Abstract
Imaging of cerebrovascular β-amyloid (cerebral amyloid angiopathy, CAA) is complicated by this pathology’s nearly universal overlap with Alzheimer pathology. We performed PET imaging with Pittsburgh Compound B (PiB) on 42-year old man with early manifestations of Iowa-type hereditary CAA, a form of the disorder with little or no plaque deposits of fibrillar β-amyloid. The results demonstrated elevated PiB retention selectively in occipital cortex, sparing regions typically labeled in Alzheimer disease. These results offer compelling evidence that PiB-PET can noninvasively detect isolated CAA prior to overt signs of tissue damage such as hemorrhage or white matter lesions.
Keywords: Cerebral amyloid angiopathy, Alzheimer disease, Noninvasive imaging
Cerebrovascular deposition of the β-amyloid (Aβ) peptide (cerebral amyloid angiopathy, CAA) is a prevalent age-associated pathology linked to spontaneous intracerebral hemorrhage, vascular cognitive impairment, and adverse responses to anti-amyloid immunotherapies.1–3 Although criteria have been validated for diagnosis of CAA during life,4 these rely on detecting the late manifestations of CAA-related vascular damage such as hemorrhage and microbleeding rather than the vascular amyloid itself. Most CAA is therefore undetectable during life. Developing noninvasive methods for determining the presence and severity of CAA would be an important step towards identifying therapies for this currently untreatable condition.
A promising imaging agent for CAA detection is the recently described lipophilic compound Pittsburgh Compound B (PiB). PiB retention in the brain has been detected by positron emission tomography (PET) in patients with Alzheimer disease (AD) in a spatial pattern (particularly frontal, temporal, cignulate, and precuneus cortex) similar to the neuropathologic distribution of Aβ-containing senile plaques.5, 6 Data from transgenic mice7 and human subjects8–10 suggest that PiB also labels vascular as well as plaque Aβ. As these two Aβ pathologies frequently co-exist, however, teasing apart the PiB signal due to CAA from that due to accompanying plaques is difficult.
To test the ability of PiB-PET to detect CAA in isolation, we imaged a subject with early manifestations of a hereditary form of CAA. Iowa-type hereditary CAA,11 like the previously described Dutch-type form,12 is characterized by severe cerebrovascular amyloid, occasional diffuse or early plaques, but few or none of the mature fibrillar plaques known to bind PiB. PiB retention in this disorder should therefore reflect strictly vascular amyloid. We investigated whether PiB would be retained in the brain of a subject with early hereditary CAA and whether the distribution of signal would demonstrate the occipital lobe predominance noted in nondemented subjects with sporadic CAA.10
Methods
All studies were performed with informed consent from the subject and with approval from the Partners Human Research Committee. Exon 17 of the gene for the amyloid precursor protein (APP) and parts of its flanking introns were amplified and sequenced from genomic DNA as described.11 Conventional CT and MRI imaging (including fast-spin echo T2-weighted, pre-and post-gadolinium T1-weighted, FLAIR-weighted, and gradient-echo sequences on a 1.5T scanner) was performed according to standard clinical protocols.
N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole (PiB; 7mCi) was administered intravenously and PET images acquired dynamically over 60 minutes as described.10 Briefly, the subject was positioned in a Siemens/CTI ECAT HR+ scanner (three dimensional mode; 63 image planes; 15.2cm axial field of view; 5.6mm transaxial resolution and 2.4mm slice interval; 69 frames: 12 × 15 seconds, 57 × 60 seconds, Knoxville, TN). PET data were reconstructed with ordered set expectation maximization, corrected for attenuation, and each frame evaluated to verify adequate count statistics and absence of head motion. PiB retention was expressed as distribution volume ratio (DVR) over the 40–60 minute interval, using the cerebellum as tissue input function. PET data were brought into registration with x-ray computed tomographic (CT) and magnetic resonance (MR) images using SPM2.
