Abstract and Introduction
Abstract
Primary sclerosing cholangitis (PSC) is a rare, chronic cholestatic liver disease of uncertain etiology characterized by the destruction of the intrahepatic and extrahepatic bile ducts through inflammation and fibrosis. This development of inflammation and fibrosis leads to biliary complications including cirrhosis and ultimately death. Given the uncertainty surrounding the pathogenesis of the disease, many different medical therapies have been studied in the treatment of PSC. However, there currently are no effective medical therapies known to halt progression of disease. Additionally, patients with PSC often develop symptoms and complications that can be managed endoscopically or surgically. This review primarily focuses on endoscopic and surgical approaches that have been studied in the treatment of patients with PSC.
Introduction
PSC is a rare, chronic cholestatic liver disease characterized by the destruction of the intrahepatic and/or extrahepatic bile ducts through chronic inflammation and fibrosis, leading to biliary complications including bile stasis, cirrhosis, and ultimately liver transplantation or death by 7-12 years after diagnosis.[1,2] The cause of PSC is unknown, and even though it is thought that the disease may have an autoimmune origin, PSC often responds unfavorably to immunosuppressive therapy.[1,2] This continued uncertainty surrounding the pathogenesis of this disease has hindered the development of effective medical management. There is still no evidence supporting a specific medical therapy capable of halting disease progression in PSC. Therefore, the only definitive therapy for patients with PSC who have advanced disease is liver transplantation.[2]
Patients with PSC often present with abnormal liver biochemistries or with symptoms typical of PSC such as fatigue, pruritus, or jaundice.[1] Presently, the management of PSC remains focused on treating the symptoms and complications that are associated with disease progression as well as close surveillance of those patients with advanced liver disease. In addition to medical management, patients with PSC are also treated endoscopically and surgically.
Endoscopic Therapy in Primary Sclerosing Cholangitis
The progressive fibrosing inflammation of PSC can lead to obliteration of the biliary tree, including large ducts. Subtotal or total stenoses of the right or left hepatic duct close to the bifurcation or of the common duct are considered dominant stenoses, which can lead to severe cholestasis by inhibiting bile flow and increasing biliary pressures. The consequences of cholestasis include jaundice, pruritus, biliary infections, stone formation above the stenosis, and parenchymal liver damage leading to cirrhosis.[3]
Dominant strictures occur in 15%-20% of patients with PSC and are often difficult to manage, particularly as the disease progresses.[4] Management of these strictures can occur through surgical, percutaneous, or endoscopic approaches. The need for repeated interventions, often required in the management of these strictures,[5] makes endoscopy the preferable intervention given its low complication rate.[3] The percutaneous approach is also effective in the treatment of symptoms associated with dominant strictures in PSC, but can be more labor-intensive and technically demanding compared with the endoscopic approach.[6]
Endoscopic therapies include stenting, balloon dilation, or nasobiliary catheter perfusion. One of the first reports of endoscopic management of bile duct strictures in a patient with sclerosing cholangitis was published in 1982.[7] Subsequently, in 1987, Johnson and colleagues[8] reported on 10 patients with sclerosing cholangitis who underwent endoscopic sphincterotomy to improve biliary drainage, and to remove biliary sludge and stones. Of these, 8 patients had balloon dilation and 3 had stent placement. Endoscopic treatment resulted in fewer episodes of cholangitis requiring hospitalization and a significant improvement in liver function test results (ie, serum bilirubin, alkaline phosphatase, and serum transaminases levels). An expanded series using endoscopic therapy in 35 patients with PSC without cirrhosis was reported by the same group in 1991.[9] Patients were again treated with endoscopic sphincterotomies, balloon dilations, stent placement, and even nasobiliary catheter perfusion. As seen in the earlier report,[8] episodes of cholangitis requiring hospitalization were significantly reduced from 2.3 hospitalizations per patient in the year before treatment to 0.4 hospitalizations per patient in the year after treatment. Such clinical improvement also translated into a significant decrease in serum bilirubin levels. Furthermore, it is of note that patients who underwent balloon dilation without stent placement had fewer complicating episodes of cholangitis compared with the patients with stents.
Further studies continued to demonstrate the effectiveness of endoscopic therapy in the management of PSC.[10–12] Enns and colleagues[13] tried to define which patients with PSC would benefit the most from endoscopic intervention. After univariate analyses, the authors reported that patients with PSC with common bile duct strictures, any dominant stricture, and those who underwent a therapeutic endoscopic retrograde cholangiopancreatography (ERCP) vs a diagnostic ERCP were most likely to have clinical and laboratory improvement. On multivariate regression analyses, the presence of a dominant stricture, endoscopic therapy, and high serum bilirubin levels were all independent predictors of a successful clinical outcome. However, deciding on the optimal endoscopic treatment has been subject to debate.[4] Even though nasobiliary catheter perfusion has been used with some success in the management of strictures in patients with PSC,[9–11] the main dispute has been about stent placement vs balloon dilation.
