Abstract
Successful aging is multidimensional, and many phenotypes have been proposed. We examined a biomarker of aging based on repeated measures of BMD for up to 15 yr and hypothesized that maintenance of BMD will be associated with low fracture risk and disability and improved survival. We studied 9704 women recruited at four U.S. clinical centers and enrolled in the Study of Osteoporotic Fractures, a longitudinal cohort study. Of these, 8224 women had at least one hip BMD measurement. Hip BMD was measured a maximum of five times over 15 yr. Random effects regression was used to determine a BMD slope for each subject. Three groups were formed—“maintained” BMD: slope ≥0, n = 724 (9%); “expected” BMD loss: slope <0 to <1 SD below mean, n = 6478 (79%); and “accelerated” BMD loss: slope ≥1 SD below mean, n = 1022 (12%). Cox proportional hazards models were used to compare the relative hazard (RH; 95% CI) of fracture, incident mobility disability, and mortality in the maintained and accelerated groups compared with the expected. A 1 SD decrease in the BMD slope was associated with an increased risk of all outcomes. In multivariate models, the RH of nonspine fracture was 0.81 (0.71–0.93) and of hip fracture was 0.36 (0.25–0.53) for women in the maintained compared with the expected group. The incidence of mobility disability was lower in the maintained versus expected group (RH = 0.70; 95% CI = 0.59–0.83), but this was largely explained by other factors. Women who experienced accelerated bone loss were more likely to develop disability (RH = 1.56; 95% CI: 1.33–1.84). Mortality risks were lower in the maintained compared with the expected group (RH = 0.49; 95% CI: 0.42–0.58). In conclusion, a subset of older women maintained their BMD up to 15 yr, suggesting that bone loss is not an inevitable consequence of aging. These women experienced a lower risk of fractures, disability, and mortality, suggesting that this phenotype may be a marker of successful aging.
Key words: successful aging, BMD, fractures, mortality, mobility disability, longitudinal follow-up study
INTRODUCTION
The landmark article by Rowe and Kahn(1) distinguished “successful” from “usual” aging, emphasizing that the physiologic, psychological, and adaptive changes that promote chronic disease, disability, and death are not the inevitable consequences of aging. Successful aging is multidimensional, and several definitions have been proposed. Although the most common definition is the absence of physical disability,(2) several physiologic biomarkers have also been proposed including hearing acuity, number of healthy teeth, absence of cardiovascular disease, systolic blood pressure, lung function, and blood glucose levels.(3,4) The osteographic scoring system, a skeletal biomarker of biological aging,(5) has been linked to mortality in younger individuals (<70 yr of age) but was not significant among subjects >70 yr of age.(6) Low BMD has also been linked to an increased risk of death.(7) These associations were, however, based on a single assessment.
We examined a biomarker of aging based on repeated measures of BMD collected over a 15-yr period. We addressed three research questions: (1) is there a group of women who maintain their BMD; (2) are there unique characteristics that differentiate these older women from others; and (3) are fracture, disability, and mortality rates lower in these women?
MATERIALS AND METHODS
Subject population
Subjects were women participating in the Study of Osteoporotic Fractures (SOF),(8,9) a prospective study of women ≥65 yr of age and older recruited at four U.S. clinics. Institutional review boards approved the SOF protocol, and all subjects provided written informed consent.
The original cohort consisted of 9704 white women recruited in 1986–1988. Total hip BMD was not included in the protocol until visit 2 (1988–1990). Hence, for this analysis, we restricted our analysis to 8224 (86%) of the cohort that did not report a prior hip fracture and had measures of hip BMD. We followed women longitudinally for up to 15 yr; the median follow-up was 8.0 yr.
BMD
BMD (g/cm2) of the total hip and the femoral neck subregion was measured using DXA (Hologic QDR 1000; Hologic, Bedford, MA, USA). Details of the measurement methods and densitometry quality control procedures have been published elsewhere.(9,10) Because the rate of bone loss has been shown to accelerate after a hip fracture,(11) we censored any BMD measure that was obtained after a hip fracture. The median number of BMD measures per subject was three (interquartile range, two to five). The number of women with BMD measures at each visit was as follows: visit 2 (1988–1990), n = 7931; visit 4 (1992–1994), n = 5960; visit 5 (1994–1996), n = 5415; visit 6 (1997–1999), n = 4496; visit 8 (2002–2004), n = 2554.
