Abstract
Pepsinogens are a class of endopeptidases that are secreted by the gastric epithelium and released into the circulation. Low serum pepsinogen I (PGI) and low serum pepsinogen I / pepsinogen II ratio (PGI/II ratio) are markers of gastric fundic atrophy, and have recently been shown to be associated with increased risk of esophageal squamous cell carcinoma (ESCC). We conducted the current study to test whether these markers are also associated with esophageal squamous dysplasia (ESD), the precursor lesion of ESCC.
We measured serum PGI and PGII, using enzyme-linked immunosorbent (ELISA) assays, in 125 case subjects (patients with moderate or severe ESD) and 250 sex-matched control subjects (no ESD) selected from an endoscopic screening study in Linxian, China. We used conditional logistic regression models adjusted for age, smoking, and place of residence to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs).
Serum PGI showed no statistically significant association with ESD, whether analyzed as a dichotomous, ordinal (quartiles), or continuous variable. Lower serum PGI/II ratio, however, showed a dose-response association with increased risk of ESD, with an adjusted OR (95% CI) of 2.12 (1.08 − 4.18), comparing the lowest versus the highest quartile. The association between lower serum PGI/II ratio and log OR of ESD was nearly linear, and the p-value for the continuous association was 0.03.
Lower serum PGI/II ratio was linearly associated with higher risk of ESD. This result is consistent with recent findings that gastric atrophy may increase the risk of ESCC.
Keywords: Esophageal cancer, Squamous dysplasia, Pepsinogen, China
Introduction
Pepsinogen I (PGI) and pepsinogen II (PGII) are two classes of endopeptidases that are secreted by the gastric epithelium and can hydrolyze peptides in acid environments.1 Some of the secreted molecules are released into the circulation and can be measured as serum PGI and serum PGII.
Serum pepsinogen levels have been used as markers of gastric fundic atrophy.2,3 As this atrophy progresses, serum PGI levels are reduced but serum PGII levels remain stable or are increased, which results in lower PGI levels and a lower serum pepsinogen I/pepsinogen II ratio (PGI/II ratio).3 Both low serum PGI and low serum PGI/II ratio have been used as markers for gastric fundic atrophy.2,3 However, low serum PGI/II is usually a more accurate marker for this atrophy than PGI alone.3 Serum pepsinogens can also predict gastric cancer risk. A large number of epidemiologic studies have shown that atrophy of gastric fundic mucosa, diagnosed histologically or by measuring serum pepsinogens, is associated with higher risk of gastric cancer.4-16 Like the association with atrophy, some studies have suggested that the serum PGI/II ratio may be a stronger risk predictor of future gastric cancer risk than serum PGI alone.5,10,11
The association between gastric fundic atrophy, defined by low serum pepsinogen levels or by histology, and risk of esophageal squamous cell carcinoma (ESCC) has been studied only within the past few years. Three recent studies have suggested that gastric atrophy may increase ESCC risk. A cohort study in Sweden showed that patients with pernicious anemia, a condition associated with severe gastric atrophy, were three times more likely to be diagnosed with ESCC than the general population.17,18 A subsequent Swedish case-control study reported that low serum PGI was associated with higher risk of esophageal squamous cell carcinoma.9 Similarly, a Japanese case-control study found that gastric atrophy, identified serologically or histologically, was associated with a higher risk of superficial ESCC.19
Linxian, a county in Henan Province, China, has some of the highest rates of ESCC in the world. Previous studies have shown that esophageal squamous dysplasia (ESD) is the precursor lesion of ESCC in Linxian.20 Compared to subjects with no dysplasia, those with moderate and severe ESD have an increased risk of ESCC of approximately 10- and 30-fold, respectively.20 In addition, we have previously shown that the risk factors for ESD are generally similar to those for ESCC in Linxian; increasing age, a positive family history of cancer, low socioeconomic status, and tooth loss are risk factors for both ESD and ESCC in this population.21-23 We conducted the current study to examine the association between gastric fundic atrophy, as measured by low serum PGI or PGI/II ratio, and the risk of moderate or severe ESD in Linxian.
