Abstract
Background
Extraintestinal manifestations (EIMs) in pediatric patients with inflammatory bowel disease (IBD) are poorly characterized. We examined the prevalence of EIMs at diagnosis, subsequent incidence, and risk factors for EIMs.
Methods
Data for 1649 patients from the PediIBD Consortium Registry, diagnosed with IBD before 18 years of age [1007(61%) with Crohn’s disease, 471(29%) with ulcerative colitis, and 171(10%) with indeterminate colitis], were analyzed using logistic regression, Kaplan-Meier, log rank tests and Cox models.
Results
EIMs were reported prior to IBD diagnosis in 97 of 1649 patients (6%). Older children at diagnosis had higher rates compared with younger children, and arthritis (26%) and aphthous stomatitis (21%) were most common. Among the 1552 patients without EIM at diagnosis, 290 developed at least one EIM. Kaplan-Meier estimates of cumulative incidence were 9% at 1 year, 19% at 5 years, and 29% at 15 years after diagnosis. Incidence did not differ by IBD type (p=0.20), age at diagnosis (p=0.22), or race/ethnicity (p=0.24). Arthritis (17%) and osteopenia/osteoporosis (15%) were the most common EIMs after IBD diagnosis.
Conclusions
In our large cohort of pediatric IBD patients, 6% had at least one EIM before diagnosis of IBD. At least one EIM will develop in 29% within 15 years of diagnosis. Incidence of EIMs both before and after diagnosis of IBD differs by type of EIM and may be slightly higher in girls, but is independent of the type of IBD, age at diagnosis, and race/ethnicity.
Keywords: children, adolescents, ulcerative colitis, Crohn’s disease, arthritis, sclerosing cholangitis
Background
Approximately 25% of all new patients with inflammatory bowel disease (IBD) are diagnosed before 20 years of age 1. Patients with IBD may present with extraintestinal manifestations (EIMs) of disease before or after diagnosis of IBD. Up to 50% of adults with IBD are reported to have at least one EIM 2-5. A higher prevalence is reported in a few studies done in children 6, 7. We explored the relationship of EIMs to age at diagnosis of IBD, type of IBD, sex, and race/ethnicity in a large cohort of children and adolescents in the Pediatric IBD Consortium Registry.
Materials and Methods
Patient population
Data were collected from January 2000 to November 2003 by the Pediatric IBD Consortium, comprised of the following centers: (1) UCSF Children’s Hospital, San Francisco, and Kaiser Permanente of Northern California; (2) University of Chicago Comer Children’s Hospital; (3) Emory University School of Medicine, Egleston Children’s Hospital, and Scottish Rite Children’s Hospital of Children’s Healthcare of Atlanta; (4) Texas Children’s Hospital, Baylor College of Medicine; (5) Children’s Hospital of Philadelphia; and (6) Mass General Hospital for Children, Boston. Each center obtained institutional review board approval for the registry protocol and complied with the Health Insurance Portability and Accountability Act. Patients and parents provided assents and consents prior to entry into the registry. Details of the establishment of the registry, enrollment, and data collection were previously reported 8. Patients were entered in the Pediatric IBD Consortium Registry when IBD was suspected or confirmed, and then followed prospectively. Of 1736 patients in the registry, we identified 1649 patients with IBD diagnosed before 18 years of age, in many cases substantially before entry into the registry. EIMs were detected both retrospectively before entry into the registry and prospectively over cohort follow-up ending on October 31, 2003.
Measurements
Age at diagnosis of IBD was based on age at which the final type of IBD was made, and was categorized as 0-5, 6-12, and 13-17 years inclusive, based on modified IBD Consortium FDA categories 8. IBD was classified as Crohn’s disease (CD), ulcerative colitis (UC), or indeterminate colitis (IC). Race/ethnicity was assessed based on self report and classified as Caucasian, African American, Asian/Pacific Islander, Hispanic and Unknown/Other. EIMs were ascertained retrospectively by history at registry entry and prospectively by history, physical examination, and radiologic study. EIMs in the registry were based on clinical documentation in the medical history during the course of follow up, referral diagnoses confirmed by the gastroenterologist, or reports from diagnostic procedure confirmed by specialist. The data included any EIMs that might have occurred before, concurrent with, or after diagnosis of IBD.