Other measurements
Information on factors that have previously been correlated to BMD,(12) rates of BMD loss,(13,14) and healthy aging(15) were collected. Body weight was measured in indoor clothing using a balance beam scale; height was measured using the Harpenden Stadiometer (Dyfed, UK). Weight and height were used to calculate body mass index (BMI, kg/m2). Percent weight change was calculated from baseline to the final clinic visit. Height loss from age 25 was calculated from self-reported height at age 25 to baseline height. Subjects completed a questionnaire and interview that collected information on medical and fracture history, falls, self-rated health, physical activity, alcohol, and smoking. Participants brought all prescription and nonprescription medications for verification of use. Participants were asked if they had any difficulty carrying out any of the following instrumental activities of daily living (walking two or three blocks, climbing up 10 steps, walking down 10 steps, preparing meals, doing heavy housework, or grocery shopping). The number of difficult activities was summed. Dietary calcium intake was assessed by a food frequency questionnaire(16) and summed with supplemental intake. Tests of physical performance included standing from a chair (without using arms five times), walking speed (over a 6-m course), and grip strength. Cognitive function was assessed with a modified version of the Mini Mental State Examination (mMMSE), which is a brief test of global cognitive functioning that evaluates concentration, language, and auditory skills; the maximum MMSE score for this modified scale is 26.(17) Prevalent vertebral fractures were identified from lateral thoracic and lumbar spine films.(18)
Outcomes
Incident nonspine fractures and deaths were identified from postcard follow-up every 4 mo; >95% of these contacts were complete. All nonspine fractures were confirmed by radiographic report. We included all fractures, including major trauma fractures, because these fractures have also been related to low BMD and an increased risk of fracture.(19) Incident disability was defined as first report of difficulty walking after baseline.(20) All deaths were confirmed by death certificate and hospital discharge summaries, if available.
Statistical analysis
Random effects regression (PROC MIXED in SAS; SAS Institute, Cary, NC, USA) was used to determine a BMD slope and intercept for each subject. Random effects regression enables estimation of population level fixed effects (overall rate of change in BMD in the entire sample) and individual level random effects (individual deviation from the overall group pattern).(21) Data from the group are used to increase precision in the estimation of individual specific quantities. Slopes were estimated in all 8224 subjects who had at least one BMD measurement until hip fracture, death, or the end of the 15-yr follow-up period. For women with one BMD measure, this method allows an estimation of their slope based on the population level effect on the overall rate of change in the entire cohort. We have used a similar analytic approach to model cognitive trajectories over time.(22) By year 15, 4043 (42%) subjects had died, and 832 (9%) had dropped out or been lost to follow-up. All slopes were modeled as linear. We analyzed the slope as a continuous variable and examined the relative hazard (RH; 95% CIs) of fracture, disability, and death per 1 SD decrease in the slope using Cox proportional hazards models. We placed women into three groups depending on their individual slope. Women were considered to have “maintained” BMD if their slope was ≥0; “expected” BMD loss if their slope was <0 to <1 SD below the mean slope, and “accelerated” BMD loss if their slope was ≥1 SD below the mean slope.
Characteristics of the three groups were compared by χ2 tests (categorical) or ANOVA (continuous). Multinomial logistic regression was used to determine the factors associated with maintenance of BMD or accelerated BMD loss. The expected group formed the referent group. The final model included all significant variables with p < 0.05 after stepwise multivariate adjustment. We calculated the age-adjusted incidence rate of nonspine and hip fractures and mortality rates across the three groups. We used Cox proportional hazards regression to estimate the RH (95% CIs) of fracture and death in the maintained and accelerated groups, in comparison with the expected. We excluded women who had a prior hip fracture from the hip fracture analyses (n = 185), and we excluded women who reported difficulty walking at baseline from the disability analyses (n = 932).
Over the follow-up period, women could have begun other medications approved for osteoporosis such as a bisphosphonates or selective estrogen receptor modulators. Thus, we further adjusted models for ever use of a bone active agent.
In secondary analyses, we excluded women who reported hormone therapy use. We also repeated our analyses limiting the cohort to women who had at least two BMD measurements (n = 6500).