Endoscopic Balloon Dilation vs Stenting
Although balloon dilation has the disadvantage of early restenosis, occlusion of a stent may lead to the development of suppurative cholangitis and sepsis.[3] In 1996, van Milligen and colleagues[14] reported on retrospective data from 25 patients with PSC who were endoscopically treated for symptoms secondary to dominant extrahepatic bile duct strictures. The median duration of stent therapy was 3 months, with the policy of electively removing the stent every 2 to 3 months. Even though endoscopic therapy was technically successful in 84% of patients, nonelective therapeutic ERCPs were still needed in 48% of the cases performed for stent exchange or removal secondary to jaundice or cholangitis attributed to stent occlusion. Therefore, long-term stenting is generally less desirable given the greater risk for the development of occlusion and cholangitis. The cholangitis seen in long-term stenting makes short-term stenting more desirable.[15]
In 1997, van Milligan and colleagues[16] reported on 16 symptomatic patients with PSC and dominant extrahepatic bile duct strictures who were treated with stent placement for a median interval of 9 days. Endoscopic therapy was successful in all 16 patients, with only a 7% incidence of transient procedure-related complications. Serum bilirubin, alkaline phosphatase, and gamma-glutamyltransferase (GGT) levels all significantly decreased after stent therapy. Also, the prevalence of fatigue, pruritus, and right upper quadrant pain all decreased significantly after stent therapy. Overall, during the median follow-up of 19 months after stent removal, 81% of patients remained asymptomatic. Additionally, none of the patients had evidence (clinical or biochemical) suggestive of cholestasis that could be attributed to stent occlusion.
Another study in favor of short-term endoscopic stenting was published in 1999.[17] In this study, 32 patients with symptomatic PSC and a dominant stricture were treated with stenting; stents were extracted after a mean of 11 days. Procedure-related complications occurred in 15% of patients, but 83% of patients had improvement in their fatigue, pruritus, and right upper quadrant pain at 2 months after short-term stent therapy. In addition, serum bilirubin, alkaline phosphatase, and GGT levels all decreased significantly when evaluated 2 months after stent therapy. Long-term follow-up showed that at 1 and 3 years, 80% and 60% of patients, respectively, still did not require repeated endobililary therapy.
Balloon dilation is another endoscopic method that can be used to avoid the complications of long-term stent occlusion. However, balloon dilation often requires multiple endoscopic sessions to achieve sustained stricture dilation.[9,11] It has even been reported that balloon dilation alone is often not sufficient for obtaining sustained stricture dilation,[14] but other investigators have reported that balloon dilation can have short- and long-term benefits in patients with PSC and biliary strictures.[18] The immediate effect of balloon dilation in PSC can be observed in Figures 1–3.
Figure 1.
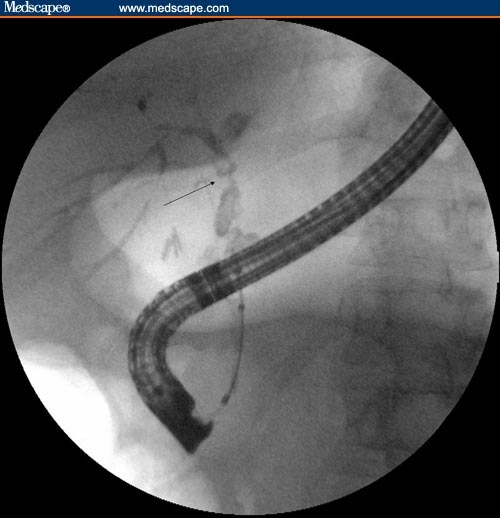
Cholangiogram obtained during ERCP showing a dominant stricture (arrow) involving the common hepatic duct/right hepatic duct.
Figure 2.
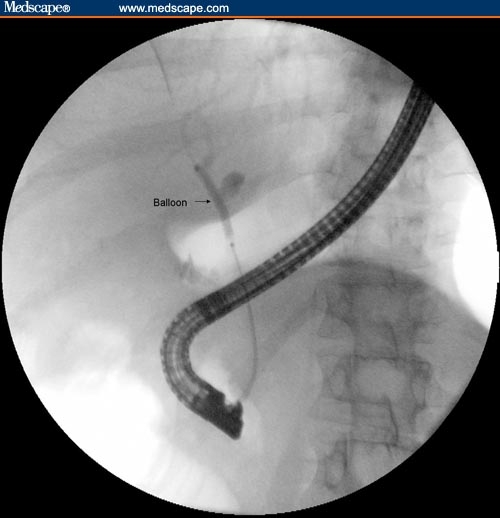
Cholangiogram showing balloon dilation of stricture of common hepatic duct.
Figure 3.
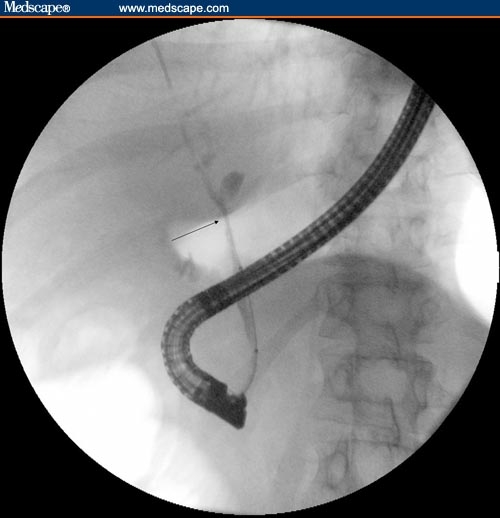
Cholangiogram obtained after balloon dilation of dominant stricture.
