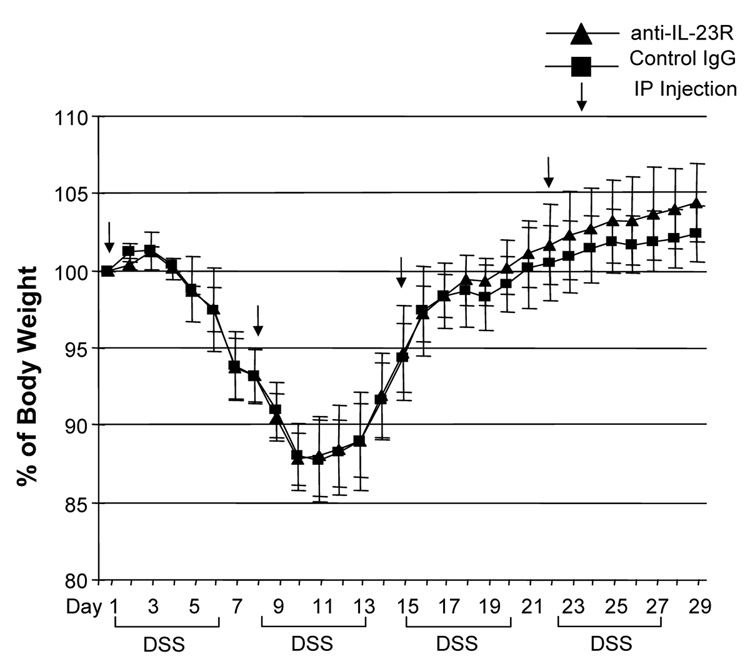
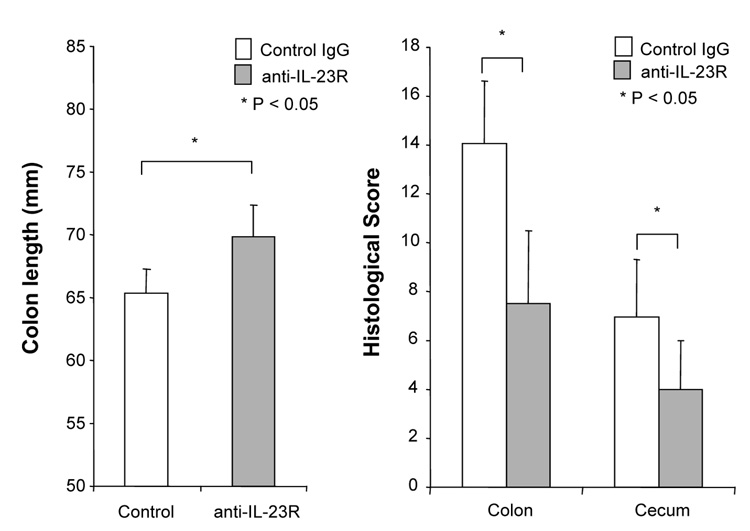
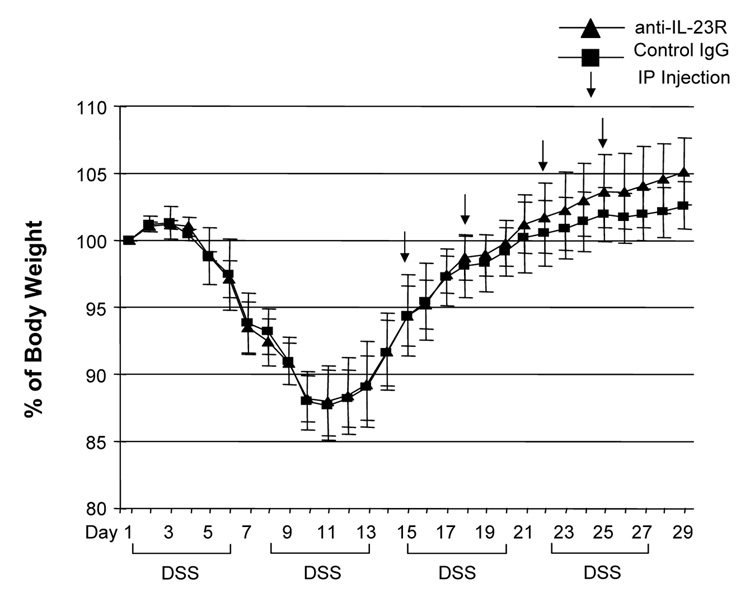
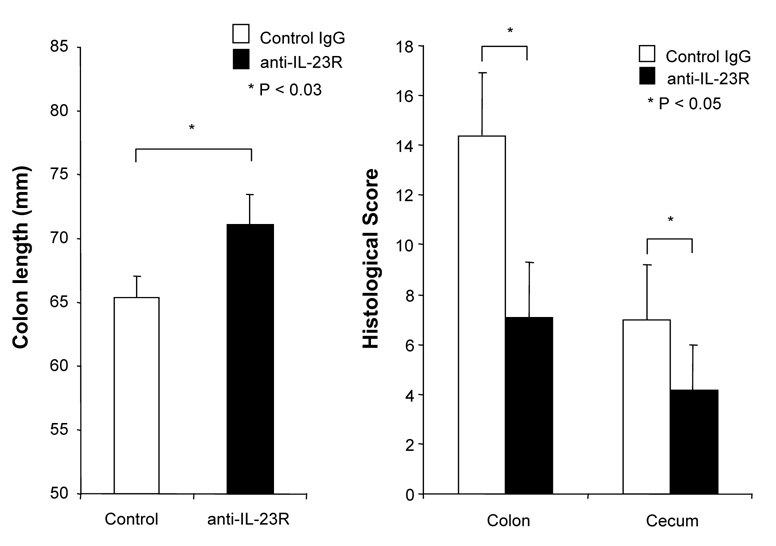
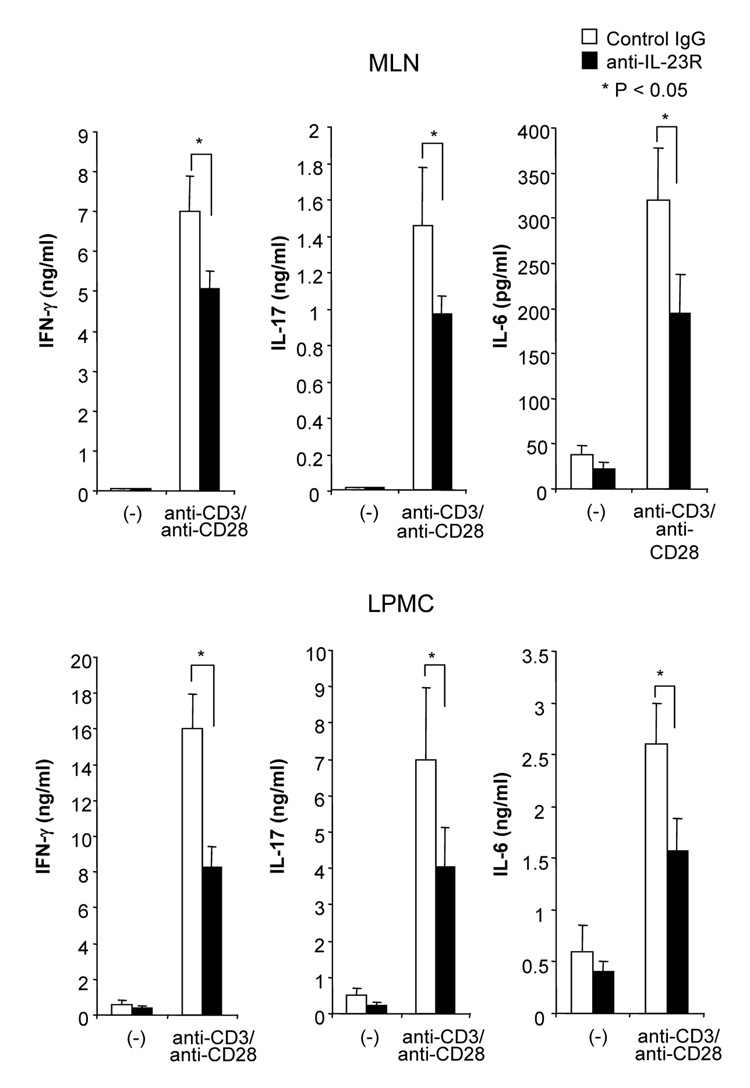
Although several studies suggested that the IL-12/IFN-γ pathway was involved in DSS-induced colitis, the role of the IL-23/IL-17 pathway is still unknown. We treated DSS-induced colitis with anti-mouse IL-23 receptor (IL-23R) mAb to investigate the role of IL-23. The neutralizing effect of anti-IL-23R mAb was assessed in vitro by stimulating MLN cells with IL-23 alone or IL-23 plus TL1A. Two µg/ml of anti-IL-23R mAb completely neutralized the effect of IL-23 on IL-17 production (Supplementary Figure 2). Next, anti-IL-23R mAb or control IgG was administered into DSS-treated mice by weekly i.p. injections (Figure 7A). Administration of anti-IL-23R mAb showed limited protective effects as indicated by minimal attenuation of weight loss in the chronic phase of colitis (Figure 7A). The colon shortening and histological scores were significantly improved in the anti-IL-23R mAb treatment group (Figure 7B). Furthermore, to investigate whether anti-IL-23R mAb could attenuate chronic intestinal inflammation after the development of colitis, anti-IL-23R mAb was injected twice per week after 2 cycles of DSS administration. Body weight in the anti-IL-23R mAb treatment group was better restored than in the control group (Figure 7C). The colon shortening and the histological scores of cecum and colon were improved by anti-IL-23R mAb (Figure 7D). Anti-IL-23R treatment induced a significant decrease of both TH1 and TH17 cytokine production (Figure 7E). Thus, in addition to an attenuation of chronic colitis, anti-IL-23R mAb suppressed the production of not only IL-17/IL-6 (TH17), but also IFN-γ (TH1) cytokines from mucosal T cells. These results suggested that IL-23 is at least partially involved in the pathogenesis of DSS-induced chronic colitis, but not acute colitis.
