Abstract
Background
Chronic kidney disease (CKD) is a widespread medical condition that is progressive in nature. As renal function declines, the disease ultimately reaches the life-threatening end stage(ESRD), which requires urgent replacement therapy, either by dialysis or transplantation. As a result, CKD patients, many of whom have comorbid medical conditions, are severely restricted in physical, psychological, and social dimensions of life. Over the past 3 decades, research has been carried out on the effects of intradialytic exercise rehabilitation on the quality of life (QoL) of CKD patients.
Aims
This review aims to critically examine the effect of exercise prescription in reducing the physical and psychological limitations encountered by CKD patients.
Method
Four studies were selected and critically appraised using specific inclusion criteria.
Results
The results of all studies suggest a causal relationship between exercise intervention and QoL of CKD patients. Exercising patients have shown improvements in physical fitness, psychological function, manual dexterity, reaction times, and lower-extremity muscle strength. All of these factors help improve QoL.
Conclusion
Evidence gathered from the studies shows that exercise training has beneficial effects on the QoL of CKD patients; however, exercise is still not routinely prescribed. Further research and robust evidence are needed to overcome the limitations encountered by previous studies to confirm the positive results of exercise prescription in management of CKD.
Introduction
Chronic kidney disease (CKD) is a progressive, debilitating condition, resulting in a long-standing deterioration in renal function that requires nursing and medical intervention.[1] It manifests as a “biochemical abnormality,” causing an irreversible reduction in renal excretory and homeostatic function over a period of months to years.[2] This eventually results in the clinical signs and symptoms of CKD, referred to as uremia. CKD may result from many causes. Hypertension, diabetic nephropathy, and glomerulonephritis are the most common causes in the developed world and account for 75% of all cases in the United States.[3]
The condition can often be diagnosed on the basis of the patient's history and physical examination and simple laboratory tests of renal function. Elevated levels of urea and creatinine found in blood serum,[4] often accompanied by anemia, proteinuria, or hypertension, indicate signs of reduced renal function.[5] The disease is characterized by a sustained, gradual decline in glomerular filtration rate to <60 mL/min/1.73 m2 for 3 or more months, which is indicative of CKD.[3]
According to the National Kidney Foundation, there are 5 categories of chronic kidney disease, based upon the GFR[6] (Table 1):
Table 1.
Stages of Chronic Renal Disease[3]
| Stage | Characteristics |
|---|---|
| 1 | Kidney damage with normal or increased GFR (>90 mL/min/1.73 m2) |
| 2 | Mild reduction in GFR (60-89 mL/min/1.73 m2) |
| 3 | Moderate reduction in GFR (30-59 mL/min/1.73 m2) |
| 4 | Severe reduction in GFR (15-29 mL/min/1.73 m2) |
| 5 | Kidney failure (GFR <15 mL/min/1.73 m2 or dialysis) |
Patients with a GFR < 15 mL/min are classified as having end-stage renal disease (ESRD). Renal replacement therapies, in the form of life-long dialysis or transplantation, are the only means of treating such patients. Withdrawal of treatment results in death.[7]
Statistical reports have shown that the incidence and prevalence of CKD seems to be increasing every year.[8] In the United States, “430,000 individuals currently live with CKD” and over “30,000 patients with CKD are under treatment in the U.K.”.[9] By 2010, it is predicted that over half a million patients worldwide will require hemodialysis.[10]
CKD patients have low functional abilities and QoL compared with healthy individuals.[11] The disease has an impact on physical status, psychological status, functional status, independence, and general well-being. Although great advances in renal replacement therapy have made it possible to save the lives of many CKD patients, more work needs to be done to improve the QoL.
A great deal of controversy surrounds the concept of “quality of life,” and lack of universal consensus on a definition makes the entity difficult to measure effectively.[12]
The World Health Organization defines “quality of life” as:
An individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns. It is a broad-ranging concept affected in a complex way by the person's physical health, psychological state, level of independence, social relationships, and their relationships to salient features of the environment.[12]
QoL is viewed as a multidimensional concept that includes such aspects as physical, psychological, and social functioning.[13] The “walk” and “sit-to-stand” test are both objective measures of functional capacity and are easily quantified. Subjective measures, such as the Short-Form 36 questionnaire, are measures of health status and satisfaction. These measures can be generic or specific.[12] It is important to assess QoL objectively and subjectively, as this provides a balanced overview with regard to a treatment intervention.