RESULTS
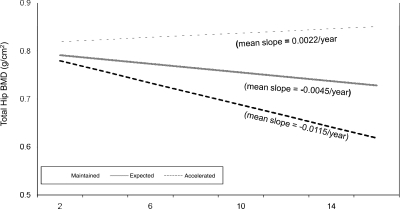
Nine percent of women (n = 724) maintained their BMD, and 12% (n = 1022) experienced an accelerated BMD loss over the 15-yr follow-up (Fig. 1). The average rate of bone loss in the accelerated group was 6 times greater in magnitude than that observed among the maintainers and 2.5 times greater than the expected group.
FIG. 1.
Total hip BMD over 15 yr of follow-up. *Estimated from random effects regression.
Women who maintained their BMD over the 15-yr period were younger, slightly taller at baseline, experienced less height loss since age 25, were more likely to report surgical menopause, and less likely to report diabetes, hypertension, or chronic obstructive pulmonary disease (COPD) than women who experienced expected or accelerated bone loss (Table 1). There was no difference in the prevalence of stroke, myocardial infarction (MI), or falling. Baseline body weight was similar in women who maintained and who experienced accelerated BMD loss, but over follow-up, maintainers gained 2.5% body weight, whereas women in the accelerated group lost 12% body weight. Congestive heart failure and Parkinson's disease were slightly more common in the accelerated group in comparison with those who maintained their BMD.
Table 1.
Baseline Characteristics of Women by Change in Total Hip BMD Over 15 yr: Maintained, Expected, and Accelerated
| Maintained | Expected | Accelerated | p for trend | |
| N (%) | 724 (8.8) | 6,478 (78.8) | 1,022 (12.4) | |
| Age, mean (SD) (yr) | 68.9 (3.6) | 71.4 (5.1) | 71.8 (5.0) | <0.0001 |
| Weight, mean (SD) (kg) | 69.2 (11.8) | 66.7 (12.0) | 69.6 (14.2) | <0.0001 |
| Percent weight change, baseline to last visit, mean (SD) | 2.5 (10.3) | −3.5 (9.6) | −11.8 (10.8) | <0.0001 |
| Height, mean (SD) (cm) | 159.8 (5.8) | 159.1 (6.0) | 159.1 (6.0) | 0.006 |
| Height loss since age 25, mean (SD) (cm) | 2.7 (2.4) | 3.2 (2.9) | 3.6 (3.1) | <0.0001 |
| BMI, mean (SD) (kg/m2) | 27.1 (4.5) | 26.4 (4.5) | 27.5 (5.3) | <0.0001 |
| Systolic blood pressure (mmHg) | 138.4 (17.4) | 141.3 (18.8) | 143.0 (19.5) | <0.0001 |
| Medical history | ||||
| Surgical menopause (%) | 15.1% | 12.6% | 10.4% | 0.02 |
| Diabetes (%) | 3.2% | 6.6% | 8.7% | <0.0001 |
| Stroke (%) | 1.8% | 2.7% | 2.7% | 0.36 |
| Hypertension (%) | 31.6% | 37.2% | 43.5% | <0.0001 |
| MI (%) | 6.0% | 6.8% | 8.2% | 0.22 |
| CHF (%) | 1.6% | 2.7% | 3.2% | 0.14 |
| Parkinson's (%) | 0.1% | 0.5% | 0.9% | 0.09 |
| COPD (%) | 5.5% | 9.2% | 9.6% | 0.003 |
| Health behaviors | ||||
| Walk for exercise (%) | 58.4% | 52.2% | 48.4% | 0.0002 |
| Total kcal/week burned, mean (SD) | 1814 (1751) | 1671 (1689) | 1546 (1576) | 0.004 |
| Alcohol consumption (%) | 76.5% | 71.0% | 70.5% | 0.006 |
| Alcohol drinks per week, mean (SD) | 1.8 (3.7) | 1.9 (4.0) | 1.8 (3.9) | 0.44 |
| Current smoker (%) | 6.0% | 9.8% | 9.4% | 0.004 |
| Calcium intake per day, mean (SD) (mg) | 744 (428) | 715 (421) | 699 (431) | 0.10 |
| Medication use (current) | ||||
| Estrogen (%) | 23.5% | 14.3% | 9.2% | <0.0001 |
| Thiazide (%) | 24.0% | 26.7% | 32.0% | 0.0003 |
| Calcium (%) | 49.0% | 42.7% | 39.4% | 0.0003 |
| Physical performance | ||||
| Chair stand without using arms (%) | 98.9% | 97.1% | 97.6% | 0.02 |
| Time of 5 chair stands, mean (SD) (s) | 11.4 (4.2) | 12.2 (4.2) | 12.5 (4.5) | <0.0001 |
| Walking speed, mean (SD) (m/s) | 1.08 (0.19) | 1.03 (0.21) | 1.02 (0.21) | <0.0001 |
| Grip strength, mean (SD) (kg) | 21.9 (3.9) | 21.0 (4.3) | 21.1 (4.2) | <0.0001 |