Methods
Study participants, questionnaire and biological sample collection
The subjects of this study were selected from the participants of the Cytology Sampling Study 2 (CSS2) in Linxian. Methods of the CSS2 study have been described elsewhere.23,24 In brief, in April 2002 we invited all apparently healthy adults aged 50−64 who lived in three villages in Yaocun commune, Linxian, to participate in a cytologic and endoscopic screening study for ESD and early ESCC. All of these residents who had no contraindications for the study procedures were eligible and were invited to sign an informed consent and enroll in the study. Participation rates among the eligible subjects in the three villages were 41%, 25%, and 14%, respectively, and 98% of the participants denied having any symptoms of esophageal cancer (e.g., pain or difficulty swallowing). Subjects were administered a structured questionnaire that included questions about personal characteristics, habits, and living conditions. Subjects also received a brief physical exam which included measuring height and weight. Fasting blood was collected and the separated serum was frozen at −70 °C for future use.
Seven hundred and forty study participants completed both an esophageal balloon cytology examination and an endoscopy examination with Lugol's iodine staining and biopsy,25 and 725 had at least one technically sufficient squamous biopsy. The biopsies were fixed in 80% ethanol, embedded in paraffin, cut in 5:m sections and stained with hematoxylin and eosin. The biopsy slides were read independently by two pathologists (NL, SMD), without knowledge of the patient's history or the visual endoscopic findings, and discrepant findings were adjudicated by joint review. The histologic criteria were based on previous descriptions.26
Subjects were classified according to their worst biopsy diagnosis as normal (n = 306), esophagitis (n = 186), mild dysplasia (n = 102), moderate dysplasia (n = 88), severe dysplasia (n = 39), or ESCC (n = 4). Because moderate and severe dysplasia are strong predictors of ESCC risk, individuals with these two diagnoses (n = 127) were chosen as cases for the current study. Two of the case subjects did not have serum for pepsinogen assays. Therefore, there were a total of 125 case subjects in this study. For each case, we selected two sex-matched controls (a total of 250 controls) from subjects with only normal biopsies, in a way that minimized age differences between cases and controls.
The design and conduct of the CSS2 study was approved by the Institutional Review Boards of Cancer Institute (Hospital), Chinese Academy of Medical Sciences and the US National Cancer Institute. All subjects provided written informed consent.
Serum pepsinogen assays
Serum PGI and PGII were measured using an enzyme-linked immunosorbent assay (Biohit ELISA kit, Finland) by an experienced laboratory scientist (LD) who was unaware of subjects’ case–control status. Serum was measured in duplicate and then the average value was used for each individual. Duplicate results were strongly correlated; Pearson's correlation coefficient was 0.996 for PGI and 0.998 for PGII.
Duplicate aliquots of the quality control samples that were provided with the kits were included on each assay plate. Based on these samples, the coefficients of variation were 4.9% and 2.1% for the PGI and PGII assays, respectively. In addition, 30 quality control samples, aliquoted from a single large pool of serum from Linxian residents, were distributed among the 10 assay plates (three samples per plate). On the basis of these samples, the coefficients of variation were 9.3% and 7.0% for PGI and PGII, respectively.
Statistical Analysis
Statistical analyses were done using Stata version 10.0 (StataCorp LP, College Station, TX), and SAS version 9.1 (SAS Institute, Cary, NC).
We used conditional logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs). Serum PGI and PGI/II ratio were analyzed as dichotomous variables, as quartiles, and as continuous variables. There are no universally accepted cutoff points for these two makers.27 Therefore, we used four different dichotomous seropositivity cutpoints (30, 50, 100, and 130 ng/ml) for PGI, and four cutpoints for PGI/II ratio (3, 4, 9, and 12) for PGI/II ratio. We selected these cutpoints because they were used by some previous studies as cutpoints to diagnose severe gastric fundic atrophy (30 for PGI and 3 for PGI/II ratio); because they were used in a previous study in Linxian (50 for PGI and 4 for PGI/II ratio); because they were close to the median of the collected data (100 for PGI and 9 for PGI/II ratio); or because they were close to the 75th percentile of the collected data (130 for PGI and 12 for PGI/II ratio). Quartiles were made based on the distribution of these serum markers among the controls. For continuous analyses, similar to previous studies,28 we chose one unit change as half the distance between the 25th and 75th percentile values among the controls (the average size of the two central quartiles).