To explore the histologic pattern of PiB binding in Iowa-type hereditary CAA, we labeled post-mortem brain tissue from a previously described11 family member diagnosed with this disorder. Paraffin-embedded sections of frontal, parietal, and hippocampal cortex were deparaffinized, incubated with 10 µM PIB (gift from W.E. Klunk, University of Pittsburgh) in 0.001%DMSO and PBS for 45 minutes, rinsed in PBS and coverslipped with GVA mounting solution (Invitrogen). Fluorescent images were taken at 10 and 20X amplification with an Olympus BX51 microscope.
Results
The subject was a 42 year-old man with first-degree relative diagnosed by genetic testing and post-mortem brain examination as having advanced CAA due to the Iowa APP mutation.11 He initially presented for neurologic evaluation at age 32 with positional tremor, migraine headaches with visual aura, and memory and word-finding complaints. Serial cognitive examinations at ages 32, 33, 34, 36, 40, and 42 showed a pattern of gradual decline across multiple domains consistent with a diagnosis of mild cognitive impairment, multiple domain type.13 Progressive impairments were evident on tests of anterograde memory, naming of common objects, visuoperceptual discrimination, and attention. Serial neurologic evaluations found no substantial change in the tremor or headaches and no other focal deficits.
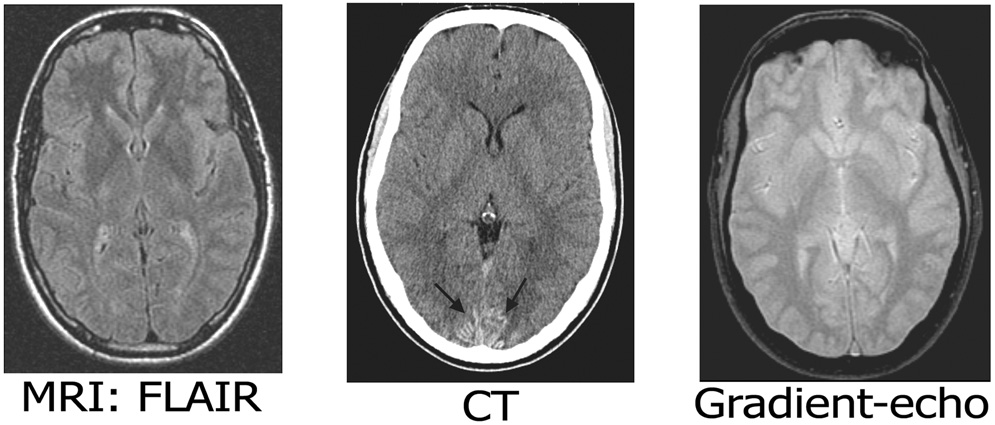
Genetic analysis showed apolipoprotein E ε3/ε4 genotype. Sequencing of APP exon 17 demonstrated the heterozygous Iowa-type G→A transition associated with substitution of asparagine for aspartic acid at APP position 694 (or Aβ position 23). Serial CT and MRI scans disclosed no areas of ischemic or hemorrhagic stroke, scant FLAIR hyperintensity posterior to the occipital horns of the lateral ventricles, and a symmetric gyral pattern of hyperdensity in the bilateral occipitotemporal cortex (Fig. 1) absent on CT scans at ages 32 and 35 but present at age 42. The gyral hyperdensities closely resembled those previously observed in Iowa-type hereditary CAA patients11 and identified pathologically as calcifications of amyloid-laden occipitotemporal leptomeningeal and cortical vessels. Gradient-echo MRI detected no microbleeds (Fig. 1).
Figure 1.
Neuroimaging of early Iowa-type hereditary CAA. Representative axial CT (top) and MRI (bottom) images are notable for occipital gyral hyperdensities (arrows), very mild periventricular FLAIR hyperintensities, and no microbleeds on gradient-echo imaging.