Exercise is often difficult to incorporate in chronically ill patients because of limited physical strength, motivation, and pain. These factors can contribute to deteriorating health and loss of independence. It is important to understand this issue and address it in specific patients. Exercise prescription based on individual needs would be the most promising way to help achieve individual independence in chronically ill patients.
Many studies have used objective and subjective measures to compare the effects of exercise prescription on QoL of CKD patients. Based on this, the review will aim to answer the following research question:
How does exercise prescription affect the QoL of patients with CKD undergoing hemodialysis therapy?
To answer this question, this review will attempt to objectively assess the effects of exercise prescription on the QoL of CKD patients and to critically appraise the literature regarding the effectiveness of exercise prescription on the QoL of CKD patients.
Methodology
Literature Search
To conduct a literature search, various methods were applied to retrieve information relevant to the effectiveness of exercise prescription on the QoL of CKD patients. Search engines, such as Google Scholar were used, in addition to several textbooks, to search for academic articles. Also, key medical journals, such as the American Journal of Kidney Disease and Nephrology Dialysis Transplantation, were found to be useful particularly in searching for full-text articles that were not available via Ovid-Medline or PubMed.
Primary medical databases, such as Ovid-Medline and Scopus, were accessed to obtain relevant primary literature. Specific keywords and limits were applied to ensure that the relevant articles that were found met the inclusion criteria. These will be discussed below.
Inclusion Criteria
Primary and secondary inclusion criteria were devised to limit the search results only to studies that were relevant to assessing the relationship of exercise prescription with QoL in CKD patients. The primary criteria (Table 2) reduced the large number of results from each database search. The secondary criteria (Table 3) refined the remaining results to those that are directly related, or specific to, the research question. Application of these criteria enables accurate comparisons of the studies and provides a form of standardization with regard to how exercise prescription affects the QoL of CKD patients.
Table 2.
Primary Inclusion Criteria
| Language | Only articles written in English were included in the review due to impracticalities in translating foreign-language articles. |
| Published | Articles published from 2000 onward were used. This helped to ensure that recent literature was reviewed to give an “up-to-date,” accurate evaluation relevant to the topic area. |
| Abstract | Due to time constraints, only articles with a clear abstract or title of investigation were obtained and reviewed through the databases. |
| Content | Articles that focused on, and investigated, the relationship between exercise prescription and QoL in CKD patients were included. This was decided upon by reading relevant abstracts. |
| Study Design | Only clinical trials, such as randomized controlled trials or uncontrolled trials, were used because these studies provide more standardized results. Secondary sources, such as reviews and publications, were eliminated. |
Table 3.
Secondary Inclusion Criteria
| Dialysis Therapy | Only articles involving patients receiving hemodialysis for management of CKD were considered. This form of renal replacement therapy is widely used and has been readily investigated. Assessing its implications would be valuable. |
| Exercise Prescription | Only trials that prescribed intradialytic exercise were used. Current research focuses on such exercise therapy and would be important to evaluate. |
| Outcome Measures | Articles that used a comparable spectrum of physiological, psychological, or functional outcome measures in the assessment of QoL in CKD patients in response to exercise prescription were considered. |
| Subjects | Only studies of adult patients were included. Renal disease in children is an individual specialty of its own and not the focus of this review. |
Search Strategy
In this review, a detailed search strategy was conducted on 2 databases: Ovid-Medline and Scopus. To ensure that the primary and secondary inclusion criteria were met, specific key words and medical subject headings (MeSH) were used.
The flow diagram below (Figure) summarizes the process of the search strategy applied to Ovid-Medline and Scopus databases.
Results
Results of Database Search Strategy
The tables below show the results obtained after applying the search strategy to the individual databases. One hundred fifty-three results were obtained from Scopus and 44 from Ovid-Medline before the application of limits (Table 4 and Table 5). The results were then limited to available abstracts and to clinical trials, thus reducing the numbers to 74 and 12 (Table 6).
Table 4.
Results Obtained from Search Strategy Applied to Scopus Database
| Keyword | Number of Hits |
|---|---|
| Chronic Renal Failure AND Exercise Prescription AND Quality of Life | 153 |
Table 5.