Our a priori model included adjustment for age (years), history of smoking (yes vs. no), and village of residence. These variables were chosen for adjustment because age is a universal risk factor for gastric atrophy, ESD, and ESCC; smoking is a risk factor for ESCC in Linxian and it has been positively (but non-significantly) associated with ESD risk there; and residence in a certain village may be associated with unknown risk factors that cannot be measured. In addition to this a priori model, we also examined models that were adjusted for age only and models that were adjusted for age, smoking, village of residence, tooth loss, family history of cancer, and per capita income (as an indicator of socioeconomic status). None of these adjustments changed the results materially, so we only present the results of the a priori model. P-values for trend were obtained from the continuous analyses. Two-sided p-values < 0.05 were considered to be statistically significant.
We also examined the shape of the association between PGI or the PGI/II ratio and the study outcome (moderate or severe ESD) using non-linear models using PROC GAM in SAS. After fitting the GAM model, we plotted the ORs on the logarithmic scale versus PGI and PGI/II ratio on the linear scale.
Results
Table 1 shows the demographic characteristics and smoking status of cases and control subjects. The two groups were matched for sex; 47.2% of cases and controls were males. Case subjects were slightly, but statistically non-significantly, older and more likely to smoke than the control subjects. Among the control subjects, PGI and PGII were strongly correlated, with a Pearson correlation coefficient of 0.69.
Table 1.
Demographic characteristics, tobacco use, and place of residence among cases and control subjects
| Cases (n =125) | Controls (n=250) | |
|---|---|---|
| Mean age in years (s.d.) | 55.4 (4.7) | 54.7 (4.3) |
| Number of males (%) | 59 (47.2) | 118 (47.2) |
| Number of smokers (%)1 | 39 (31.2) | 67 (26.8) |
| Village of residence | ||
| Fentou | 44 (35.2) | 89 (35.6) |
| Jingwan | 30 (24.0) | 51 (20.4) |
| Xifeng | 51 (40.8) | 110 (44.0) |
Ever smoking cigarettes for six or more months
Table 2 shows the ORs (95% CIs) for the associations between PGI and ESD, adjusted for age, smoking and village of residence. The ORs for the more extensively adjusted model were similar (data not shown). Results are presented for dichotomous categories, quartiles, and continuous measures. In dichotomous analyses, serum PGI was not significantly associated with risk of ESD, regardless of the choice of cutpoint. For a cutpoint of 100, for example, 56 (44.8%) of the case subjects and 119 (47.6%) of the control subjects had low PGI values, and the adjusted OR (95% CI) for the association between low PGI and ESD was 0.88 (0.55 − 1.39). Analysis of the data by quartiles showed no statistically significant association either, and no substantial change in risk was observed across the quartiles. The adjusted OR (95% CI) for a 28.5 ng/ml decrease in PGI (half the distance between the 25th and 75th percentiles) was 1.03 (0.89 − 1.18), with a corresponding p-value of 0.71.
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) for the associations between serum pepsinogen I (PGI) and serum pepsinogen I/II ratio (PGI/II ratio) with moderate or severe esophageal squamous dysplasia
| Cases n (%) |
Controls n (%) |
Adjusted OR (95% CI)1 | |
|---|---|---|---|
| PGI | |||
| Dicohotomous | |||