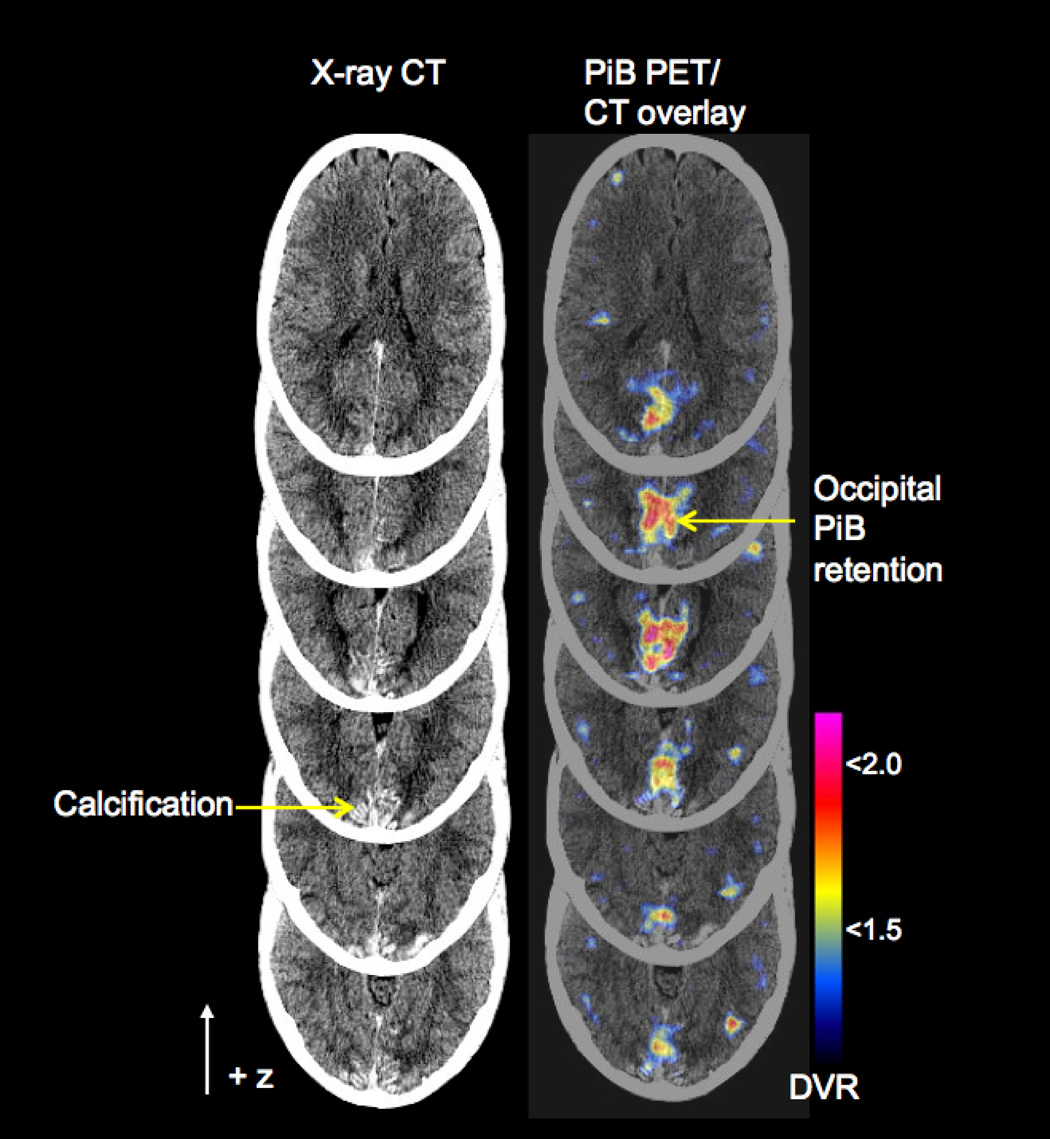
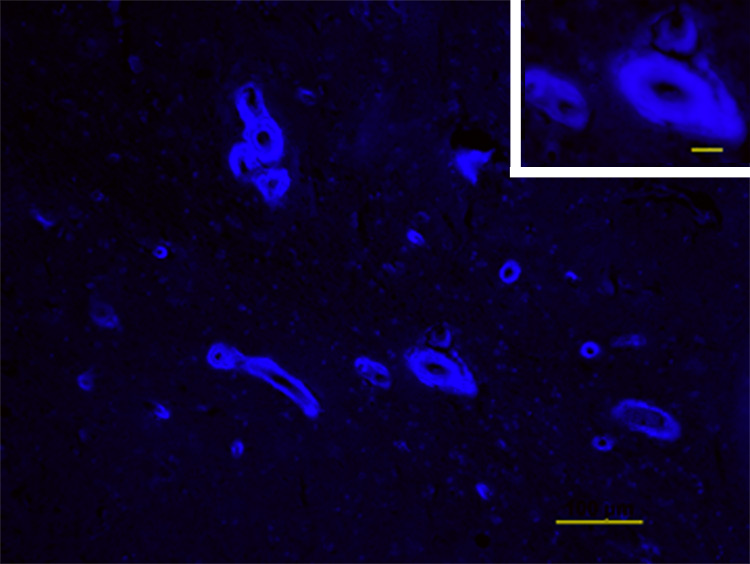
Imaging of fibrillar Aβ by PiB-PET demonstrated elevated PiB retention (DVR > 1.6) in bilateral occipital cortex anterior to the gyral CT hyperdensities (Fig. 2). Areas prominently labeled by PiB in patients with AD such as frontal, temporal, cingulate, and precuneus cortex were largely spared (DVR < 1.2). To define further whether PiB signal in this subject was due strictly to vascular rather than plaque amyloid, we used PiB to label brain sections from a related patient with Iowa-type CAA (Fig. 3). These images demonstrated exclusively vascular labeling, without evidence of plaque-associated PiB.
Figure 2.
PiB-PET in Iowa-type hereditary CAA. Serial axial PET images are overlaid on co-registered CT images (right column) to illustrate the relationship between PiB retention and occipital calcification. DVR = distribution volume ratio
Figure 3.
PiB staining in Iowa-type hereditary CAA. A representative image is shown of PiB staining in a section of hippocampal cortex from an affected member of the subject’s pedigree. PiB signal (blue) is exclusively localized to thickened vessel walls. Scale bar=100µm and 20µm (inset).
Discussion
The primary finding from this study is positive PiB-PET imaging in a young subject with early Iowa-type hereditary CAA. As the fibrillar amyloid deposits in Iowa-type CAA appear to be entirely vascular (Fig. 3), this study offers a unique opportunity to image CAA in isolation and thus provides compelling evidence that PiB-PET detects CAA as well as AD plaque pathology. This possibility had previously been suggested by studies showing robust PiB signal in vessels as well as plaques of transgenic mice,7 the observation of vascular PiB staining in human brain