Results Obtained from Search Strategy Applied to Ovid-Medline Database
| Search Number | MeSH Term | Number of Hits |
|---|---|---|
| 1 | Chronic Renal Failure | 37,351 |
| 2 | Exercise Prescription | 34,951 |
| 3 | Term 1 AND Term 2 | 200 |
| 4 | Quality of Life | 46,919 |
| 5 | Term 3 AND Term 4 | 44 |
Table 6.
Results from Database Searches After Application of Limits and Inclusion Criteria
| Results | Scopus | Ovid-Medline |
|---|---|---|
| Total number of hits before limitations | 153 | 44 |
| Limits applied | 74 | 12 |
| Primary inclusion criteria | 25 | 8 |
| Secondary inclusion criteria | 3 | 3 |
| Removal of duplicates | 1 | 3 |
| TOTAL | 1 | 3 |
From the search strategy, the following 4 articles[14–17] met the secondary inclusion criteria and were found to be most relevant for further critical analysis:
Painter P, Carlson L, Carey S, Paul S, Myll J. Physical functioning and health-related quality of life changes with exercise training in haemodialysis patients. Am J Kidney Dis. 2000;35:482-492.
Van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141-146.
Oh-Park M, Fast A, Gopal S, et al. Exercise for the dialyzed: Aerobic and strength training during haemodialysis. Am J Phys Med Rehabil. 2002;81:814-821.
Painter P, Moore G, Carlson L, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39:252-265.
Objective 1: Literature Review
Study A: Physical Functioning and Health-Related Quality-of-Life Changes with Exercise Training in Hemodialysis Patients
Painter and colleagues conducted a study that was designed to evaluate the feasibility and effects of 2 approaches to exercise programming on the levels of physical activity, physical functioning, and self-reported health status in hemodialysis patients.
A total of 286 patients were recruited for participation and were allocated to an intervention or a control group. Patients in the control group received no exercise and were tested at baseline and at 2-month intervals. Patients receiving the intervention were individually prescribed exercise for 8 weeks of independent (IND) home exercise, followed by 8 weeks of in-center cycling (ICC) during dialysis (Table 7).
Table 7.
Description of the 2 Exercise Programs
| Individually Prescribed Exercise | In-Center Cycling |
|---|---|
|
|
At each stage of the study, patients were assessed and separated into 4 groups according to their ability to perform certain physical activities (Table 8).
Table 8.
Categorization of Patient Levels of Physical Activity
| Group | Description of Patients' Physical Ability |
|---|---|
| 1 | No participation of additional activities. Only activities of daily living.. |
| 2 | Strengthening or stretching activity. |
| 3 | Low cardiovascular exercise (cycling or walking) at a level less than that recommended by the Surgeon General's report (i.e., <3 times weekly and/or <20 min/session). |
| 4 | Recommended cardiovascular exercise in which patients reported doing at least 3 times weekly for 20 minutes or more per session. |
To assess the changes in the self-reported domains of health status, the Short Form-36 questionnaire and well-established physical functioning tests (the gait speed test, the sit-to-stand test, and the 6-minute walk test) were done at baseline and during both IND and ICC approaches.
The results of the study showed that in all cases, the intervention group had increased self-reported physical scale and physical functioning scores compared with the control group. In the intervention group, improvements were seen in gait speed (P < .001), the 6-minute walk test (P = .05), and the sit-to-stand test (P = .05). Significant positive findings were also found in the following scales of the SF-36: general health (P = .05), physical functioning (P = .004), physical role (P < .01), and bodily pain (P = .03). In conclusion, exercise prescription showed beneficial effects on the QoL in patients receiving hemodialysis.
In evaluation of the study, many advantages and disadvantages were encountered.
Advantages
A wide range of participants, varying in age and comorbidities, were included in the study, thus allowing better representation of the general hemodialysis population.
A large study sample was used, which increased the reliability of the results.
The study was a randomized, controlled trial (RCT) that had both intervention and control groups. This is the most reliable study design in clinical research.
Exercise adherence was aided by the IND approach, which took place in patients' homes. Patients were more comfortable with this convenient approach and thus more likely to exercise.
Disadvantages
A total of 49% of all tests were incomplete. This was primarily due to orthopedic limitations and medical concerns. Consequently, sample sizes were smaller and results may have been amplified to give an incomplete evaluation.
Many physical function tests were missed. The reasons for this were accounted for and included lack of indoor space for the 6-minute walk test (outdoor facilities had to be used, and the tests were subject to disruption by unsuitable weather).