Figure 7. Neutralizing IL-23R mAb attenuates DSS-induced chronic colitis.
100 µg anti-mouse IL-23R mAb or control IgG were injected i.p. once per week into mice receiving DSS-drinking water (n=5 per group). (A) Body weights as percentage of initial weight at day 1. (B) Colon length and histological scores.
100 µg anti- IL-23R mAb or control IgG i.p. injections twice per week were started at day 15 (n=5 per group). (C) Body weights as percentage of initial weight at day 1 were shown. (D) Colon length and histological score. (E) Cells from MLN and LP from mice receiving control IgG, or anti-IL-23R mAb from day 15 were cultured with or without anti-CD3ε and anti-CD28 Ab for 72 h. Cytokine productions were measured by ELISA (n=5 per group).
Discussion
This study demonstrates for the first time that TL1A plays a causal role in the development and perpetuation of T cell-mediated chronic colitis by enhancing both IFN-γ and IL-17 production from mucosal CD4+ T cells. TL1A and DR3 were significantly up-regulated in the intestine of DSS-treated mice, which confirms previous studies in human CD and murine ileitis.9, 11 We and others have also shown that TL1A was expressed on monocytes and CD11chigh MHC class II+ DCs and transmembrane DR3 was expressed on activated lymphocytes.9, 14 TL1A preferentially acts on CD4+CD45RBlow memory T cells since they have high expression of the TL1A receptor DR39, 14. The percentage of CD4+CD45RBlow memory T cells in LP is higher than MLN cells, resulting in TL1A being more effective in LP than in MLN. Early therapeutic trials support the role for both the IFN-γ 5 as well as the IL-23 pathways 6 in the pathogenesis of subsets of CD. Therefore, inhibiting an important molecule for mucosal production of both cytokines could be a very effective therapeutic, targeting a broader population of CD patients.
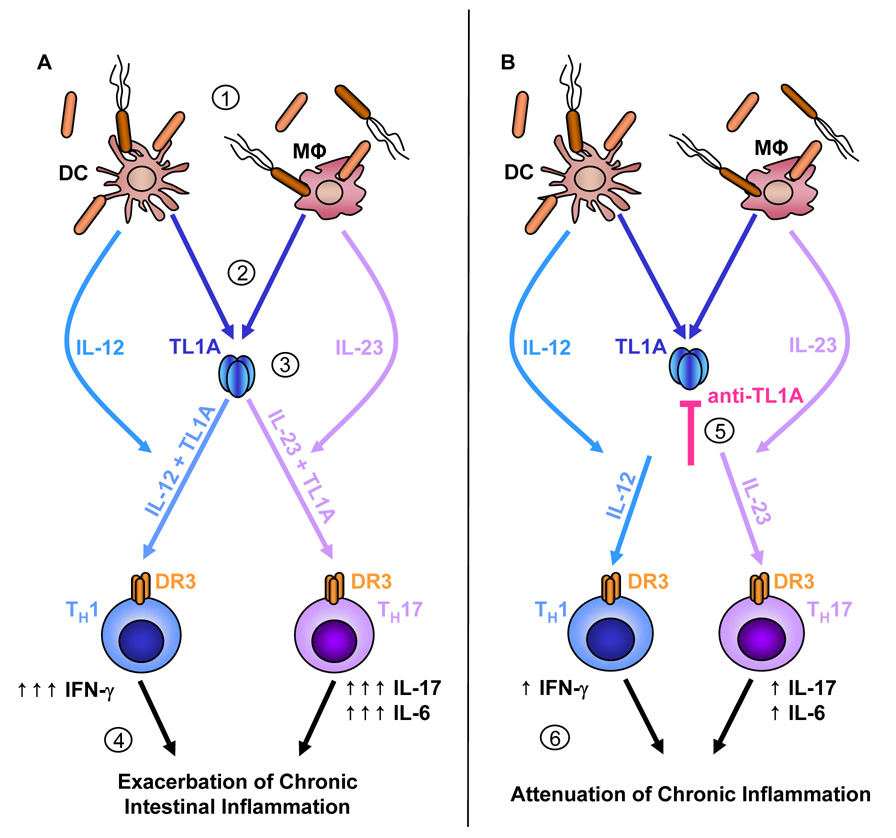
IL-23 has been demonstrated to be required for survival and TH17 effector function rather than TH17 differentiation.26, 30 Interestingly, a small number of IFN-γ/IL-17 double positive CD4+ T cells was generated by stimulation with IL-23 and IL-23 plus TL1A. Although some studies showed the presence of a small number of IFN-γ/IL-17 double positive CD4+ T cells induced by IL-23, the function of this population has not been elucidated.25, 31 In contrast, IFN-γ induced by IL-12 inhibited IL-17 production from CD4+ T cells, which is consistent with a previous publication.31 Taken together, these findings indicated that this T cell population was induced by the stimulation of IL-23, but not IL-12, indicating it might arise from TH17 cells. The activation of the IL-23/IL-17 pathway is suppressed by IFN-γ, even though both the IL-12/IFN-γ pathway and the IL-23/IL-17 pathway have been implicated in autoimmune diseases including IBD. In spite of this discrepancy, TL1A appears to directly affect both TH1 and TH17 cells and produce both IFN-γ and IL-17. These findings identified TL1A as a central cytokine that augments TH1 and TH17 activation in conjunction with IL-12 and IL-23, particularly in host/bacterial pathogen mucosal responses in intestinal inflammation (Figure 8).
Figure 8. TL1A plays a pivotal role in the development of TH1 and TH17 responses during chronic colitis.