tissue,8, 9 and the finding of increased PiB retention in an occipital-predominant pattern in 6 nondemented subjects diagnosed with advanced sporadic CAA.10 Previous PiB-PET studies have shown a striatal-predominant pattern in 10 subjects with pre-symptomatic or early symptomatic mutations of presenilin-1,14 striatal-and posterior cingulate-predominant PiB retention in 2 subjects with symptomatic APP locus duplication,15 and an AD-like pattern in a subset of nondemented (presumably pre-symptomatic) sporadic elderly.6 The current case differs from these previous reports by offering a situation of isolated CAA that allows the independent contribution of vascular amyloid to be delineated.
A second notable finding is that vascular deposition of Aβ at a level sufficient to produce elevated PiB-PET signal appears to be a relatively early step in the pathogenesis of CAA. Radiographic findings such as extensive microbleeding and white matter hyperintensities that appear in members of the same family with advanced symptoms11 were not present in the current subject, suggesting that PiB retention precedes these radiographic signs of vascular disease. This subject did demonstrate two other sets of findings: occipital gyral CT hyperdensities (likely representing calcification of amyloid-laden vessels in the leptomeninges and superficial cortex11, 12) and mild cognitive impairment. The mild cognitive deficits in this subject appeared not to be driven by either widespread amyloid deposition or radiographically visible tissue damage, and might instead relate to other non-visualized processes associated with the Iowa APP mutation. Migraine headaches with visual aura, another element of this individual’s presentation, have not previously been reported as characteristic of Aβ-related CAA.