EIMs were recorded based upon standards established by all study coordinators for each EIM at the inception of the data collection, although the study is limited by initial clinical impression of the gastroenterologist.
EIMs were subdivided into the following categories: colitis related, specifically skin, eye, joint, and mouth, where the activity of the EIM parallels the activity of the underlying intestinal disease; hepatobiliary; impaired growth; EIMs secondary to complications ofor as direct extensions of bowel disease, more frequently noted in patients with CD than with UC and including nephrolithiasis, obstructive uropathy, cholelithiasis, and pancreatitis; iatrogenic EIMs such as drug-induced bone marrow suppression, pancreatitis, and corticosteroid-associated myopathy, and EIMs that cannot be categorized clearly in one of the other groups, such as amyloidosis and cancer 9. In accordance to the above classification we defined EIMs as signs and symptoms extrinsic to the gastrointestinal system, including (1) dermatologic (erythema nodosum, psoriasis, and pyoderma gangrenosum), (2) ophthalmologic (iritis and uveitis), (3) musculoskeletal (arthritis; axial and peripheral, compression fractures and osteopenia/osteoporosis), (4) renal (renal calculi), (5) hepatobiliary (primary sclerosing cholangitis [PSC], autoimmune hepatitis), (6) oral (aphthous stomatitis), (7) pancreatitis, and (8) iatrogenic (anemia, papilledema, corneal infiltrate, and glaucoma). This classification is controversial because anemia, glaucoma, and pancreatitis may also be considered complications of therapy and not as primary EIMs.
Follow-up for this study was censored on October 30, 2003.
Statistical Methods
Descriptive statistics were used to characterize the cohort for our data analyses. Kaplan-Meier methods were used to estimate cumulative incidence of EIMs after diagnosis of IBD. The curves were compared by age at diagnosis of IBD and type of EIM using the log rank test. Two regression models were used to identify risk factors for developing EIMs. First, we fit a logistic model for history of EIM at diagnosis of IBD. Second, we applied a Cox proportional hazards model for time from diagnosis of IBD to first EIM among patients without any history of EIM at diagnosis of IBD. Because of some controversy of inclusion of osteopenia/osteoporosis, glaucoma, papilledema and anemia as EIMs, a sensitivity analysis excluding these EIMs was also performed. Observations with missing dates of diagnosis of IBD or EIM were excluded from the analysis. Registry data were collected and stored in Microsoft Access 2000. Data were analyzed using STATA, Version 9.2 (Stata Corp, College Station, TX) and are expressed as Mean ± SD.
Results
The study population was 54% male and 81% Caucasian (Table 1). About 28% of the patients were 6 years old or younger at IBD diagnosis. Mean (median) age at diagnosis was 11.1±4.15 (11.8) years. The majority of patients (61%) had Crohn’s disease.
Table 1.
Characteristics of the study sample
| N | % | |
|---|---|---|
| Gender | ||
| Male | 893 | 54 |
| Female | 756 | 46 |
| Race/Ethnicity | ||
| Caucasian | 1337 | 81 |
| African American | 153 | 9 |
| Hispanic | 45 | 3 |
| Asian/Pacific Islander | 36 | 2 |
| Other/unknown | 78 | 5 |
| Age at IBD diagnosis (years) | ||
| 0-5 | 292 | 18 |
| 6-12 | 900 | 54 |
| 13-17 | 457 | 28 |
| IBD type | ||
| Crohn’s disease | 1007 | 61 |
| Ulcerative colitis | 471 | 29 |
| Indeterminate colitis | 171 | 10 |
Overall 387(24%) children developed at least one EIM by the end of follow up. Of these EIMS, 33% were musculoskeletal, 7.5% dermatologic, 7% ophthalmologic, 7.8% hepatobiliary, 13.7% oral, and 13.4% iatrogenic (Table 2). No overlap syndrome was identified.