| No. of IADL difficulties, mean (SD) | 0.49 (1.02) | 0.68 (1.23) | 0.72 (1.25) | 0.0001 |
| Fall past 12 months (%) | 29.8% | 29.3% | 28.9% | 0.92 |
| Fracture any bone since age 50 (%) | 30.7% | 34.8% | 36.4% | 0.04 |
| Prevalent vertebral fracture (%) | 18.2% | 18.9% | 20.8% | 0.28 |
| Excellent/good self-rated health (%) | 87.7% | 84.5% | 84.2% | 0.06 |
| Baseline mMMSE score, mean (SD) | 25.1 (1.2) | 24.7 (1.6) | 24.7 (1.5) | <0.0001 |
| Baseline total hip BMD, mean (SD) (g/cm2) | 0.80 (0.14) | 0.76 (0.13) | 0.76 (0.14) | <0.0001 |
Maintainers were more likely to walk for exercise, had higher physical activity, were more likely to drink alcohol (although number of drinks per week did not differ), were less likely to smoke, and had higher calcium intake. Estrogen use was more common and thiazide diuretic use was less common in the maintainer than other groups. Baseline physical performance measures were consistently better in maintainers and fewer of these women reported difficulty with any instrumental activity of daily living (IADL) compared with the others. A slightly greater proportion of women who maintained their BMD reported excellent/good health status at baseline. Scores on cognitive function tests and baseline total hip BMD were higher in maintainers, but there was no difference between the expected or accelerated groups.
In multivariable adjusted models, older age, lower body weight, weight loss, having diabetes, and smoking were associated with a lower likelihood of maintaining BMD. Estrogen use and better neuromuscular and cognitive function was associated with a greater likelihood of maintaining BMD (Table 2). Higher body weight, weight loss and height loss, and diabetes were associated with an increased likelihood and estrogen use was associated with a lower likelihood of experiencing accelerated bone loss. Physical activity, hypertension, COPD, alcohol consumption, use of thiazide diuretics or calcium supplements, fracture history, and BMD were not significantly related to either group in the multivariable models.
Table 2.
Factors Independently Associated With Maintaining BMD and Accelerated Bone Loss in Multivariate Models*
| Maintained (N = 724) | Accelerated (N = 1,022) | |
| Age (per 5-yr increase) | 0.62 (0.55–0.70) | 1.00 (0.93–1.08) |
| Baseline weight (per 1 SD decrease) | 0.77 (0.71–0.83) | 0.91 (0.85–0.98) |
| Percent weight change (per 1 SD decrease) | 0.54 (0.49–0.58) | 2.40 (2.22–2.59) |
| Height loss since age 25 (per 1 SD increase) | 1.02 (0.93–1.12) | 1.11 (1.04–1.20) |
| Diabetes | 0.48 (0.31–0.74) | 1.24 (0.96–1.61) |
| Health behaviors Current smoker | 0.47 (0.33–0.65) | 1.08 (0.84–1.38) |
| Medication use (current) Estrogen | 1.65 (1.36–2.01) | 0.65 (0.51–0.83) |
| Chair stand (per 1 SD decrease) | 1.14 (1.03–1.26) | 0.99 (0.92–1.06) |
| Baseline mMMSE (per 1 SD increase) | 1.19 (1.08–1.31) | 1.06 (0.99–1.14) |
* Each row represents OR with 95% CI from multivariate multinomial logistic regression model with women who experienced expected bone loss as the reference group.
Fracture
A 1 SD decrease in the slope for BMD over the 15 yr was associated with an increased risk of any nonspine fracture (RH = 1.14; 95% CI = 1.11–1.18) and hip fracture (RH = 1.37; 95% CI = 1.29–1.44). Further adjustment for other factors reduced the magnitude of the RH, but they remained statistically significant (p = 0.0006 and p = 0.0005, respectively). Analyses limited to women with at least two BMD measures and to women who were not using estrogen at entry showed similar results.