A total of 61 patients were lost from baseline and between the IND and ICC programs. This was due to unavoidable causes, such as medical complications or death. In addition, some were lost due to apparent disinterest, highlighting the need to make a study appealing to keep patient withdrawals to a minimum. No losses were accounted for in the control group.
Poor follow-up results.
Study B: The Effects of a Low-to-Moderate Intensity Pre-conditioning Exercise Programme Linked With Exercise Counselling for Sedentary Haemodialysis Patients in the Netherlands: Results of a Randomized Clinical Trial
Study B was designed to evaluate the effects of exercise on the QoL of sedentary hemodialysis patients in the Netherlands. Tests examined physical fitness, behavior change, and health-related QoL. The program consisted of 12 weeks of low- and moderate-intensity preconditioning exercise, composed of strength training before dialysis and cycling and exercise counseling during dialysis.
A total of 96 patients were recruited on the basis of an inclusion criterion assessing their physical activity status. Fifty-three participants were allocated to an exercise group and 43 were assigned to the control group.
The 30-minute training sessions for the exercise group were held 2 to 3 times per week in the first 2 hours of dialysis. The level of exercise intensity was prescribed on an individual basis using the rate of perceived exertion of the Borg Scale.[18] To help secure lower dropout rates, exercise counselors and motivational support were provided during the intervention.
The physical fitness components were examined by measuring exercise capacity (VO2 peak), reaction time, manual dexterity, and lower-extremity muscle strength (sit-to-stand test). To allow for better comparison and significant change, the MOS Short-Form General Health Survey (RAND-36) was used to measure the general health-related QoL of patients.
The results from the exercise program test displayed both physical and psychological benefits. The physical benefits included improvement in “reaction time, lower-extremity muscle strength, Kt/V and 3 components of QoL” with short-term exercise activity. Exercising led to significant improvements in the QoL assessment of the RAND-36 components of vitality (P = .001), general health perception (P = .001), and health change (P = .02) for the exercise group. Patients also developed a stronger belief about their capacity for behavior change from the exercise counseling and psychological benefits.
In evaluation of the study, many advantages and disadvantages were encountered.
Advantages
The study also was an RCT. This reliable design helps to eliminate spurious causality and confounding bias that otherwise may be present.
A statistical measure of D scores enabled a more effective evaluation of exercise therapy.
To compensate for the effects of dropout, additional patients were recruited to make the study stronger.
Exclusion of patients with comorbidities allowed a better comparison of the results.
An inclusion criterion was applied so that only patients on hemodialysis who had no other illnesses were allowed to participate in the study. This is because comorbidity could affect the results.
Disadvantages
The study population was not representative of the general hemodialysis population. Patients with comorbidities, such as cardiovascular or orthopedic problems, which are common in CKD patients, were not included in the study.
The study used the RAND-36 health related QoL measure, which differs slightly from the SF-36 questionnaire used by the other studies. Consequently, direct comparisons and standardization among the studies may be difficult.
Study C: Exercise for the Dialyzed: Aerobic and Strength Training During Hemodialysis
The purpose of this study was to evaluate aerobic and strength training during hemodialysis for CKD patients and to investigate the impact of these exercises on function status, cardiac fitness, and muscle strength.
Eighteen of the 22 patients completed a 12-week exercise training program, which was carried out during the first 2 hours of dialysis. Regular health monitoring was performed at baseline and before and after the intervention. Training, consisting of knee-strengthening exercises and cycling, occurred 2 or 3 times a week during dialysis. Patients took a 6-minute walk test and cycle stress testing, in which cardiac function (blood pressure, peak heart rate, and peak O2) were measured before and after the intervention.
The widely used SF-36 questionnaire was used in this study to assess the mental and physical status of the patients, thus indicating changes in their QoL. The results of the study showed statistically significant improvements in the physical component summary scales (36 ± 8.7 vs. 44.9 ± 8.1, P = .0003) and the mental component summary scales (48.7 ± 10.2 vs. 55 ± 7.8, P = .004) of this questionnaire.
In evaluation of the study, many advantages and disadvantages were encountered.
Advantages
The study showed that an aerobic and strength training program, under correct supervision, can improve the mental and physical components of QoL in patients with CKD.
The study highlighted the importance of patient education and encouragement in maintaining adherence and success in such an exercise program.
The study incorporated strength-building exercises, which also play a role in the level of independence and physical fitness of patients, thus affecting QoL.