Table 2.
Classification of First EIMs before and after IBD diagnosis
| First EIMs | N | % |
|---|---|---|
| Dermatologic | 29 | 7.5 |
| Erythema nodosum | 21 | 5.4 |
| Pyoderma gangrenosum | 6 | 1.6 |
| Psoriasis | 2 | 0.5 |
| Ophthamologic | 27 | 7.0 |
| Iritis | 16 | 4.1 |
| Uveitis | 1 | 0.3 |
| Papilledema/corneal infiltrate | 7 | 1.8 |
| Musculoskeletal | 129 | 33.3 |
| Axial arthritis | 12 | 3.1 |
| Peripheral arthritis | 45 | 11.6 |
| Axial and peripheral arthritis | 14 | 3.6 |
| Compression fracture | 7 | 1.8 |
| Osteopenia/osteoporosis | 51 | 13.2 |
| Renal calculi | 21 | 5.4 |
| Gallstones | 9 | 2.3 |
| Pancreatitis | 37 | 9.6 |
| Hepatobiliary | 30 | 7.8 |
| Primary sclerosing cholangitis | 24 | 6.2 |
| Gallstones | 9 | 2.3 |
| Autoimmune hepatitis | 6 | 1.6 |
| Oral | 53 | |
| Aphthous stomatitis | 53 | 13.7 |
| Iatrogenic | ||
| Anemia | 52 | 13.4 |
| 387 | 100 |
At least one EIM was reported in 97(6%) patients at IBD diagnosis; these children accounted for 25% of the total of 387 developing at least one EIM by the end of the follow-up period. Prevalence was 3% among children diagnosed with IBD before age 6 years and 6-7% among those who were older at IBD diagnosis (p<.05 after adjustment for covariates using the logistic model; Table 3). No other risk factors for prevalent EIMs were identified in the logistic analysis. Arthritis (26%) and aphthous stomatitis (21%) were the most common EIMs before IBD diagnosis.
Table 3.
Logistic Model for History of EIM before IBD Diagnosis (N= 1649)
| Odds-Ratio | 95% CI | p-value | p-value (heterogeneity) | |
|---|---|---|---|---|
| Gender | 0.38 | |||
| Female | 1.00 | - | - | |
| Male | 1.21 | 0.80-1.83 | 0.38 | |
| Race/Ethnicity | 0.33 | |||
| Caucasian | 1.00 | - | - | |
| African American | 1.28 | 0.68-2.43 | 0.45 | |
| Hispanic | 2.22 | 0.84-5.83 | 0.11 | |
| Asian/Pacific Islander | 1.07 | 0.25-4.58 | 0.93 | |
| Other/unknown | 0.43 | 0.10-1.79 | 0.24 | |
| Age at IBD diagnosis | 0.07 | |||
| 0-5 | 1.00 | - | - | |
| 6-12 | 2.33 | 1.09-5.01 | 0.030 | |