(A) During chronic colitis, the integrity of the intestinal epithelial barrier is compromised allowing commensal bacteria to enter LP where they interact with APC present (1). Stimulation of APC, LP tissue macrophages or DC, by commensal bacteria leads to an up-regulation of TL1A (2). APC also secrete IL-12 and IL-23 upon encountering commensal bacteria. TL1A synergistically enhances the potential of IL-12 or IL-23 to induce IFN-γ or IL-17/IL-6 secretion by TH1 or TH17 cells, respectively (3). Overwhelming production of these cytokines leads to exacerbation of chronic intestinal inflammation (4). (B) Administration of neutralizing TL1A antibodies during chronic inflammation diminishes the secretion of IFN-γ, IL-17 and IL-6 by TH1 or TH17 cells, respectively (5). Thus, blocking TL1A leads to attenuation of chronic inflammation while maintaining the ability to clear pathogenic bacteria (6).
Several studies in animal models of IBD support the role of the IL-12/IFN-γ pathway in TH1-mediated injury in the intestine.4, 32, 33 In DSS colitis, intestinal inflammation was ameliorated in IL-12p35−/− and IFN-γ−/− mice.21, 34 Recent studies demonstrated that a pathogenic IL-23-dependent TH17 CD4+ T cell population characterized by the production of IL-17 and IL-6 is essential for the establishment of organ-specific inflammation associated with autoimmunity and IBD.24, 25 However, its role in DSS-induced acute colitis is controversial. One study showed that the IL-17/IL-17R pathway was involved in intestinal epithelial damage in DSS-induced acute colitis.20 In contrast, another study reported IL-23 was not required for the development of acute colitis, because colitis was exaggerated in IL-23p19−/− mice.35 IL-23 has been shown to be necessary for promoting chronic intestinal inflammation in C3H/HeJBir CD4+ T cell transfer model.36 It is therefore possible that IL-23 is required for the development of DSS-induced chronic colitis, but not acute colitis and our data presented here support this conclusion. Treatment of DSS-induced colitis with anti-IL-23R mAb had no effect on weight loss during the acute phase of colitis and minimal effect on weight loss during the chronic phase of colitis. The limited effects of anti-IL-23R mAb in this chronic colitis model suggests that not only the IL-23/IL-17 pathway, but also the IL-12/IFN-γ pathway seem to be important for the development of chronic intestinal inflammation. Treatment with p40 antibodies suggests that suppression of both IL-12 and IL-23 is required for prevention of colitis in human CD and animal models of IBD.35, 37 For these findings, both the IL-12/IFN-γ pathway and the IL-23/IL-17 pathway seem to be important for the development of intestinal inflammation. Administration of anti-TL1A mAb attenuated chronic colitis by significantly decreasing the production of IFN-γ, IL-17, and IL-6 in GALT. This effect was likely due to the fact that TL1A markedly enhanced IL-12 and IL-23 induced IFN-γ and IL-17 production from CD4+ T cells of the GALT. However, colitis was not completely eliminated. The most plausible explanation for our observation is that anti-TL1A mAb only suppresses IFN-γ and IL-17 production to the degree that their production is enhanced by TL1A, without affecting basal IL-12, or IL-23-induced IFN-γ or IL-17 production (Figure 8). Therefore, IL-12 and IL-23 still could induce some, albeit less, IFN-γ and IL-17 production. Given the need for IL-12 and IL-23 for host protection of intra and extra cellular mucosal pathogens, this residual capacity to produce IFN-γ and IL-17 could avoid infection complications while attenuating mucosal inflammation.