One retrospective study published in 2001 compared balloon dilation with balloon dilation plus stenting.[19] In this study, 34 patients were treated with endoscopic balloon dilation alone and 37 patients were treated with endoscopic balloon dilation plus stent placement. Stents were removed or changed after 3-4 months, or when jaundice or cholangitis occurred. Both balloon dilation and balloon dilation with stenting improved biliary drainage, with a decrease in serum bilirubin, but this improvement occurred for up to 2 years in the balloon dilation group compared with only 1 year in the stenting group. Although biliary drainage improved in both groups, no statistically significant benefit in regard to pruritus or abdominal pain occurred in either group in the long-term follow-up. Additionally, more statistically significant complications occurred in the endoscopic stenting group compared with the balloon dilation group. One caveat is that patients in the stenting group may have had more severe disease given that stenting only took place when balloon dilation did not sufficiently improve biliary drainage. Overall, there was no significant difference between the 2 groups with respect to the number of patients who died, required additional therapeutic intervention, or underwent orthotopic liver transplantation. On the basis of this retrospective study, it was the authors' conclusion that stenting did not add benefit after balloon dilation in the treatment of dominant strictures in patients with PSC. Other investigators have also preferred endoscopic balloon dilation over stenting.[20–22] At times, balloon dilation may be the only option because stenting may not even be possible, particularly in patients with a dominant stricture close to the bifurcation, where the stenting of one hepatic duct could hinder the bile flow of the other hepatic duct.[3]
Ursodeoxycholic Acid in Combination With Endoscopic Therapy
Data supporting the use of ursodeoxycholic acid (UDCA) in patients with PSC have been encouraging. Both low-dose (8-15 mg/kg/day) and high-dose (25-30 mg/kg/day) UDCA have been studied and found to lead to improvement in serum liver biochemistries. However, it is clear that low-dose UDCA does not confer survival benefit, as evidenced by a large randomized placebo-controlled study conducted by Lindor.[23] High-dose UDCA, on the other hand, appears to lead to histologic and endoscopic improvement,[24,25] and more recent studies show a trend towards a survival benefit as well.[26,27] A large placebo-controlled study is currently underway at the Mayo Clinic to address this issue.
The efficacy of UDCA has been examined in combination with endoscopic therapy in the management of dominant strictures in patients with PSC. Some study authors have reported no significant differences in improvement of liver biochemistry values after stent therapy between patients receiving vs not receiving UDCA.[16] Stiehl and colleagues[20] studied survival in 65 patients with PSC who were treated with UDCA (750 mg/day) and by endoscopic therapy (consisting of repeated balloon dilatations) when necessary. The mean follow-up period was 45 months. Eighteen percent of patients had major duct stenosis at study entry and another 17% developed major duct stenosis during UDCA treatment which required endoscopic intervention. Thus, the study authors concluded that UDCA given at a dose of 750 mg/day did not prevent major duct stenosis. During follow-up, only 1 patient died due to the development of cholangiocarcinoma and 1 patient required liver transplantation. Both of these patients had a major duct stenosis. The actuarial survival without liver transplantation after treatment with UDCA + endoscopic therapy was significantly improved compared with the predicted survival.
The same group reported similar data in 2002 in a prospective study of 106 patients with PSC treated for up to 13 years with UDCA (8.8-17.4 mg/kg/day) to assess the development of major bile duct stenoses and the efficacy of endoscopic therapy.[21] At study entry, 10% of patients had major duct stenoses, and during median follow-up of 5 years, another 43 patients developed a dominant stenosis. Endoscopic therapy (balloon dilation or stent therapy) was used to treat 98% of the dominant stenoses. Five years after the first endoscopic dilation of a dominant stenosis, the actuarial survival probabilities free of orthotopic liver transplantation were 100%, 72% and 50% in patients with histologic stage 2, 3, and 4 liver disease (at study entry), respectively. Again, the study authors concluded that UDCA was unable to prevent major duct stenosis, which might have been related to the low dose of UDCA used in these cases. Baluyut and colleagues[22] studied the effect of endoscopic therapy on survival in 63 patients with PSC and a dominant stenosis. All patients were on 600-1200 mg/day of UDCA, and the survival of patients with PSC subjected to repeated endoscopic intervention was significantly higher than predicted based on the Mayo Risk Score.