| 13-17 | 2.55 | 1.14-5.69 | 0.022 | |
| IBD type | 0.29 | |||
| Crohn’s disease | 1.00 | - | - | |
| Ulcerative colitis | 1.12 | 0.57-2.19 | 0.75 | |
| Indeterminate colitis | 0.68 | 0.41-1.15 | 0.15 |
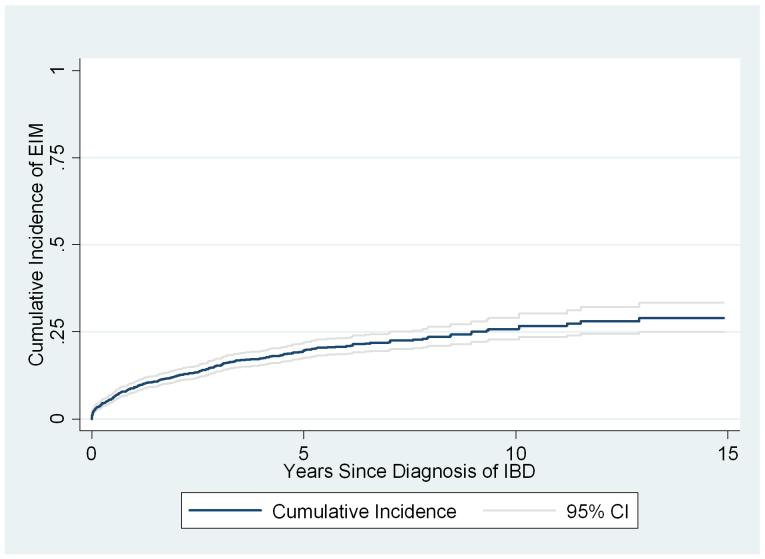
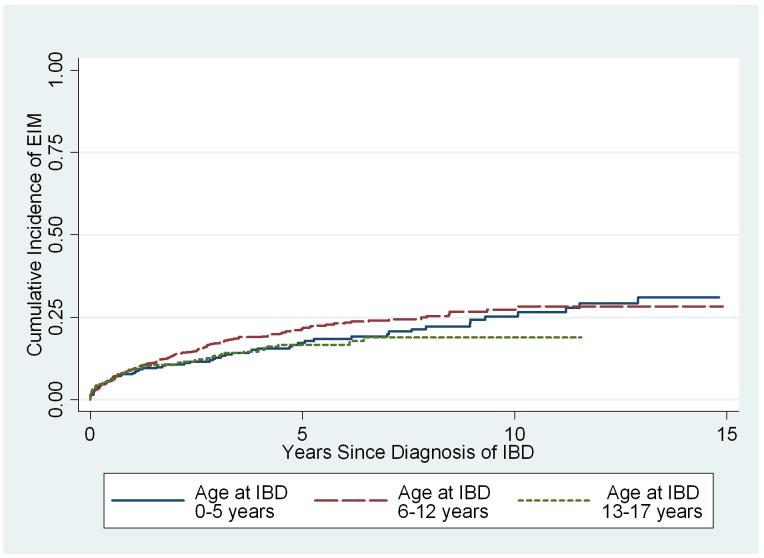
Among the 1552 patients with no history of EIM at the time of IBD diagnosis, 290(18%) subsequently developed a first EIM; of these, 52 developed a second EIM, and 13 developed a third. Osteopenia/osteoporosis, anemia and arthritis were the most common EIMs post IBD diagnosis. Kaplan-Meier estimates of the cumulative incidence were 9% (95% CI = 8%-11%) at 1 year, 19% (95% CI = 17%-22%) at 5 years and 29% (95% CI= 25%-33%) at 15 years from time of diagnosis of IBD (Figure 1). Follow-up ended at least 15 years after diagnosis of IBD for 68 of these patients. Cox analysis (Table 4) revealed weak evidence for greater risk of developing EIM among girls than boys (Hazard Ratio 1.22, 95% CI 0.97-1.54, p = 0.09), but did not differ by age of IBD diagnosis (p = 0.22; Figure 2), IBD type (p=0.20) or race (p=0.24).
Figure1.
Kaplan Meier estimates of the cumulative incidence of EIM after diagnosis of IBD among patients with no history of EIM.
Table 4.
Cox Model for Time from Diagnosis of IBD to Subsequent EIM (N = 1552)
| Hazard-Ratio | 95% CI | p-value | p-value (heterogeneity) | |
|---|---|---|---|---|
| Gender | 0.09 | |||
| Male | 1.00 | - | - | |
| Female | 1.22 | 0.97-1.54 | 0.09 | |
| Race/Ethnicity | 0.24 | |||
| Caucasian | 1.00 | - | - | |
| African American | 1.18 | 0.80-1.74 | 0.41 | |
| Hispanic | 0.56 | 0.21-1.50 | 0.25 | |
| Asian/Pacific Islander | 0.45 | 0.14-1.41 | 0.17 | |
| Other/unknown | 0.68 | 0.36-1.29 | 0.24 | |
| Ages (y) | 0.22 | |||
| 0-5 | 1.00 | - | - | |
| 6-12 | 1.13 | 0.83-1.54 | 0.44 | |
| 13-17 | 0.87 | 0.60-1.27 | 0.47 | |
| IBD type | 0.20 | |||
| Crohn’s disease | 1.00 | - | - | |
| Ulcerative colitis | 0.70 | 0.44-1.10 | 0.12 | |
| Indeterminate colitis | 1.08 | 0.83-1.40 | 0.56 |
Figure 2.