Disadvantages
Small sample size may have affected the outcome and reliability of the results.
There was no control group. The true benefits of an exercise program in CKD patients may be difficult to assess.
This was an experimental study, unlike the RCT design used in the other 3 trials, and is more likely to have spurious causality and confounders, which may affect the outcome.
Study D: Effects of Exercise Training Plus Normalization of Hematocrit on Exercise Capacity and Health-Related Quality of Life
The purpose of this study was to establish whether exercise training and complete correction of hematocrit levels affected exercise capacity and the QoL of hemodialysis patients.
A total of 65 patients were randomized and stratified according to demographic variables (age and sex) into 4 groups Table 9. Ten patients withdrew, resulting in only 48 participants having completed pre- and postexercise measurements.
Table 9.
Participants of the Four Study Groups
| Study | Description |
|---|---|
| 1 | Usual-care hematocrit (30%-33%) with no exercise training. (UH) |
| 2 | Usual-care hematocrit (30%-33%) with exercise. (UHX) |
| 3 | Normalized hematocrit (40%-42%) with no exercise (NH) |
| 4 | Normalized hematocrit (40%-42%) with exercise training. (NHX) |
A dialysis nurse administered a defined intravenous dose of R-hu-recombinant erythropoietin 3 times per week to each patient according to their target hematocrit ranges. Testing was carried out at baseline, and measures were repeated at 3 and 5 months.
Intradialytic exercise training was performed by using a stationary cycle. Similar to study B, the intensity of the individual exercise prescription was calculated using the rate-of-perceived-exertion scale. Each exercise session was monitored by dialysis staff, which also provided moral support and encouragement.
A treadmill exercise was used to objectively measure the exercise capacity and was done at baseline and at 5 months after the intervention. Blood pressure, peak VO2, and ECG recordings were all measured to assess the impact of exercise at each stage.
To investigate the effects of exercise on QoL, all patients completed the SF-36 questionnaire. A statistical significance, similar to study A, was found (P ≤ .05). In the intervention groups, peak VO2 levels had increased by 15.7% ± 11.3% in the UHX group and by 9.6% ± 21.1% in the NHX group. Results from these groups showed that normalized hematocrit levels had no effect on the VO2 levels, which is discordant with the marked improvements of over 10% shown in other studies.[19]
In terms of QoL, the study indicated that low-level exercise training resulted in an improvement in the physical functioning scales (P = .015) of the SF-36 questionnaire for both exercise groups (UHX and NHX).
In evaluation of the study, many advantages and disadvantages were encountered.
Advantages
A strong RCT study design was used. Random allocation of participants by age and sex into the intervention and control groups enabled elimination of confounders and reduction of selection bias.
Large study sample size made the results more reliable.
Differences in demographic variables between the participants at baseline were adjusted and accounted for, thus enabling accurate comparisons to be made.
To gain better representation of the hemodialysis population as a whole, less strict exclusion/inclusion criteria were used.
Disadvantages
The scores from all the component scales on the SF-36 questionnaire, excluding the mental component summary scale and general health, were averaged much higher than reported values. As a consequence, the study may not have faithfully represented the CKD population.
Over 26% of the participants did not complete the study, and 15% withdrew. This reduces the sample size and affects the validity of the results.
Objective 2: Critical Appraisal
A critical appraisal tool consisting of 10 questions was applied to each of the 4 studies. Three options were provided for each of the questions: (Y)es, (N)o or (U)nsure. The tool, adapted by Guyatt and coworkers,[20] is necessary to assess the relevance and quality of literature, providing a better appraisal of the validity of the results. The findings of the critical appraisal criteria and a score to show their quality is shown in Table 10.
Table 10.
The Critical Appraisal Tool
| Study | |||||
|---|---|---|---|---|---|
| Critical Appraisal Questions | A | B | C | D | |
| 1 | Did the study state a clear focused question? | Y | Y | Y | Y |
| 2 | Was an appropriate method used to answer the focused question? | Y | Y | N | Y |
| 3 | Were patients assigned to an intervention and control group by means of random allocation? | U | Y | N | Y |
| 4 | Were patients, staff, and medical professionals “blinded” to the intervention study group? | N | N | N | N |
| 5 | Were measures taken to adjust patient characteristics at baseline? | Y | Y | Y | Y |
| 6 | Was an adequate follow-up and data collection method used in the same way for all participants? | Y | Y | Y | Y |
| 7 | Were all patients who originally entered the trials accounted for in the conclusion of the study? | Y | Y | Y | Y |
| 8 | Did the study have enough participants to reduce the play of chance? | Y | Y | N | U |
| 9 | Is the outcome of the results of significant relevance to the effect of the intervention? | Y | Y | Y | Y |
| 10 | Were all important outcome measures considered to apply these results to the general CKD population? | Y | Y | N | N |
| TOTAL | 17 | 18 | 10 | 15 | |
Y=2; N=0; U=1
Discussion
Results from the 4 studies indicate a positive relationship between intradialytic exercise rehabilitation programs and QoL of CKD patients. Exercise-induced improvements have been reported in a variety of physiological, functional, and psychological aspects of life in CKD patients.