When dominant strictures occur in patients with PSC, their clinical course can deteriorate quickly, thus making endoscopic management a valuable therapy. Given that dominant strictures often require repeated interventions, endoscopy with its lower complication rate is the preferable method vs surgical or percutaneous approaches.[3] The complication rate after endoscopic therapy for dominant strictures in patients with PSC has been reported to range between 7% and 18%.[10, 13–15, 17] Complications include cholangitis, sepsis, pancreatitis, bleeding, perforation of the biliary tract, and biliary leak, all of which are typically transient, and without related mortality.[12, 14–17, 19, 20]
It appears that long-term stenting should generally be avoided in patients with PSC and dominant strictures given the increased risk for suppurative cholangitis. Balloon dilation and short-term stenting are 2 other options that have been shown to have positive results in the management of PSC in patients with a dominant stricture. The addition of UDCA with endoscopic therapy in this patient population has been shown to improve survival in some studies.[20–22] Unfortunately, randomized trials comparing the various endoscopic therapeutic options and their efficacy in maintaining patency in biliary strictures are not available. Although we cannot yet provide strong recommendations regarding the optimal endoscopic intervention for the management of dominant strictures in PSC,[28] it is clear that antibiotics should be given routinely in connection with endoscopic intervention.[29] It is also imperative not to overlook the possibility of cholangiocarcinoma, particularly in patients with long-segment strictures.[3,11]
Surgical Management of Primary Sclerosing Cholangitis
Surgery also has a role in the management of PSC. Liver transplantation is the procedure of choice in patients with diffuse ductal involvement with deteriorating liver function and cirrhosis.[2] However, some experts have advocated extrahepatic resection in symptomatic patients with PSC with a dominant extrahepatic stricture.[30] In 1998, Ahrendt and colleagues[31] examined the results of extrahepatic biliary resection and long-term transhepatic stenting (50 patients), liver transplantation (21 patients), nonoperative endoscopic biliary dilation with or without percutaneous stenting (54 total patients with 19 patients only receiving percutaneous stenting), and medical therapy (28 patients) in the treatment of patients with PSC. In general, the extrahepatic biliary resections included extrahepatic biliary tree and hepatic duct bifurcation resections, followed by intrahepatic biliary tree dilations and stenting with cholangiojejunostomies performed between individual intrahepatic ducts and a Roux-en-Y loop of jejunum. In the noncirrhotic patients (75%), bilirubin levels were significantly reduced 1 and 3 years after operative resection and long-term stenting, but not significantly reduced after nonoperative endoscopic or percutaneous management. In addition, in the noncirrhotic patients, survival until death or transplantation (82% vs 46%) and overall 5-year survival (85% vs 59%) were significantly improved (P < .05) in the operative group with long-term stenting vs nonoperative dilation with or without stenting. Another interesting finding was that cholangiocarcinoma did not develop in the surgically resected patients during follow-up. As expected in the cirrhotic patients, liver transplantation led to longer survival compared with operative resection (3-year survival of 80% vs 38%). In patients who underwent transplantation, prior biliary surgeries led to longer operative times during transplant, but operative mortality was not statistically different between patients with prior biliary surgery vs patients without prior biliary surgery. Even though the surgically treated patients had improved survival compared to the nonoperative patients, more procedure-related complications and longer hospital stays occurred in this group compared with the endoscopically treated patients. However, patients with percutaneous stenting actually had more procedure-related complications (42% vs 32%) and deaths (10.5% vs 6%) than the surgically resected patients.
Other study authors have also reported positive results, including improvement in jaundice and survival, with extrahepatic biliary resection or bypass with or without stenting in noncirrhotic patients with PSC.[32–36] Overall, endoscopic management should be the initial approach to patients with PSC and dominant strictures. However, when endoscopy fails, then extrahepatic biliary resection with transhepatic stenting can be considered for noncirrhotic patients in centers with appropriate expertise.[37]
Liver Transplantation in Primary Sclerosing Cholangitis
Although medical therapy, balloon dilation, or stenting may help to alleviate some of the symptoms associated with PSC, the only definitive treatment for patients with end-stage liver disease due to PSC is orthotopic liver transplantation (OLT).[38,39] Patients with PSC account for approximately 5%-10% of patients undergoing OLT in North America.[39,40] The long-term outcomes of patients with PSC undergoing OLT have been favorable, with 5-year survival rates reported at 83%-89%.[38,39,41,42] However, complications can still arise after OLT, including infection, hepatic artery thrombosis, biliary leaks, acute and chronic rejection, biliary strictures (anastomotic and nonanastomotic), and recurrent PSC.[38,39] Both anastomotic (8.1%-16.2%) and nonanastomotic (5.4%-27.2%) biliary strictures have been reported to occur frequently after OLT.[38,39] Due to the association of biliary strictures in patients with PSC, Roux-en-Y biliary reconstruction has been the preferred surgical method during OLT in these patients.[38,43] Duct-to-duct anastomosis during OLT has also been reported to be effective in patients with PSC[44] and the presence of previous biliary surgery has been reported to have no effect on the survival of patients with PSC after OLT.[38,39,42] One complication that has been reported to affect graft survival after OLT in patients with PSC is the development of recurrent PSC,[38] although some studies have disputed this finding.[39,41,45,46]
The incidence of recurrent PSC following OLT has been reported to range from 9% to 20%,[39, 42,45–50] to as high as 50% after living donor liver transplantation.[51] Graziadei and colleagues[45] defined recurrent PSC after liver transplantation by confirmation of PSC prior to transplantation and either the development of intrahepatic and/or extrahepatic biliary stricturing, beading, and irregularity 90 days after transplantation, or liver histology with fibrous cholangitis and/or fibro-obliterative lesions with or without ductopenia, biliary fibrosis, or biliary cirrhosis. However, given sampling variability on histology and the difficulty in differentiating recurrent PSC from ongoing chronic rejection, a histologic gold standard in diagnosing recurrent PSC is lacking; therefore, the diagnosis is based primarily on cholangiographic findings.[52] Exclusion criteria for the diagnosis of recurrent PSC include hepatic artery stenosis, established ductopenic rejection, anastomotic stricture alone, nonanastomotic strictures before posttransplantation day 90, and ABO incompatibility between donor and recipient.[45] Graziadei and colleagues[45] found the mean time to the development of cholangiographic and histologic evidence of recurrent PSC was 421 days and 1380 days, respectively. The study authors also found no clinical differences in patients with and without recurrent PSC after OLT. Although 92% of patients with recurrent PSC had associated inflammatory bowel disease (IBD) compared with only 76% of the remainder of patients with PSC, this finding did not reach statistical significance. The study authors also reported that patients with recurrent PSC tended to have significantly elevated serum alkaline phosphatase levels compared with the nonrecurrent group. Other reported risk factors for recurrent PSC after OLT include the presence of a specific human leukocyte antigen (HLA-DRB1*08), acute cellular rejection, steroid-resistant acute cellular rejection, multiple episodes of rejection, presence of a cytomegalovirus infection, maintenance steroids for ulcerative colitis (> 3 months) after OLT, the presence of cholangiocarcinoma prior to OLT, the use of OKT3 (anti-CD3 antibody) during episodes of rejection, male sex, and intact colon before transplantation.[47–50, 53–56]