Kaplan Meier estimates of the cumulative incidence of EIM after diagnosis of IBD among patients with no history of EIM, stratified by age at IBD diagnosis.
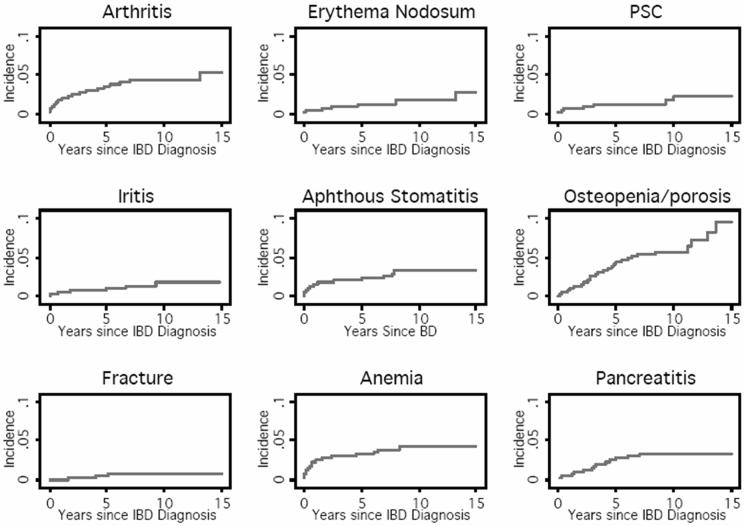
In contrast, in analyses including second and third EIMs, EIM incidence did clearly differ by type of EIM (log rank p= 0.0005, Figure 3). Kaplan-Meier estimates of the cumulative incidence of the most common EIMs at 1, 5, and 10 years post IBD are shown in Table 5. Osteopenia/osteoporosis had the highest cumulative incidence at 5 and 10 years.
Figure 3.
Kaplan Meier estimates of the cumulative incidence of the most common EIMs after diagnosis of IBD.
Table 5.
Cumulative incidence of the most common EIMs after diagnosis of IBD
| EIMs | Cumulative Incidence (%) | ||
|---|---|---|---|
| 1 yr | 5 yrs | 10 yrs | |
| Osteopenia/osteoporosis | 1.0 | 4.4 | 5.8 |
| Peripheral Arthritis | 1.7 | 3.6 | 4.2 |
| Aphthous stomatitis | 1.6 | 2.1 | 3.4 |
| Pancreatitis | 0.5 | 2.6 | 3.2 |
| Iritis | 0.5 | 1 | 1.8 |
| Erythema Nodosum | 0.4 | 1.1 | 1.6 |
| PSC | 0.7 | 1.2 | 1.6 |
| Compression Fracture | 0 | 0.5 | 0.7 |
Kaplan-Meier estimates of the cumulative incidence of PSC 5 years after diagnosis of IBD were 0.5% (95% CI 0.2%-1.3%) for children with CD, 2.7% (95% CI 1.5%-4.8%) for those with UC, and 1.3% (95% CI 0.03%-5.0%) for those with IC. In a Cox model, children with UC were at increased risk compared to those with CD (Hazard Ratio 5.25, 95% CI 1.84-14.9, p=0.002). However, risk was similar among children with CD and IC (p = 0.31), and did not differ significantly by gender (p = 0.14).