In summary, our data provide the first evidence that TL1A acts as a central regulator in the development of T cell mediated chronic intestinal inflammation by directly enhancing IFN-γ production from TH1 cells, and IL-17 and IL-6 production from TH17 cells alone and in combination with IL-12 and IL-23, respectively. Accumulating evidence from our current study and previously published data suggest that TL1A is a relevant target for the treatment of IBD, especially CD.
Supplementary Material
Acknowledgments
The authors would like to thank CoGenesys Inc. (Rockville, MD) for generously providing us with the 19E06A hybridoma. We would also like to thank Dr. Charles Elson (University of Alabama at Birmingham, Birmingham) for providing us with anti-IL-23R antibody.
Grant Support; This work was supported by the US National Institute of Health grants R01 DK056328 (S.R.T.) and DK046763 (S.R.T., J.B.).
Abbreviations
- APC
Antigen-presenting cell
- DC
dendritic cell
- DSS
dextran sodium sulphate
- DR
death receptor
- ELISA
enzyme linked immunosorbent assay
- GALT
gut-associated lymphoid tissue
- IFN
interferon
- IL
interleukin
- LP
lamina propria
- mAb
monoclonal antibodies
- MC
mononuclear cells
- MHC
major histocompatibility complex
- MLN
mesenteric lymph node
- rm
recombinant mouse
- RT-PCR
real-time polymerase chain reaction
- TH
T helper cell
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures; The authors declare no competing financial interests.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Hommes DW, Mikhajlova TL, Stoinov S, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 7.Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 8.Prehn JL, Thomas LS, Landers CJ, et al. The T Cell Costimulator TL1A Is Induced by Fc{gamma}R Signaling in Human Monocytes and Dendritic Cells. J Immunol. 2007;178:4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 9.Bamias G, Mishina M, Nyce M, et al. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadakis KA, Zhu D, Prehn JL, et al. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174:4985–4990. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]
- 11.Prehn JL, Mehdizadeh S, Landers CJ, et al. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Cassatella MA, da Silva GP, Tinazzi I, et al. Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis. J Immunol. 2007;178:7325–7333. doi: 10.4049/jimmunol.178.11.7325. [DOI] [PubMed] [Google Scholar]
- 13.Bamias G, Martin C, 3rd, Marini M, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 14.Papadakis KA, Prehn JL, Landers C, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 15.Picornell Y, Mei L, Taylor K, et al. TNFSF15 is an ethnic-specific IBD gene. Inflamm Bowel Dis. 2007;13:1333–1338. doi: 10.1002/ibd.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki K, McGovern D, Ragoussis J, et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn's disease. Hum Mol Genet. 2005;14:3499–3506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 17.Dieleman LA, Ridwan BU, Tennyson GS, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 18.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 21.Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–338. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Yoshizaki K, Kishimoto T, et al. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 23.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 24.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Hornquist CE, Lu X, Rogers-Fani PM, et al. G(alpha)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J Immunol. 1997;158:1068–1077. [PubMed] [Google Scholar]
- 29.Wei B, Velazquez P, Turovskaya O, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Flavell RA, et al. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 31.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 32.Neurath MF, Fuss I, Kelsall BL, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson SJ, Shah S, Comiskey M, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takagi H, Kanai T, Okazawa A, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 35.Becker C, Dornhoff H, Neufert C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, Cong Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 37.Fuss IJ, Becker C, Yang Z, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn's disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006;12:9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 38.Yoshihara K, Yajima T, Kubo C, et al. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut. 2006;55:334–341. doi: 10.1136/gut.2005.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aranda R, Sydora BC, McAllister PL, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.