Overall, recurrent PSC can be a challenging problem after OLT. Campsen and colleagues[49] found that once recurrent PSC was diagnosed, the median survival without receiving a second liver transplantation was 39.1 months. Our group[56] also reported that recurrent PSC significantly affects graft survival, but overall patient survival is not different between OLT patients with and without recurrent PSC. Currently, there are no known therapies to delay the presentation or progression of recurrent PSC in the transplanted liver.[57]
Inflammatory Bowel Disease and Primary Sclerosing Cholangitis
In addition to the risk for recurrent PSC and other complications after OLT, patients with PSC also pose other clinical dilemmas. Seventy-five percent of PSC patients will have concomitant IBD.[2,58] A colectomy prior to transplant may lead to severe hepatic decompensation, hindering the patient's availability to undergo OLT.[59] The preferred surgical procedure for patients with PSC and concomitant ulcerative colitis who need a colectomy is a proctocolectomy with ileal pouch anal anastomosis (IPAA). Even though pouchitis is a concern with IPAA, the decreased bleeding risk makes IPAA a more favorable surgical technique compared with a Brooke ileostomy with its higher incidence of peristomal varices.[60,61] However, 2 of the main concerns in patients with PSC and ulcerative colitis undergoing evaluation for OLT are determining if a colectomy is needed and the timing of the colectomy. Patients with ulcerative colitis and PSC have been reported to be at a higher risk for the development of colorectal dysplasia or cancer compared with patients with ulcerative colitis alone.[62,63] Vera and colleagues[64] reported 152 patients with PSC who underwent 173 liver transplantations. They found that the incidence of colorectal cancer in patients with PSC and ulcerative colitis with an intact colon after OLT was 5.3%, with a cumulative risk of developing colorectal cancer of 14% and 17% at 5 and 10 years, respectively. A multivariate analysis revealed that colonic dysplasia after transplantation, duration of colitis greater than 10 years, and pancolitis were all significant risk factors for developing colorectal cancer. Some study authors have also reported that ulcerative colitis can have a more aggressive course after OLT in patients with PSC with preexisting ulcerative colitis (ie, before transplantation),[65] although data regarding this relationship have been conflicting.[66]
Given these factors, some experts have advocated colectomy before or during OLT in patients with PSC and concomitant ulcerative colitis, particularly in those with pancolitis or long-standing ulcerative colitis.[64] Therefore, not all patients who have PSC and ulcerative colitis before undergoing OLT need a prophylactic proctocolectomy, but patients with PSC and ulcerative colitis after OLT do require aggressive colonic surveillance.[58,64,67,68] Then, if low- or high-grade dysplasia is found during colonic surveillance, a colectomy with or without IPAA can be recommended.[67]
Even in the non-transplant setting, any patient with a new diagnosis of PSC and without known IBD should undergo a colonoscopy with multiple biopsies to evaluate for asymptomatic IBD. If negative, a repeat colonoscopy should be done only on the basis of symptoms/clinical presentation. On the other hand, patients with known PSC and IBD should have annual surveillance colonoscopies regardless of IBD duration due to the increased risk of developing colorectal cancer.[66]
Hepatobiliary Malignancies and Primary Sclerosing Cholangitis
In addition to the risk of developing colorectal cancer in patients with PSC and ulcerative colitis, the risk for hepatobiliary malignancies (hepatocellular carcinoma [HCC], gallbladder carcinoma, and cholangiocarcinoma) can represent additional challenges in treating patients with PSC, particularly during evaluation for liver transplantation. It has been reported that as many as 20% of patients with PSC accepted for the liver transplant waiting list were found to have a hepatobiliary malignancy.[69] In 1 study, a recent diagnosis of PSC, history of colorectal cancer, no UDCA treatment, and high clinical suspicion were found to be predictors of hepatobiliary malignancy.[69]
Although the diagnosis of gallbladder carcinoma and HCC in patients with PSC can usually be made through imaging studies, diagnosis of cholangiocarcinoma can be very difficult. The diagnosis of cholangiocarcinoma is often made through a combination of diagnostic modalities, including imaging and cholangiograhic findings, brush cytology from a stricture of the biliary tract, and laboratory data including tumor markers.[69] However, increases in tumor markers such as CA19-9 can frequently be seen in patients with PSC without bile duct carcinoma, particularly in patients with dominant strictures.[70,71]
The survival rates of patients with PSC and either HCC or early stage gallbladder carcinoma after liver transplantation are greater than patients with PSC and cholangiocarcinoma.[69] By following the Milan criteria for patients with PSC and HCC undergoing liver transplantation (single tumor 5 cm or less, or no more than 3 tumor nodules, each 3 cm or less in diameter), an 85% 4-year survival rate after liver transplantation has been reported.[72] In comparison, a 35% 5-year survival rate has been reported following liver transplantation in patients with PSC and localized cholangiocarcinoma prior to transplant.[69] To improve survival prior to transplant in patients with unresectable cholangiocarcinoma or cholangiocarcinoma arising in the setting of PSC, some experts have advocated neoadjuvant therapy.[73,74] Sudan and colleagues[75] reported a 45% long-term survival (median, 7.5 years) in patients with cholangiocarcinoma who received biliary brachytherapy and chemotherapy until transplantation. However, presently, there are no randomized controlled studies using neoadjuvant therapy and liver transplantation for patients with cholangiocarcinoma and PSC. With rare exceptions, given the decreased overall survival as well as a higher likelihood for cholangiocarcinoma recurrence following transplantation, the discovery of cholangiocarcinoma is often considered a contraindication for liver transplantation and treatment decisions are individualized.[69,76]
Conclusion
PSC is a rare, progressive cholestatic liver disease of unclear etiology. Many different therapies have been evaluated as potential options for the management of these patients. Although there is still no definitive evidence that UDCA can improve survival in patients with PSC, data are promising and many experts choose to initiate high-dose UDCA on the basis of the available literature. There is still no evidence supporting a specific medical therapy capable of halting disease progression in PSC. At present, the management of PSC remains focused on treating the symptoms and complications that are associated with disease progression as well as on close surveillance of those patients with advanced liver disease. In addition to the medical management of the associated symptoms, these patients are also managed endoscopically and surgically on an individual basis. Although balloon dilation or stenting may help to alleviate some of the symptoms associated with PSC, the only definitive form of therapy for patients with end-stage liver disease due to PSC is OLT.