The risk of developing other EIMs did not differ by IBD types except for anemia (log rank p = 0.003). In sensitivity analyses excluding 94 EIMs (osteopenia/osteoporosis, anemia, papilledema and glaucoma), history of at least one of the other EIMs was recorded among 85/1649 patients (5.2%) at diagnosis of IBD, compared with 97 (6%) when those EIMs are included. The number of patients with at least one post IBD EIM dropped from 290 to 208 and the Kaplan-Meier estimates of cumulative incidence were consequently lower: 6% at 1 year after IBD diagnosis, 14% at 5 years, and 21% at 15 years. Results of both risk factor analyses were similar.
Discussion
In this study from a large multicenter pediatric IBD registry, EIMs were reported in 6% before diagnosis of IBD, accounting for 25% of the total first EIMs reported by the end of the study follow-up period. EIMs before diagnosis of IBD were found more frequently in children who were older than 5 years at diagnosis. Subsequent EIM incidence after diagnosis of IBD differed by type of EIM, but not by age at IBD diagnosis, race/ethnicity or IBD type. Cumulative incidence was similar to the range reported in prior adult studies 2-5, 10. However our data show a much lower incidence among children compared with prior (and smaller) pediatric reports. Grossman et al, reported that 68% of 41 children and adolescents with IBD had EIMs. In a study of 184 children with IBD, Stawarski et al found that 50% of those with CD and 80% of those with UC had at least one EIM 6, 7. However while both investigators included osteopenia, they also added growth delay as an EIM.
A prevalence study of 873 adults revealed that age at presentation did not affect the likelihood of EIM occurrence, similar to our finding in the 1552 patients without EIMs at IBD diagnosis3. However, comparisons with prior studies are problematic because of differences in patient population size, demographics and methods. A report by Monsen et al. excluded stomatitis and episcleritis from the EIMs, while Bernstein et al excluded arthropathies from their study 11, 12. No large cohort cumulative incidence study has been reported to date.
Our data reveal a non-significant but clinically relevant trend for higher risk of EIMs among girls. This finding could possibly be explained by the hypothesis that autoimmune diseases are more common in girls and is consistent with our earlier report of gender differences among children with Crohn’s disease 13. A report by Lakatos et al on 873 adult patients also found EIMs more prevalent in females than males 14. Bernstein et al, found gender variation related to types of EIMs 11.
Our finding of no difference in risk by race/ethnicity is consistent with Eidelwein et al, who found no differences in symptom presentation and EIMs between Caucasians and African American pediatric patients 15.
While our analysis did not reveal strong evidence of variation in the overall incidence of EIMs by type of IBD, specific EIMs were more prevalent in patients with CD 3, 6, 9, 11, 16. Previous reports of prevalence of EIMs have also documented increased risk in patients with CD compared with UC, with the exceptions of PSC and ocular manifestations 2, 4, 9, 14 17. The cumulative incidence of PSC was 2.1% in this study, within the range reported in prior prevalence adult studies 3, 11, 18, 19. The cumulative incidence of PSC was higher in UC than CD, similar to other studies 9, 11, 14, 20. The incidence in children may be higher than described in this study because clinical presentation of PSC in childhood is frequently different from that of PSC in adults and a high index of suspicion is required to make diagnosis of PSC in children. Though, our study did not show any gender difference in the incidence of PSC, other studies have shown that males have increased risk compared to females 11, 20-24.
Arthritis and aphthous stomatitis were the most frequent EIMs before diagnosis of IBD, while osteopenia/osteoporosis was the most common EIM after diagnosis of IBD. Peripheral arthritis was more common than axial arthritis. The cumulative incidence of peripheral arthritis at 10 years was 4.2%, lower than in three previous studies 9, 25, 26. The difference in results may be explained by differences in methods, study population and the type of referral centers. The high incidence of osteopenia/osteoporosis may be due to malabsorption of calcium and/or vitamin D, low body mass index, corticosteroid exposure, disease activity and elevation of inflammatory cytokines 27, 28. The pathogenesis of osteopenia/osteoporosis is poorly understood, and patients with CD may develop bone-mineralization disorders even without exposure to corticosteroids. 29
As a multicenter registry, our study had several innate limitations. Although our study population was drawn primarily from tertiary care referral centers, most pediatric IBD patients are followed in such centers, so our findings should be representative for pediatric patients with IBD. Information on EIMs at diagnosis of IBD was retrospective. No a priori standard criteria were developed for the definition of each EIM. Risk of EIMs in relation to disease severity could not be assessed with this dataset. The effects of therapy of the underlying IBD on EIMs were not evaluated in this study. Some clinical presentations related directly to therapy of IBD may have been included as EIMs. Impaired growth, unique to the pediatric age group, was also not evaluated in this study, as the influence of the primary disease including disease activity could not be clarified from our data.