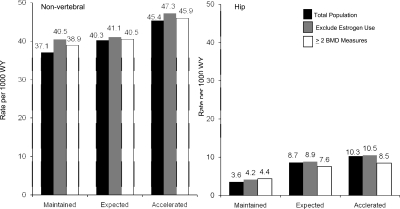
The age-adjusted incidence of nonspine and hip fracture was lowest in women who maintained their BMD (Fig. 2). Maintenance of BMD was associated with 30% lower likelihood of experiencing a nonspine fracture and a 75% lower risk of a hip fracture in comparison with women who experienced the expected rate of bone loss (Table 3). The association was greater than the effects of age alone for both nonspine and hip fractures. In the fully adjusted models, maintenance of BMD was associated with a 19% lower risk of nonspine fracture and a 64% lower risk of hip fracture (Table 3). Further adjustment for ever use of a bone active agent over the follow-up period had little effect on our results.
FIG. 2.
Age-adjusted incidence rate (per 1000 women-years) of nonspine fracture and hip fracture: women who maintained hip BMD, experienced expected hip bone loss, or experienced accelerated bone boss.
Table 3.
RH (95% CI) of Incident of Nonspine Fractures and Hip Fracture by Age and Among Women Who Maintained Their BMD or Experienced Accelerated Loss in BMD
|
Total population |
Exclude estrogen users |
Women with ≥2 BMD measures |
||||
| RH | 95% CI | RH | 95% CI | RH | 95% CI | |
| Nonspine fractures | ||||||
| Age alone (per 5-yr decrease) | 0.81 | (0.78–0.83) | 0.81 | (0.78–0.84) | 0.81 | (0.78–0.85) |
| Unadjusted models | ||||||
| Maintained | 0.70 | (0.62–0.79) | 0.73 | (0.64–0.84) | 0.74 | (0.67–0.82) |
| Accelerated | 1.15 | (1.05–1.27) | 1.16 | (1.05–1.28) | 1.16 | (1.04–1.29) |
| Age-adjusted models | ||||||
| Maintained | 0.75 | (0.66–0.85) | 0.80 | (0.69–0.91) | 0.78 | (0.70–0.86) |
| Accelerated | 1.12 | (1.02–1.24) | 1.13 | (1.03–1.25) | 1.11 | (1.00–1.24) |
| Age- and BMD-adjusted models | ||||||
| Maintained | 0.78 | (0.69–0.89) | 0.81 | (0.70–0.94) | 0.76 | (0.68–0.84) |
| Accelerated | 1.14 | (1.03–1.26) | 1.16 | (1.04–1.28) | 1.14 | (1.03–1.28) |
| Multivariate models* | ||||||
| Maintained | 0.81 | (0.71–0.93) | 0.83 | (0.71–0.96) | 0.78 | (0.70–0.87) |
| Accelerated | 1.08 | (0.97–1.20) | 1.10 | (0.98–1.22) | 1.08 | (0.96–1.21) |
| Multivariate models† | ||||||
| Maintained | 0.79 | (0.69–0.90) | 0.80 | (0.69–0.92) | 0.75 | (0.67–0.84) |
| Accelerated | 1.09 | (0.98–1.21) | 1.11 | (0.99–1.23) | 1.09 | (0.98–1.23) |
| Hip fractures | ||||||
| Age alone (per 5-yr decrease) | 0.55 | (0.52–0.58) | 0.55 | (0.52–0.59) | 0.55 | (0.52–0.59) |
| Unadjusted models | ||||||
| Maintained | 0.25 | (0.18–0.36) | 0.27 | (0.18–0.40) | 0.44 | (0.35–0.56) |
| Accelerated | 1.23 | (1.04–1.46) | 1.22 | (1.02–1.46) | 1.31 | (1.07–1.59) |
| Age-adjusted models | ||||||
| Maintained | 0.31 | (0.22–0.45) | 0.34 | (0.23–0.50) | 0.50 | (0.39–0.64) |
| Accelerated | 1.13 | (0.95–1.33) | 1.12 | (0.94–1.34) | 1.13 | (0.93–1.38) |
| Age- and BMD-adjusted models | ||||||
| Maintained | 0.34 | (0.24–0.49) | 0.36 | (0.24–0.53) | 0.49 | (0.38–0.63) |
| Accelerated | 1.16 | (0.98–1.38) | 1.17 | (0.97–1.40) | 1.20 | (0.98–1.47) |
| Multivariate models* | ||||||
| Maintained | 0.36 | (0.25–0.53) | 0.37 | (0.25–0.56) | 0.51 | (0.40–0.67) |
| Accelerated | 1.06 | (0.88–1.27) | 1.09 | (0.90–1.31) | 1.05 | (0.85–1.30) |
| Multivariate models† | ||||||
| Maintained | 0.38 | (0.26–0.55) | 0.39 | (0.26–0.59) | 0.53 | (0.41–0.68) |
| Accelerated | 1.04 | (0.87–1.25) | 1.07 | (0.89–1.30) | 1.05 | (0.84–1.29) |
Expected group forms the referent group.