Footnotes
Reader Comments on: Endoscopic and Surgical Management of Primary Sclerosing Cholangitis See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at Anthony.michaels@medicine.ufl.edu or to Peter Yellowlees, MD, Deputy Editor of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: peter.yellowlees@ucdmc.ucdavis.edu
Contributor Information
Anthony Michaels, Division of Gastroenterology, Hepatology and Nutrition, University of Florida, Gainesville, Florida Author's email: Anthony.michaels@medicine.ufl.edu.
Cynthia Levy, Division of Gastroenterology, Hepatology and Nutrition, Malcolm Randall VA Medical Center, Gainesville, Florida, and University of Florida, Gainesville Author's email: levyc@medicine.ufl.edu.
References
- 1.Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A sngle center study. Am J Gastroenterol. 2007;102:107–114. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 2.LaRusso NF, Shneider BL, Black D, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–764. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 3.Stiehl A. Primary sclerosing cholangitis: the role of endoscopic therapy. Semin Liver Dis. 2006;26:62–68. doi: 10.1055/s-2006-933564. [DOI] [PubMed] [Google Scholar]
- 4.Cullen SN, Chapman RW. Review article: current management of primary sclerosing cholangitis. Aliment Pharmacol Ther. 2005;21:933–948. doi: 10.1111/j.1365-2036.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 5.Silvis SE, Nelson DB, Meier PB. Ten-year response to stenting in a patient with primary sclerosing cholangitis. Gastrointest Endosc. 1998;47:83–87. doi: 10.1016/s0016-5107(98)70306-2. [DOI] [PubMed] [Google Scholar]
- 6.May GR, Bender CE, LaRusso NF, Wiesner RH. Nonoperative dilatation of dminant strictures in primary sclerosing cholangitis. AJR. 1985;145:1061–1064. doi: 10.2214/ajr.145.5.1061. [DOI] [PubMed] [Google Scholar]
- 7.Huibregtse K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of large bore bile duct endoprosthesis. Gut. 1982;23:371–375. doi: 10.1136/gut.23.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson GK, Geenen JE, Venu RP, Hogan WJ. Endoscopic treatment of biliary duct strictures in sclerosing cholangitis: follow-up assessment of a new therapeutic approach. Gastrointest Endosc. 1987;33:9–12. doi: 10.1016/s0016-5107(87)71475-8. [DOI] [PubMed] [Google Scholar]
- 9.Johnson GK, Geenen JE, Venu RP, Schmalz MJ, Hogan WJ. Endoscopic treatment of biliary tract strictures in sclerosing cholangitis: a larger series and recommendations for treatment. Gastrointest Endosc. 1991;37:38–43. doi: 10.1016/s0016-5107(91)70618-4. [DOI] [PubMed] [Google Scholar]
- 10.Lee JG, Schutz SM, England RE, Leung JW, Cotton PB. Endoscopic therapy of sclerosing cholangitis. Hepatology. 1995;21:661–667. [PubMed] [Google Scholar]
- 11.Wagner S, Gebel M, Meier P, et al. Endoscopic management of biliary tract strictures in primary sclerosing cholangitis. Endoscopy. 1996;28:546–551. doi: 10.1055/s-2007-1005552. [DOI] [PubMed] [Google Scholar]
- 12.Linder S, Söderlund C. Endoscopic therapy in primary sclerosing cholangitis: outcome of treatment and risk of cancer. Hepatogastroenterology. 2001;48:387–392. [PubMed] [Google Scholar]
- 13.Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Baillie J. Predictors of successful clinical and laboratory outcomes in patients with primary sclerosing cholangitis undergoing endoscopic retrograde cholangiopancreatography. Can J Gastroenterol. 2003;17:243–248. doi: 10.1155/2003/475603. [DOI] [PubMed] [Google Scholar]
- 14.van Milligen de Wit AW, van Bracht J, et al. Endoscopic stent therapy for dominant extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1996;44:293–299. doi: 10.1016/s0016-5107(96)70167-0. [DOI] [PubMed] [Google Scholar]
- 15.Parlak E, Kuran SO, Disibeyaz S, Ciçek B, Oguz D, Sahin B. Endoscopic treatment of primary sclerosing cholangitis. Turk J Gastroenterol. 2004;15:144–148. [PubMed] [Google Scholar]
- 16.van Milligen de Wit AW, Rauws EA, van Bracht J, et al. Lack of complications following short-term stent therapy for extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1997;46:344–347. doi: 10.1016/s0016-5107(97)70123-8. [DOI] [PubMed] [Google Scholar]
- 17.Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:2403–2407. doi: 10.1111/j.1572-0241.1999.01364.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson GK, Saeian K, Geenen JE. Primary sclerosing cholangitis treated by endoscopic biliary dilation: review and long-term follow-up evaluation. Curr Gastroenterol Rep. 2006;8:147–155. doi: 10.1007/s11894-006-0011-y. [DOI] [PubMed] [Google Scholar]
- 19.Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 20.Stiehl A, Rudolph G, Sauer P, et al. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol. 1997;26:560–566. doi: 10.1016/s0168-8278(97)80421-7. [DOI] [PubMed] [Google Scholar]
- 21.Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151–156. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 22.Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308–312. doi: 10.1016/s0016-5107(01)70403-8. [DOI] [PubMed] [Google Scholar]