In our large cohort of pediatric IBD patients, EIMs were relatively uncommon before diagnosis of IBD, but substantially increase after diagnosis. Race and type of IBD provided no information about risk, but risk may be slightly higher among girls. Because many EIMs may be treatment-related and can significantly complicate treatment, effects of IBD therapy on EIMs deserve further prospective investigation.
Acknowledgements
This project was supported in part by NIH grants DK060617 (mbh), DK53708 (bdg), DK006544 (bdg) and DK007762 (mbh/fj), and the Crohn’s and Colitis Foundation of America. The authors want to thank Joel Cutler for his vision and leadership in raising awareness and support for issues relevant to children with inflammatory bowel disease. The registry was supported by the indispensable efforts of a group of local study coordinators: Jennifer Cooper, Catherine Geraci, Rachel Kreh, and Amy York. Advice and guidance pertaining to database development and management was provided by Traci Clemens, PhD, Emmes Corporation, Rockville, MD. We are grateful for local support, including generous support from the Wallace Family (hsw), Jim Brooks (hsw). The Barnett Family (bsk), the Nathan Cummings Foundation (bsk), John Fullerton and family (mbh), and the Marcus Foundation (sac). The authors would like to acknowledge the generous collaboration of the following associates who referred patients for our registry: Jeffrey Blumental, Tim Buie, Robert Cannon, Conrad Cole, Michael Durant, Mark Gilger, Ranjana Gokhale, Stefano Guandalini, Colleen Hadigan, Stephen Hardy, Jay Hochman, Alison Hoppin, Sandy Hwang, Esther Israel, Crain Jensen, Seiji Kitagawa, Ronald Kleinman, William Klish, Jeffrey Lewis, Larry Glen Lewis, Carlos Lifshitz, Petar Mamula, Marjorie McCracken, William Meyers, Kathleen Motil, William Mow, Anthony Olive, Dinesh Patel, David Piccoli, Edith Pilzer, Rene Romero, Philip Rosenthal, Gary Russell, Larry Saripkin, Bess Schoen, Robert Shulman, John Snyder, Gayathri Tenjalra, Ritu Verma, Xavier Villa, Qian Yuan.
References
- [1].Calkins BM, Mendeloff AI. Epidemiology of inflammatory bowel disease. Epidemiol Rev. 1986;8:60–91. doi: 10.1093/oxfordjournals.epirev.a036296. [DOI] [PubMed] [Google Scholar]
- [2].Bernstein CN. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2001;3:477–83. doi: 10.1007/s11894-001-0068-6. [DOI] [PubMed] [Google Scholar]
- [3].Lakatos L, Pandur T, David G, et al. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–7. doi: 10.3748/wjg.v9.i10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mendoza JL, Lana R, Taxonera C, Alba C, Izquierdo S, Diaz-Rubio M. [Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn’s disease and ulcerative colitis] Med Clin (Barc) 2005;125:297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- [5].Repiso A, Alcantara M, Munoz-Rosas C, et al. Extraintestinal manifestations of Crohn’s disease: prevalence and related factors. Rev Esp Enferm Dig. 2006;98:510–7. doi: 10.4321/s1130-01082006000700004. [DOI] [PubMed] [Google Scholar]
- [6].Grossman BJ, DeBenedetti CD. Extraintestinal manifestations of chronic inflammatory bowel disease in children. Proc Inst Med Chic. 1970;28:119. [PubMed] [Google Scholar]
- [7].Stawarski A, Iwanczak B, Krzesiek E, Iwanczak F. [Intestinal complications and extraintestinal manifestations in children with inflammatory bowel disease] Pol Merkur Lekarski. 2006;20:22–5. [PubMed] [Google Scholar]