QoL assessment for dialysis patients was based upon subjective and objective measurements. All studies used the self-administered SF-36 questionnaire or adapted versions to assess the 8 health concepts: limitations in physical activities because of health problems; limitations in social activities because of physical or emotional problems; limitations in usual role activities because of physical health problems; bodily pain; general mental health (psychological distress and well-being); limitations in usual activities because of emotional problems; vitality; and general health perceptions.[21]
Previous studies have shown SF-36 scores to be consistent and reliable. However, despite this reliability, the SF-36 is a subjective measure; thus, results can be difficult to compare because different individuals may perceive their health differently. That said, a subjective measurement does have merit, as it takes a more individual account that is often lost by groupings in many studies.
Results from all studies showed improvements in the physical component scales of the SF-36 questionnaire for exercising patients compared with nonexercising patients. However, only study C showed improvements in the mental component scales (48.7 ± 10.2 vs. 55 ± 7.8, P = .004) of this questionnaire. This indicates that psychological limitations are not overcome by exercise training but suggests that overall exercise therapy has beneficial effects on QoL. Future studies could improve QoL measurements, as psychological limitations in CKD patients are important in addition to physical aspects.
Objective and performance-based measures were also used to assess exercise capacity. Study D used a treadmill exercise to objectively assess cardiorespiratory fitness while other studies used the cycle ergometer. CKD patients who are not prescribed exercise therapy have documented low cardiac function. Results from all studies indicated improvements in cardiac and respiratory functions with increased VO2 levels – another good indicator of the positive effects of exercise therapy.
Physical functioning tests showed improvements in gait speed, the sit-to-stand test, and the 6-minute walk test. Study B showed improvements in lower-extremity muscle strength. Muscle atrophy is a significant problem in dialysis patients and a “major contributor to limitations in physical functioning.”[22]
These tests indicate improvements in physical fitness and suggest that patients are more able to perform activities of daily living with exercise prescription and are potentially able to lead an independent life. Independence tends to be restricted in dialysis patients because of the sedentary lifestyle caused by the disease and inability to perform such basic activities as sitting down. Exercise therapy has shown to help reduce these limitations and reduce the risk for physical disability later in life.[23,24]
Regular physical activity may benefit a number of conditions, such as cardiovascular risk, muscle wasting, and chronic inflammation, that affect patients in the general CKD population.
Although all the studies agreed in their conclusions regarding exercise prescription and QoL, common issues among the studies were highlighted by the critical appraisal tool, such as small sample size. Before a fair evaluation is made, the limitations posed by such issues must be considered.
Sample size varied greatly among the 4 studies. Studies C and D assessed a much smaller cohort, with just 22 and 65 individuals, respectively, participating in the trial. Study C had an overall dropout rate of 18%, and 2 patients did not complete the 3-month exercise regimen due to unrelated medical events. Similarly, in study D there was an overall dropout rate of 26% due to unavoidable medical reasons and transplants. However, some patients from the NHX group withdrew due to insufficient training stimulus and lack of motivation. Both could have been avoided if more time and effort were devoted to encouraging exercise and providing moral support to increase patient adherence. Such small samples may affect the outcomes and may mean that the results are due not to the effects of exercise prescription but to chance. Amplifications of small discrepancies are more likely to occur, thus giving imprecise and unreliable results.
In comparison, 286 participants were included in study A and 96 in study B. Larger study sizes are key to improving the accuracy and reliability of the results. In studies C and D, the numbers of participants were too small to be representative of the general CKD population.
Study A had an unequal distribution of men and women; this also may not accurately represent the CKD population.