- 23.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodexycholic Acid Study Group. N Engl J Med. 1997;336:691–665. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 24.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558–1562. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SA, Bansi DS, Hunt N, et al. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900–907. doi: 10.1053/gast.2001.27965. [DOI] [PubMed] [Google Scholar]
- 26.Olsson R, Boberg KM, de Muckadell OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Cullen SN, Rust C, Fleming K, et al. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792–794. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis-Aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.01.020. Feb 11 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Olsson R, Björnsson E, Bäckman L, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol. 1998;28:426–432. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 30.Eckhauser FE, Colleti LM, Knol JA. The changing role of surgery for sclerosing cholangitis. Dig Dis. 1996;14:180–191. doi: 10.1159/000171549. [DOI] [PubMed] [Google Scholar]
- 31.Ahrendt SA, Pitt HA, Kalloo AN, et al. Primary sclerosing cholangitis: resect, dilate, or transplant. Ann Surg. 1998;227:412–423. doi: 10.1097/00000658-199803000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitt HA, Thompson HH, Tompkins RK, Longmire WP., Jr Primary sclerosing cholangitis: results of an aggressive surgical approach. Ann Surg. 1982;196:259–268. doi: 10.1097/00000658-198209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron JL, Pitt HA, Zinner MJ, et al. Resection of hepatic duct bifurcation and transhepatic stenting for sclerosing cholangitis. Ann Surg. 1988;207:614–622. doi: 10.1097/00000658-198805000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myburgh JA. Surgical biliary drainage in primary sclerosing cholangitis. The role of the Hepp-Couinaud approach. Arch Surg. 1994;129:1057–1062. doi: 10.1001/archsurg.1994.01420340071012. [DOI] [PubMed] [Google Scholar]
- 35.Hirai I, Ishiyama S, Fuse A, Kuzu H, Sakurai F, Kimura S, Kimura W. Primary sclerosing cholangitis successfully treated by resection of the confluence of the hepatic duct. J Hepatobiliary Pancreat Surg. 2001;8:169–173. doi: 10.1007/s005340170042. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Hirohashi K, Kubo S, et al. Surgery for segmental primary sclerosing cholangitis. Hepatogastroenterology. 2004;51:668–671. [PubMed] [Google Scholar]
- 37.Ahrendt SA. Surgical approaches to strictures in primary sclerosing cholangitis. J Gastrointest Surg. 2008;12:423–425. doi: 10.1007/s11605-007-0342-5. [DOI] [PubMed] [Google Scholar]
- 38.Solano E, Khakhar A, Bloch M, et al. Liver transplantation for primary sclerosing cholangitis. Transplant Proc. 2003;35:2431–2434. doi: 10.1016/j.transproceed.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Graziadei IW, Wiesner RH, Marotta PJ, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121–1127. doi: 10.1002/hep.510300501. [DOI] [PubMed] [Google Scholar]
- 40.Chapman WC. Primary sclerosing cholangitis: role of liver transplantation. J Gastrointest Surg. 2008;12:426–428. doi: 10.1007/s11605-007-0343-4. [DOI] [PubMed] [Google Scholar]
- 41.Farges O, Malassagne B, Sebagh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995;117:146–155. doi: 10.1016/s0039-6060(05)80078-9. [DOI] [PubMed] [Google Scholar]
- 42.Goss JA, Shackleton CR, Farmer DG, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg. 1997;225:472–481. doi: 10.1097/00000658-199705000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz V, Neumann UP, Puhl G, Tran ZV, Neuhaus P, Langrehr JM. Surgical complications and long-term outcome of different biliary reconstructions in liver transplantation for primary sclerosing cholangitis-choledochoduodenostomy versus choledochojejunostomy. Am J Transplant. 2006;6:379–385. doi: 10.1111/j.1600-6143.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 44.Heffron TG, Smallwood GA, Ramcharan T, et al. Duct-to-duct biliary anastomosis for patients with sclerosing cholangitis undergoing liver transplantation. Transplant Proc. 2003;35:3006–3007. doi: 10.1016/j.transproceed.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Graziadei IW, Wiesner RH, Batts KP, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–1056. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 46.Oldakowska-Jedynak U, Nowak M, Mucha K, et al. Recurrence of primary sclerosing cholangitis in patients after liver transplantation. Transplant Proc. 2006;38:240–243. doi: 10.1016/j.transproceed.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 47.Alexander J, Lord JD, Yeh MM, Cuevas C, Bakthavatsalam R, Kowdley KV. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:245–251. doi: 10.1002/lt.21394. [DOI] [PubMed] [Google Scholar]
- 48.Cholongitas E, Shusang V, Papatheodoridis GV, et al. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138–143. doi: 10.1002/lt.21260. [DOI] [PubMed] [Google Scholar]