| ≤ 30 ng/ml | 2 (1.6) | 4 (1.6) | 1.01 (0.18 − 5.69) |
| > 30 | 123 (98.4) | 246 (98.4) | 1.00 |
| ≤ 50 ng/ml | 9 (7.2) | 12 (4.8) | 1.70 (0.66 − 4.38) |
| > 50 | 116 (92.8) | 238 (95.2) | 1.00 |
| ≤ 100 ng/ml | 56 (44.8) | 119 (47.6) | 0.88 (0.55 − 1.39) |
| > 100 | 69 (55.2) | 131 (52.4) | 1.00 |
| ≤ 130 ng/ml | 88 (70.4) | 185 (74.0) | 0.83 (0.50 − 1.40) |
| > 130 | 37 (29.6) | 65 (26.0) | 1.00 |
| Quartiles2 | |||
| Quartile 1 (<75.4 ng/ml) | 33 (26.4) | 62 (24.8) | 0.99 (0.52−1.90) |
| Quartile 2 (75.4 −104 ng/ml) | 28 (22.4) | 63 (25.2) | 0.84 (0.44−1.60) |
| Quartile 3 (104 −132 ng/ml) | 30 (24.0) | 62 (24.8) | 0.88 (0.46−1.66) |
| Quartile 4 (>132 ng/ml) | 34 (27.2) | 63 (25.2) | 1.00 |
| Continuous decrease3 | -------- | -------- | 1.03 (0.89 − 1.18) |
| PGI/II ratio | |||
| Dichotomous | |||
| ≤ 3.0 | 6 (4.8) | 7 (2.8) | 1.80 (0.58 − 5.53) |
| > 3.0 | 119 (95.2) | 243 (97.2) | 1.00 |
| ≤ 4.0 | 9 (7.2) | 15 (6.0) | 1.17 (0.50 − 2.73) |
| > 4.0 | 116 (92.8) | 235 (94.0) | 1.00 |
| ≤ 9.0 | 73 (58.4) | 114 (45.6) | 1.88 (1.15 − 3.07) |
| > 9.0 | 52 (41.6) | 136 (54.4) | 1.00 |
| ≤ 12.0 | 99 (79.2) | 177 (70.8) | 1.78 (1.02 − 3.11) |
| > 12.0 | 26 (20.8) | 73 (29.2) | 1.00 |
| Quartiles2 | |||
| Quartile 1 (< 6.51) | 38 (30.4) | 62 (24.8) | 2.12 (1.08−4.18) |
| Quartile 2 (6.51 − 9.33) | 39 (31.2) | 63 (25.2) | 2.01 (1.02−3.96) |
| Quartile 3 (9.33 − 12.8) | 27 (21.6) | 62 (24.8) | 1.41 (0.72−2.74) |
| Quartile 4 (>12.8) | 21 (16.8) | 63 (25.2) | 1.00 |
| Continuous decrease3 | -------- | -------- | 1.22 (1.02 − 1.46) |
Odds ratios (ORs) and 95% confidence intervals (95% CIs) were obtained from the conditional logistic regression models adjusted for age, cigarette smoking, and place of residence.
Cutpoints (75th, 50th, and 25th percentiles) were 132, 104, and 75.4 ng/ml for PGI; and 12.8, 9.33, and 6.51 for PGI/II ratio.
Each continuous unit was equal to half the distance between the 25th and 75th percentile values in the control subjects. The units were 28.5 ng/ml for PGI and 3.13 for the PGI/II ratio.
Results for the PGI/II ratio are also shown in Table 2. In dichotomous analyses, results varied by cutpoint. For cutpoints of 3, 4, 9, and 12, the ORs were 1.80, 1.17, 1.88, and 1.78, respectively, and the latter two ORs were significantly different from one. Analysis of data by quartiles showed a monotonic increase in ESD risk with lower PGI/II ratio values, and the adjusted OR (95% CI) for the 1st (lowest) versus the 4th (highest) quartile was 2.12 (1.08 − 4.18). Similarly, analysis of the PGI/II ratio as a continuous variable showed a significant association with ESD. The adjusted OR (95% CI) for the association between a 3.13 unit decrease in the PGI/II ratio (half the distance between the 25th and 75th percentiles) was 1.22 (1.02 − 1.46), with a corresponding p-value of 0.03. Figure 1 shows the results of fitting a smoothed non-linear model of the association between the PG I/II ratio and risk of ESD. The model predicted an essentially linear association between the PGI/II ratio and the log OR.
Figure 1.
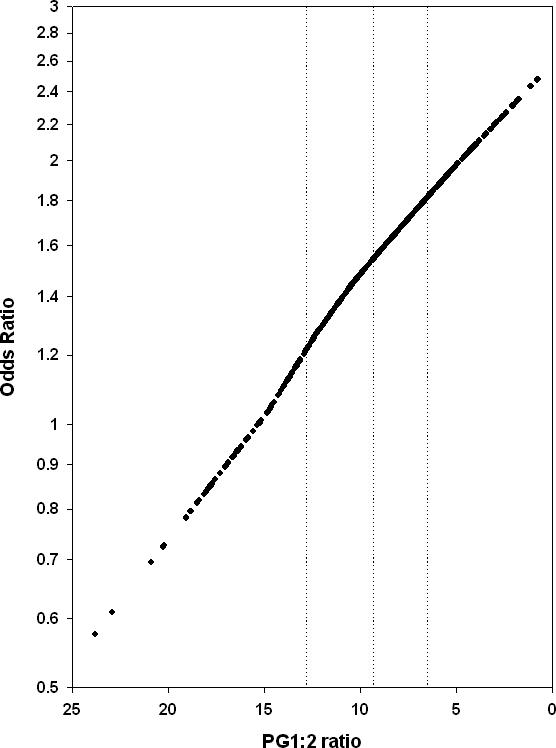
The association between the pepsinogen I/II ratio and the risk of moderate or severe esophageal squamous dysplasia. The pepsinogen I/II ratio was plotted on a linear scale, from high to low, and the odds of ESD was plotted on a logarithmic scale. The vertical dotted lines demarcate the quartiles (the 75th, 50th, and 25th percentiles, respectively) of the PGI/II ratio values.