* Multivariate models: age, total hip BMD, current smoking, physical activity, weight change, hypertension, diabetes, calcium supplement use, and health status. MV models in the total population also include estrogen use.
† Multivariate models: additional adjustment for any estrogen, bisphosphonate, or SERM use after baseline.
Accelerated bone loss was associated with a 15% increased risk of nonspine fracture and a 23% increase in hip fractures. However, adjustment for other factors including use of bone active agents attenuated these associations, and they were no longer statistically significant.
Disability
A 1 SD decrease in the slope for BMD over the 15 yr of follow-up was associated with an increased risk of developing walking disability (unadjusted model: HR = 1.28; 95% CI: 1.22–1.35). Further multivariable adjustment reduced the magnitude of this association, but it remained statistically significant (HR = 1.17; 95% CI: 1.10–1.24).
A 5-yr increase in age was associated with about a 50% increased likelihood of developing incident disability, as defined by self-report walking difficulty (Table 4). Maintenance of BMD was associated with a 30% lower risk of developing disability in unadjusted analyses in comparison with women in the expected group. However, in multivariate models, the association between maintenance of BMD and incident disability was no longer statistically significant. In contrast, accelerated bone loss was associated with a 75% increased likelihood of disability in unadjusted models in comparison with women in the expected group. In multivariate adjusted models, this association was attenuated slightly; but nevertheless, women who experienced accelerated bone loss were 56% more likely to experience incident disability, independent of other factors.
Table 4.
RH (95% CI) of Walking Disability by Age and Among Women Who Maintained Their BMD or Experienced Accelerated Loss in BMD
|
Total population |
Exclude estrogen users |
Women with ≥2 BMD measures |
||||
| RH | 95% CI | RH | 95% CI | RH | 95% CI | |
| Age alone (per 5-yr decrease) | 0.66 | (0.62–0.69) | 0.66 | (0.62–0.69) | 0.66 | (0.62–0.70) |
| Age alone (per 5-yr increase) | 1.52 | (1.44–1.60) | 1.53 | (1.45–1.62) | 1.52 | (1.43–1.62) |
| Unadjusted models | ||||||
| Maintained | 0.70 | (0.59–0.83) | 0.71 | (0.59–0.86) | 0.77 | (0.67–0.89) |
| Accelerated | 1.75 | (1.52–2.03) | 1.73 | (1.48–2.01) | 1.65 | (1.40–1.93) |
| Age-adjusted models | ||||||
| Maintained | 0.84 | (0.71–1.00) | 0.87 | (0.71–1.06) | 0.85 | (0.74–0.98) |
| Accelerated | 1.71 | (1.48–1.99) | 1.69 | (1.45–1.98) | 1.53 | (1.30–1.81) |
| Age- and BMD-adjusted models | ||||||
| Maintained | 0.83 | (0.70–0.99) | 0.88 | (0.72–1.08) | 0.85 | (0.74–0.99) |
| Accelerated | 1.74 | (1.49–2.02) | 1.72 | (1.47–2.02) | 1.53 | (1.30–1.81) |
| Multivariate models* | ||||||
| Maintained | 0.92 | (0.76–1.11) | 0.96 | (0.77–1.18) | 0.96 | (0.82–1.12) |
| Accelerated | 1.56 | (1.33–1.84) | 1.57 | (1.32–1.86) | 1.38 | (1.16–1.65) |
| Multivariate models† | ||||||
| Maintained | 0.89 | (0.74–1.08) | 0.93 | (0.75–1.15) | 0.94 | (0.80–1.10) |
| Accelerated | 1.58 | (1.34–1.86) | 1.58 | (1.33–1.88) | 1.40 | (1.17–1.67) |
The expected group forms the referent group.