- 49.Campsen J, Zimmerman MA, Trotter JF, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008;14:181–185. doi: 10.1002/lt.21313. [DOI] [PubMed] [Google Scholar]
- 50.Brandsaeter B, Schrumpf E, Bentdal O, et al. Recurrent primary sclerosing cholangitis after liver transplantation: a magnetic resonance cholangiography study with analyses of predictive factors. Liver Transpl. 2005;11:1361–1369. doi: 10.1002/lt.20444. [DOI] [PubMed] [Google Scholar]
- 51.Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int. 2007;27:86–94. doi: 10.1111/j.1478-3231.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 52.Graziadei IW. Recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2002;8:575–581. doi: 10.1053/jlts.2002.33952. [DOI] [PubMed] [Google Scholar]
- 53.Brandsaeter B, Schrumpf E, Bentdal O, et al. Recurrent primary sclerosing cholangitis after liver transplantation: a magnetic resonance cholangiography study with analyses of predictive factors. Liver Transpl. 2005;11:1361–1369. doi: 10.1002/lt.20444. [DOI] [PubMed] [Google Scholar]
- 54.Kugelmas M, Spiegelman P, Osgood MJ, et al. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727–732. doi: 10.1053/jlts.2003.50143. [DOI] [PubMed] [Google Scholar]
- 55.Vera A, Moledina S, Gunson B, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943–1944. doi: 10.1016/S0140-6736(02)11861-7. [DOI] [PubMed] [Google Scholar]
- 56.Levy C, Zein CO, Chen C, Nelson DR. Allograft survival is decreased in patients with recurrent primary sclerosing cholangitis. Hepatology. 2007;46:509A. [Google Scholar]
- 57.Gordon F. Recurrent primary sclerosing cholangitis: Clinical diagnosis and long-term management issues. Liver Transpl. 2006;12:S73–S75. doi: 10.1002/lt.20948. [DOI] [PubMed] [Google Scholar]
- 58.MacLean AR, Lilly L, Cohen Z, O'Connor B, McLeod RS. Outcome of patients undergoing liver transplantation for primary sclerosing cholangitis. Dis Colon Rectum. 2003;46:1124–1128. doi: 10.1007/s10350-004-7291-9. [DOI] [PubMed] [Google Scholar]
- 59.Bjøro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69–79. doi: 10.1055/s-2006-933565. [DOI] [PubMed] [Google Scholar]
- 60.Kartheuser AH, Dozois RR, LaRusso NF, Wiesner RH, Ilstrup DM, Schleck CD. Comparison of surgical treatment of ulcerative colitis associated with primary sclerosing cholangitis: ileal pouch-anal anastomosis versus Brooke ileostomy. Mayo Clin Proc. 1996;71:748–756. doi: 10.4065/71.8.748. [DOI] [PubMed] [Google Scholar]
- 61.Grucela AL, Steinhagen RM. Restorative proctocolectomy and ileal pouch-anal anastomosis for ulcerative colitis after liver transplant for primary sclerosing cholangitis: case report and review of literature. Am Surg. 2005;71:362–365. [PubMed] [Google Scholar]
- 62.Lindberg BU, Broomé U, Persson B. Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study. Dis Colon Rectum. 2001;44:77–85. doi: 10.1007/BF02234825. [DOI] [PubMed] [Google Scholar]
- 63.Gorgun E, Remzi FH, Manilich E, Preen M, Shen B, Fazio VW. Surgical outcome in patients with primary sclerosing cholangitis undergoing ileal pouch-anal anastomosis: a case-control study. Surgery. 2005;138:631–637. doi: 10.1016/j.surg.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Vera A, Gunson BK, Ussatoff V, et al. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983–1988. doi: 10.1097/01.TP.0000058744.34965.38. [DOI] [PubMed] [Google Scholar]
- 65.Papatheodoridis GV, Hamilton M, Mistry PK, Davidson B, Rolles K, Burroughs AK. Ulcerative colitis has an aggressive course after orthotopic liver transplantation for primary sclerosing cholangitis. Gut. 1998;43:639–644. doi: 10.1136/gut.43.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31–41. doi: 10.1055/s-2006-933561. Review. [DOI] [PubMed] [Google Scholar]
- 67.Loftus EV, Jr, Aguilar HI, Sandborn WJ, et al. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis following orthotopic liver transplantation. Hepatology. 1998;27:685–690. doi: 10.1002/hep.510270308. [DOI] [PubMed] [Google Scholar]
- 68.Higashi H, Yanaga K, Marsh JW, Tzakis A, Kakizoe S, Starzl TE. Development of colon cancer after liver transplantation for primary sclerosing cholangitis associated with ulcerative colitis. Hepatology. 1990;11:477–480. doi: 10.1002/hep.1840110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandsaeter B, Isoniemi H, Broomé U, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol. 2004;40:815–822. doi: 10.1016/j.jhep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 71.Petersen-Benz C, Stiehl A. Impact of dominant stenoses on the serum level of the tumor marker CA19-9 in patients with primary sclerosing cholangitis. Z Gastroenterol. 2005;43:587–590. doi: 10.1055/s-2005-858105. [DOI] [PubMed] [Google Scholar]
- 72.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 73.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 74.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 76.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]