Discussion
It has long been known that gastric atrophy is an indicator of increased risk of gastric cancer.29 In Pelayo Correa's model for the pathogenesis of intestinal-type gastric cancer, gastric atrophy is considered one of the precursor lesions of gastric cancer.4,29 Therefore, when low serum PGI and low serum PGI/II ratio were established as sensitive and specific markers for gastric fundic atrophy, a large number of studies examined the association between these markers and risk of gastric cancer, and they found a consistent association. However, the association between gastric atrophy or serum pepsinogens and other cancers, including ESCC, has not been studied until recently.
The first indication that gastric atrophy might be associated with ESCC came from a cohort study in Sweden that compared the risk of ESCC in pernicious anemia patients, who have severe gastric atrophy, with the risk of this tumor in the general population, and found a three-fold increased risk in the pernicious anemia patients.17,18 Then, in 2004, Ye and colleagues published the first evaluation of the association between serum pepsinogens and ESCC risk, in a Swedish case-control study, and found a four-fold increased risk of ESCC in patients with low serum PGI.9 Another recently published case-control study from Japan also found that gastric atrophy, diagnosed serologically or histologically, was associated with a four-fold or higher increased risk of superficial ESCC.19
Our study is the first that has examined the association between gastric atrophy, as measured by serum pepsinogens, and risk of ESD, the precursor lesion of ESCC. Our results show a continuous increase in risk of ESD associated with lower values of the serum PGI/II ratio, with a two-fold increased risk in the lowest compared to the highest quartile of this ratio. These results support the recent findings that suggest that gastric fundic atrophy increases the risk of ESCC. Gastric fundic atrophy results in reduced acid secretion, a higher luminal pH, and proliferation of bacteria in the stomach.4 These bacteria, in turn, may increase the production of carcinogens such as acetaldehyde and nitrosamines, which may underlie all of the observed associations between gastric atrophy and gastric and esophageal neoplasia.
We found a significant association in this study between ESD and low serum PGI/II ratio, but no association between ESD and low serum PGI, even though there was a strong correlation between PGI and PGII values (Pearson's r = 0.69). One possible reason for this apparent discrepancy is that the PGI/PGII ratio is a more sensitive and specific maker of gastric atrophy than PGI alone. Several previous studies suggest that the PGI/II ratio is a more accurate marker for both gastric atrophy 3 and future risk of cancer 5,10,11 than PGI alone. The superiority of the PGI/II ratio may be explained by the changes in the gastric mucosa during progression toward atrophy. In severe atrophy the fundic mucosa, which secretes both PGI and PGII, is replaced by antral-type mucosa, 30 which secretes only PGII. As a result, in subjects with severe atrophic gastritis, serum PGI levels are reduced, by up to one order of magnitude, while serum PGII levels remain constant or may even increase.3 Therefore, the PGI/II ratio may be a marker of atrophy (via its PGI component) that is adjusted for a person's total capacity of pepsinogen secretion (via its PGII component). While most studies have shown that PGI/II ratio is superior to PGI alone as a marker of atrophy, this is not a universal finding. For example, a recent study31 reported that serum PGI was more strongly correlated than PGI/II ratio with gastric maximal acid output, another functional measure of gastric fundic atrophy. Therefore, another possible explanation for the apparent discrepancy in PGI and PGI/II ratio results in this study is that the association between PGI:II and ESD is at least partly due to unknown mechanisms unrelated to atrophy.