Major factors that affect the ability to exercise and the extent to which individuals can perform exercise are their age and their gender. Study A showed large numbers of women in the intervention group (57.1%) in comparison to study B (36.7%). This was similar for the control groups in study A and study B, in which the results were 65.4% and 30.2%, respectively. These variations could cause the groups to be sufficiently dissimilar to the CKD population and result in skewed or incorrect trial results.
In addition to the issues of sample size and patient demographics, none of the 4 studies was blinded and consequently there is a chance of assessment bias. All studies in this respect were open trials, in which the patients, clinical staff, and health care professionals were fully aware of which group was receiving the intervention, physical exercise. Blinding patients would have been very difficult, as those not exercising would know they were not receiving the intervention.
In all studies, staff members were responsible for supervising and collecting data from functional and aerobic tests, and as such, they were also aware of which group was receiving the intervention. This increases the chance for observer bias. In study B, clinical staff provided exercise counseling and support for patients in the exercise program and therefore were also aware of which group was receiving the intervention.
Patients from study C were not randomly allocated to a control group. It is unclear from study A how patients were assigned to the intervention and control groups. No data regarding numbers of participants in each group are provided. Intervention units were chosen if both the nurse manager and 75% of the dialysis staff were willing to participate in the project. Control units were chosen in which dialysis staff were not willing to participate in the project.
In studies B and D, participants were randomly allocated to both groups. Randomization is important as it equalizes and accounts for all confounding variables among the study groups and reduces selection bias. Studies A, B, and D used an RCT study design, which is considered to be the “gold standard” in epidemiologic research. Researchers randomly assigned participants to an intervention (exercise) group or a control (nonexercise) group. The RCT is an effective type of trial that is designed to minimize bias. The use of control groups, random testing, and other such methods as double-blind tests, reduce error and avoid the introduction of biased results.[25] In contrast, study C was an experimental study with no control group. All participants were given the exercise intervention and observed. Using this type of study can cause exaggerated results, as there is no “neutral” control group for comparison.
All the studies used similar inclusion/exclusion criteria with which patients had to comply. This ensured, to an extent, that the baseline characteristics were similar among participants. However, by setting rigid criteria on patients with comorbidities, which are commonly associated with CKD, many patients were not allowed to participate. Therefore, it can be argued that this limitation may have resulted in a misleading representation of the CKD population as a whole. Patients excluded on the basis of higher numbers of comorbidities are likely to be the sickest patients and may not have responded as well to treatment. This may have skewed the results toward positive outcomes. In all studies, patients who showed evidence of orthopedic and musculoskeletal problems were excluded because of the likelihood that these difficulties may prevent them from participating in exercise treatment testing.
The evidence gathered from this structured review suggests a causal relationship between adequate amounts of exercise intervention and QoL of CKD patients. Planned exercise has been shown to enhance physiological, psychological, and functional aspects of an individual's life.
Based on the discrepancies identified in previous studies, thorough and standardized reporting of exercise prescription in CKD patients should be done in the future.
Figure.
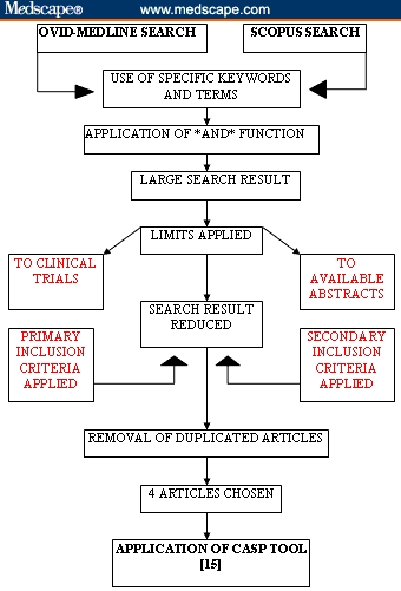
Search strategy for Ovid-Medline and Scopus databases.
Footnotes
Reader Comments on: The Effectiveness of Intradialytic Exercise Prescription on Quality of Life in Patients With Chronic Kidney Disease See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at M.Takhreem@student.liverpool.ac.uk or to Peter Yellowlees, MD, Deputy Editor of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: peter.yellowlees@ucdmc.ucdavis.edu
References
- 1.Cleary J, Drennan J. Quality of life of patients on haemodialysis for end-stage renal disease. J Adv Nurs. 2005;51:577–586. doi: 10.1111/j.1365-2648.2005.03547.x. [DOI] [PubMed] [Google Scholar]
- 2.Boon NA, Colledge NR, Walker BR. Davidson's Principles and Practice of Medicine. 20th ed. Oxford: Churchill Livingstone; 2006. Chronic renal failure; pp. 485–493. [Google Scholar]
- 3.Chronic renal failure. Available at: http://www.emedicine.com/med/topic374.htm Accessed September 9, 2007.