- [8].Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- [9].Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–12. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- [10].Rogler G, Scholmerich J. [Extraintestinal manifestations of inflammatory bowel disease] Med Klin (Munich) 2004;99:123–30. doi: 10.1007/s00063-004-1003-2. [DOI] [PubMed] [Google Scholar]
- [11].Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–22. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- [12].Monsen U, Sorstad J, Hellers G, Johansson C. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711–6. [PubMed] [Google Scholar]
- [13].Gupta N, Cohen SA, Bostrom AG, et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology. 2006;130:1069–77. doi: 10.1053/j.gastro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [14].Lakatos L, Pandur T, David G, et al. [Extra-intestinal manifestation of IBD in Veszprem county (of Hungary): results of a 25-years follow-up study] Orv Hetil. 2003;144:1965–75. [PubMed] [Google Scholar]
- [15].Eidelwein AP, Thompson R, Fiorino K, Abadom V, Oliva-Hemker M. Disease presentation and clinical course in black and white children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:555–60. doi: 10.1097/MPG.0b013e3180335bb3. [DOI] [PubMed] [Google Scholar]
- [16].Orchard T. Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5:512–7. doi: 10.1007/s11894-003-0042-6. [DOI] [PubMed] [Google Scholar]
- [17].Danzi JT. Extraintestinal manifestations of idiopathic inflammatory bowel disease. Arch Intern Med. 1988;148:297–302. [PubMed] [Google Scholar]
- [18].Olsson R, Danielsson A, Jarnerot G, et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–23. [PubMed] [Google Scholar]
- [19].Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332:924–33. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- [20].Rasmussen HH, Fallingborg J, Mortensen PB, et al. Primary sclerosing cholangitis in patients with ulcerative colitis. Scand J Gastroenterol. 1992;27:732–6. doi: 10.3109/00365529209011174. [DOI] [PubMed] [Google Scholar]
- [21].Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: A population-based cohort study. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.02.017. [DOI] [PubMed] [Google Scholar]
- [22].Lindor KD, Wiesner RH, MacCarty RL, Ludwig J, LaRusso NF. Advances in primary sclerosing cholangitis. Am J Med. 1990;89:73–80. doi: 10.1016/0002-9343(90)90101-i. [DOI] [PubMed] [Google Scholar]
- [23].Parlak E, Kosar Y, Ulker A, Dagli U, Alkim C, Sahin B. Primary sclerosing cholangitis in patients with inflammatory bowel disease in Turkey. J Clin Gastroenterol. 2001;33:299–301. doi: 10.1097/00004836-200110000-00008. [DOI] [PubMed] [Google Scholar]
- [24].Schrumpf E, Elgjo K, Fausa O, Gjone E, Kolmannskog F, Ritland S. Sclerosing cholangitis in ulcerative colitis. Scand J Gastroenterol. 1980;15:689–97. doi: 10.3109/00365528009181516. [DOI] [PubMed] [Google Scholar]
- [25].Palm O, Moum B, Jahnsen J, Gran JT. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001;40:1256–61. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- [26].Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. doi: 10.1097/00004836-199607000-00009. [DOI] [PubMed] [Google Scholar]
- [27].Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut. 1987;28:410–5. doi: 10.1136/gut.28.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vestergaard P. Prevalence and pathogenesis of osteoporosis in patients with inflammatory bowel disease. Minerva Med. 2004;95:469–80. [PubMed] [Google Scholar]
- [29].Thearle M, Horlick M, Bilezikian JP, et al. Osteoporosis: an unusual presentation of childhood Crohn’s disease. J Clin Endocrinol Metab. 2000;85:2122–6. doi: 10.1210/jcem.85.6.6640. [DOI] [PubMed] [Google Scholar]