The PGI and PGI/II ratio data were analyzed dichotomously, as quartiles, and as continuous variables. There are no universally accepted cutoff points for these two markers and, in fact, previous studies have used several different cutoff points.27 Because PGI and PGI/II ratio are indicators of gastric atrophy, one might assume that the best cutoff point might be determined by plotting Receiver Operating Characteristic (ROC) curves of these markers versus histologically confirmed gastric atrophy. However, this assumption may be misleading. Most gastric atrophy is endoscopically inapparent, and untargeted biopsies may fail to find patchy atrophic lesions, so there is no gold standard diagnostic method to which we can compare the serum markers. In fact, the serum markers may be more accurate than biopsies, because they integrate changes across the entire gastric mucosa. In our study, PGI was not associated with risk of ESD, regardless of the cutpoint used. For all but one cutpoint (4.0), the ORs found for the dichotomized PGI/II ratio values were close to 1.80. Indeed, fitting smoothed non-linear models to risk in our study suggested that risk of ESD increased continuously, and almost linearly, with lower PGI/II ratio levels. Therefore, our results support the hypothesis that progression toward gastric atrophy and increased risk of cancer is a continuous phenomenon; that using a continuous serological marker to diagnose atrophy may be more useful than using untargeted biopsies; and that using cutoff points for the PGI/II ratio, rather than continuous measurements, may reduce the power of this measurement to detect associations.
We cannot find obvious sources of bias or laboratory error that could lead to the observed associations. Ninety-eight percent of the subjects were asymptomatic at study enrollment, so it is unlikely that they changed their eating habits or other behaviors because of their disease prior to enrollment in this study. Studies with a cross-sectional design have some inherent limitations, such as evaluating temporal relationships, but these problems do not undermine our observed associations. The laboratory scientist who performed the assays was unaware of the patients’ clinical diagnoses, and calibration material suggested that the test kits functioned well. In addition, we found a correlation of 0.69 between PGI and PGII, which is similar to several previous studies, and this also suggests that the kits worked well. As always, chance findings and unknown confounders cannot be totally ruled out.
The strengths of this study include measurement of both PGI and PGII; modeling the associations multiple ways, using cutoff points, quartiles and continuous measures; the availability of data on potential confounders; and accurate classification of the ESD lesions. Limitations may include moderate sample size, the presence of unknown confounders, and imperfect correlation between PGI or PGI/II ratio and the true severity of gastric fundic atrophy.
In summary, this study showed an increased risk of moderate or severe ESD with lower levels of the serum PGI/II ratio, which is a marker of gastric fundic atrophy. This result supports recent findings that gastric fundic atrophy increases the risk of ESCC. The mechanisms by which gastric atrophy increases the risk of ESD and ESCC need to be explored further.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute and by NIH contract N01-RC-47702.
Financial Support: This study was supported by intramural research funds from the Division of Cancer Epidemiology and Genetics, NCI, NIH.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
References
- 1.Samloff IM, Taggart RT. Pepsinogens, pepsins, and peptic ulcer. Clin Invest Med. 1987;10:215–21. [PubMed] [Google Scholar]
- 2.Ley C, Mohar A, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, Parsonnet J. Screening markers for chronic atrophic gastritis in Chiapas, Mexico. Cancer Epidemiol Biomarkers Prev. 2001;10:107–12. [PubMed] [Google Scholar]
- 3.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83:204–9. [PubMed] [Google Scholar]
- 4.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 5.Fukuda H, Saito D, Hayashi S, Hisai H, Ono H, Yoshida S, Oguro Y, Noda T, Sato T, Katoh M, Terada M, Sugimura T. Helicobacter pylori infection, serum pepsinogen level and gastric cancer: a case-control study in Japan. Jpn J Cancer Res. 1995;86:64–71. doi: 10.1111/j.1349-7006.1995.tb02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokkola A, Louhimo J, Puolakkainen P, Alfthan H, Haglund C, Rautelin H. Helicobacter pylori infection and low serum pepsinogen I level as risk factors for gastric carcinoma. World J Gastroenterol. 2005;11:1032–6. doi: 10.3748/wjg.v11.i7.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura AM, Kolonel LN, Miki K, Stemmermann GN, Wilkens LR, Goodman MT, Perez-Perez GI, Blaser MJ. Helicobacter pylori, Pepsinogen, and Gastric Adenocarcinoma in Hawaii. J Infect Dis. 2005;191:2075–81. doi: 10.1086/430353. [DOI] [PubMed] [Google Scholar]