- 4.The Merck Manuals. Chronic renal failure. Available at: http://www.merck.com/mmpe/sec17/ch233c.html#CHDFHEDG Accessed September 9, 2007.
- 5.Longmore M, Wilkinson IB, Rajagopalan S. Oxford Handbook of Clinical Medicine. 6th ed. Oxford: Oxford University Press; 2004. Chronic renal failure (CRF) pp. 276–279. [Google Scholar]
- 6.Kolewaski CD, Mullally MC, Parsons TL, Paterson ML, Toffelmire EB, King-VanVlack CE. Quality of life and exercise rehabilitation in end stage renal disease. CANNT J. 2005;5:22–29. [PubMed] [Google Scholar]
- 7.Forrest GP. Inpatient rehabilitation of patients requiring haemodialysis. Arch Phys Med Rehabil. 2004;85:51–53. doi: 10.1016/s0003-9993(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheema BSB, Fiatarone Singh MA. Exercise training in patients receiving maintenance haemodialysis: A systematic review of clinical trials. Am J Nephrol. 2005;25:352–364. doi: 10.1159/000087184. [DOI] [PubMed] [Google Scholar]
- 9.McGee H, Bradley C. Quality of Life Following Renal Failure. Newark, NJ: Harwood Academic Publishers; 1994. Options in the medical management of end-stage renal failure; pp. 15–33. [Google Scholar]
- 10.Tawney KW, Tawney PJW, Kovach J. Disablement and rehabilitation in end-stage renal disease. Semin Dial. 2003;16:447–452. doi: 10.1046/j.1525-139x.2003.16097.x. [DOI] [PubMed] [Google Scholar]
- 11.Knight EL, Ofsthun N, Teng M, Lazarus M, Curhan GC. The association between mental health, physical function, and haemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 12.Guillemin F. Functional disability and quality-of-life assessment in clinical practice. Rheumatology. 2000;39(suppl. 1):17–23. doi: 10.1093/oxfordjournals.rheumatology.a031489. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri-Brennan J, Steele RJ. Measurement of quality of life in surgery. J R Coll Surg Edinb. 1999;44:252–259. [PubMed] [Google Scholar]
- 14.Painter P, Carlson L, Carey S, Paul S, Myll J. Physical functioning and health-related quality of life changes with exercise training in haemodialysis patients. Am J Kidney Dis. 2000;35:482–492. doi: 10.1016/s0272-6386(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 15.Van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in the Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141–146. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- 16.Oh-Park M, Fast A, Gopal S, et al. Exercise for the dialyzed: Aerobic and strength training during haemodialysis. Am J Phys Med Rehabil. 2002;81:814–821. doi: 10.1097/00002060-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Painter P, Moore G, Carlson L, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39:252–265. doi: 10.1053/ajkd.2002.30544. [DOI] [PubMed] [Google Scholar]
- 18.Miller WR, Rollnick S. Preparing People to Change Addictive Behaviour. New York: Guilford Press; 1991. Motivational interviewing. [Google Scholar]
- 19.Metra M, Cannella G, LaCanna G, et al. Improvement in exercise capacity after correction of anaemia in patients with end stage renal failure. Am J Cardiol. 1991;68:1060–1066. doi: 10.1016/0002-9149(91)90496-8. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Sackett DL, Cook DL. Users' guides to the medical literature. How to use an article about therapy or prevention. JAMA. 1993;270:2598–2601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 21.Ware JJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 22.Meriel EM, Campbell S. Measuring quality of life. Arch Dis Child. 1997;77:347–354. doi: 10.1136/adc.77.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Painter P. Physical functioning in end-stage renal disease patients: Update 2005. Haemodial Int. 2005;9:218–235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg AP, Hagberg J, Delmez JA. The metabolic and psychological effects of exercise training in haemodialysis patients. Am J Clin Nutr. 1980;33:1620–1628. doi: 10.1093/ajcn/33.7.1620. [DOI] [PubMed] [Google Scholar]
- 25.Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]