The observation of PiB retention apparently preceding radiographically evident tissue lesions supports the idea that the brain injury associated with CAA is a secondary consequence of vascular Aβ deposition. The pathogenic role of Aβ deposition in CAA contrasts somewhat with the situation in AD, where soluble oligomeric species of Aβ may be a primary pathogenic agent.16 Previous studies have found extent of vascular amyloid deposition to correlate with risk of intracerebral hemorrhage17 and with impaired cerebrovascular reactivity in a transgenic animal model.18
A false-positive increase in PiB retention in this subject, though impossible to exclude, is highly unlikely. Studies have shown very high correlation between PiB retention and reduced cerebrospinal Aβ4219 or regional plaque Aβ,20 indicating high specificity of PiB-PET for cerebral amyloid deposition. Although only small numbers of PiB-PET scans have been reported for healthy younger subjects, all have been negative for PiB retention, including 8 of 8 subjects under age 55 scanned at University of Pittsburgh21 and 16 of 16 under age 60 at Washington University.6 Another distinctive feature of the PiB signal in the current subject is its striking localization to occipital cortex. This feature is consistent with the known occipital predilection of CAA22 and the increased relative occipital PiB found in nondemented subjects with sporadic CAA compared to those with AD.10
These data highlight the intriguing possibility that vascular amyloid can be noninvasively detected before it triggers intracerebral hemorrhage or other overt small vessel brain injury. If sensitive and specific methods for early detection of CAA could be established, they theoretically be applied to areas such as the decision of whether to anticoagulate or the design of future therapeutic trials aimed at primary prevention of CAA-related hemorrhage. Noninvasive measurement of CAA also raises the prospect of defining CAA’s contribution to such key issues as a patient’s risk of vascular cognitive impairment1 and response to anti-amyloid immunotherapy.2, 3
Acknowledgments
We are grateful for Dr. Steven Anderson (University of Iowa) for critical review of neuropsychometric data. This work was supported by grants from the National Institutes of Health (R01 AG026484) and the Alzheimer’s Association (IIRG-06-26331).
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer M, Boncristiano S, Bondolfi L, et al. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 3.Nicoll JA, Wilkinson D, Holmes C, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston Criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 6.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 7.Bacskai BJ, Hickey GA, Skoch J, et al. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci U S A. 2003;100:12462–12467. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch. Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 9.Lockhart A, Lamb JR, Osredkar T, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann. Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski TJ, Cho HS, Vonsattel JPG, et al. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann. Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 12.Vinters HV, Natte R, Maat-Schieman ML, et al. Secondary microvascular degeneration in amyloid angiopathy of patients with hereditary cerebral hemorrhage with amyloidosis, Dutch type (HCHWA-D) Acta Neuropathol. 1998;95:235–244. doi: 10.1007/s004010050793. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 14.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J. Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remes AM, Laru L, Tuominen H, et al. Carbon 11-labeled pittsburgh compound B positron emission tomographic amyloid imaging in patients with APP locus duplication. Arch. Neurol. 2008;65:540–544. doi: 10.1001/archneur.65.4.540. [DOI] [PubMed] [Google Scholar]
- 16.Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 17.Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann. Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 18.Shin HK, Jones PB, Garcia-Alloza M, et al. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- 19.Fagan A, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008 doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizenstein HJ, Nebes RD, Saxton JA, et al. Amyloid deposition is frequent and often is not associated with significant cognitive impairment in the elderly. Arch. Neurol. 2008 doi: 10.1001/archneur.65.11.1509. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]