- 8.Shiotani A, Iishi H, Uedo N, Kumamoto M, Nakae Y, Ishiguro S, Tatsuta M, Graham DY. Histologic and serum risk markers for noncardia early gastric cancer. Int J Cancer. 2005;115:463–9. doi: 10.1002/ijc.20852. [DOI] [PubMed] [Google Scholar]
- 9.Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyren O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388–96. doi: 10.1093/jnci/djh057. [DOI] [PubMed] [Google Scholar]
- 10.You WC, Blot WJ, Zhang L, Kneller RW, Li JY, Jin ML, Chang YS, Zeng XR, Zhao L, Fraumeni JF, Jr., Xu GW, Samloff MI. Serum pepsinogens in relation to precancerous gastric lesions in a population at high risk for gastric cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:113–7. [PubMed] [Google Scholar]
- 11.Stemmermann GN, Samloff IM, Nomura AM, Heilbrun LK. Serum pepsinogens I and II and stomach cancer. Clin Chim Acta. 1987;163:191–8. doi: 10.1016/0009-8981(87)90022-2. [DOI] [PubMed] [Google Scholar]
- 12.Parsonnet J, Samloff IM, Nelson LM, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori, pepsinogen, and risk for gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1993;2:461–6. [PubMed] [Google Scholar]
- 13.Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, Shimizu Y, Takeshita T, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–43. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 14.Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–8. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oishi Y, Kiyohara Y, Kubo M, Tanaka K, Tanizaki Y, Ninomiya T, Doi Y, Shikata K, Yonemoto K, Shirota T, Matsumoto T, Iida M. The Serum Pepsinogen Test as a Predictor of Gastric Cancer. Am J Epidemiol. 2006;163:629–37. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Kurata JH, Mizuno S, Mukai M, Inokuchi H, Miki K, Ozasa K, Kawai K. Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig Dis Sci. 1997;42:1383–7. doi: 10.1023/a:1018833819860. [DOI] [PubMed] [Google Scholar]
- 17.Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, Fraumeni JF., Jr. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer. 1993;71:745–50. doi: 10.1002/1097-0142(19930201)71:3<745::aid-cncr2820710316>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Ye W, Nyren O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut. 2003;52:938–41. doi: 10.1136/gut.52.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iijima K, Koike T, Abe Y, Inomata Y, Sekine H, Imatani A, Nakaya N, Ohara S, Shimosegawa T. Extensive gastric atrophy: an increased risk factor for superficial esophageal squamous cell carcinoma in Japan. Am J Gastroenterol. 2007;102:1603–9. doi: 10.1111/j.1572-0241.2007.01257.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847–54. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 22.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–63. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 23.Wei WQ, Abnet CC, Lu N, Roth MJ, Wang GQ, Dye BA, Dong ZW, Taylor PR, Albert P, Qiao YL, Dawsey SM. Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut. 2005;54:759–63. doi: 10.1136/gut.2004.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan QJ, Roth MJ, Guo HQ, Kochman ML, Wang GQ, Henry M, Wei WQ, Giffen CA, Lu N, Abnet CC, Hao CQ, Taylor PR, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol. 2008;52:14–23. doi: 10.1159/000325430. [DOI] [PubMed] [Google Scholar]
- 25.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–31. [PubMed] [Google Scholar]
- 26.Dawsey SM, Yu Y, Taylor PR, Li JY, Shen Q, Shu YJ, Liu SF, Zhao HZ, Cao SG, Wang GQ, Li B. Esophageal cytology and subsequent risk of esophageal cancer. A prospective follow-up study from Linxian, China. Acta Cytol. 1994;38:183–92. [PubMed] [Google Scholar]
- 27.Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev. 2006;15:1083–94. doi: 10.1158/1055-9965.EPI-05-0931. [DOI] [PubMed] [Google Scholar]
- 28.Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301–6. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 29.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [PubMed] [Google Scholar]
- 30.Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology. 1972;63:584–92. [PubMed] [Google Scholar]
- 31.Derakhshan MH, El-Omar E, Oien K, Gillen D, Fyfe V, Crabtree JE, McColl KE. Gastric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobacter pylori. J Clin Pathol. 2006;59:1293–9. doi: 10.1136/jcp.2005.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]