* Adjusted for age, total hip BMD, current smoking, physical activity, weight change, hypertension, diabetes, calcium supplement use, and health status. MV models in total population also include estrogen use.
† Multivariate models: additional adjustment for any estrogen, bisphosphonate, or SERM use after baseline.
Mortality
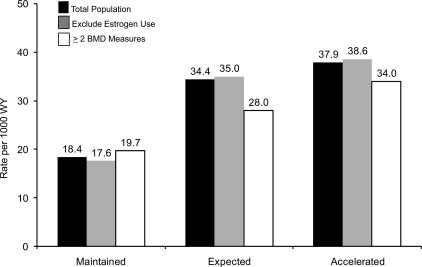
A 1 SD decrease in BMD slope was associated with a 27% increased risk of mortality (RH = 1.27; 95% CI = 1.24–1.31). This association was attenuated slightly in the multivariable model (RH = 1.11; 95% CI = 1.07–1.15). Mortality rates were lowest in women who maintained their BMD (Fig. 3). Maintenance of BMD was associated with a 63% lower likelihood of dying in unadjusted models (Table 5). This association was greater in magnitude than the effect of age alone. In the fully adjusted model, including whether a woman experienced an incident fracture as a time-dependent covariate or initiated use of a bone active agent, maintenance of BMD was associated with a 51% lower risk of dying. Accelerated loss of BMD was associated with a 18% increased risk of dying but this association was not significant after multivariable adjustment.
FIG. 3.
Age-adjusted mortality rate (per 1000 women-years): women who maintained hip BMD, experienced expected bone loss, or experienced accelerated bone boss.
Table 5.
RH (95% CI) of Death by Age and Among Women Who Maintained Their BMD or Experienced Accelerated Loss in BMD
|
Total population |
Exclude estrogen users |
Women with ≥2 BMD measures |
||||
| RH | 95% CI | RH | 95% CI | RH | 95% CI | |
| Age alone (per 5-yr decrease) | 0.55 | (0.53–0.56) | 0.55 | (0.53–0.57) | 0.54 | (0.52–0.56) |
| Unadjusted models | ||||||
| Maintained | 0.37 | (0.32–0.43) | 0.35 | (0.30–0.42) | 0.53 | (0.47–0.59) |
| Accelerated | 1.18 | (1.08–1.28) | 1.17 | (1.07–1.28) | 1.38 | (1.25–1.52) |
| Age-adjusted models | ||||||
| Maintained | 0.46 | (0.40–0.53) | 0.45 | (0.38–0.53) | 0.60 | (0.54–0.67) |
| Accelerated | 1.08 | (0.99–1.17) | 1.08 | (0.99–1.18) | 1.19 | (1.07–1.31) |
| Age- and BMD-adjusted models | ||||||
| Maintained | 0.46 | (0.39–0.53) | 0.44 | (0.37–0.52) | 0.60 | (0.53–0.68) |
| Accelerated | 1.06 | (0.97–1.16) | 1.07 | (0.97–1.17) | 1.20 | (1.08–1.33) |
| Multivariate models* | ||||||
| Maintained | 0.49 | (0.42–0.58) | 0.48 | (0.40–0.58) | 0.63 | (0.56–0.72) |
| Accelerated | 0.98 | (0.89–1.08) | 0.99 | (0.89–1.09) | 1.12 | (1.00–1.25) |
| Multivariate models† | ||||||
| Maintained | 0.53 | (0.45–0.62) | 0.53 | (0.44–0.63) | 0.66 | (0.58–0.76) |
| Accelerated | 0.95 | (0.87–1.05) | 0.97 | (0.88–1.07) | 1.10 | (0.98–1.23) |
The expected group forms the referent group.
* Adjusted for age, total hip BMD, current smoking, physical activity, weight change, hypertension, diabetes, calcium supplement use, health status, and any incident fracture over follow-up. MV models in total population also include estrogen use.
† Multivariate models: additional adjustment for any estrogen, bisphosphonate, or SERM use after baseline.
Secondary analyses
Exclusion of women who reported current estrogen use at baseline showed similar results for fracture (Fig. 2; Table 3), disability (Table 4), and mortality (Fig. 3; Table 5).
We also limited our analyses to women with at least two BMD measures. This resulted in similar findings with respect to all nonspine fractures and disability (Tables 3 and 4). For hip fractures, there was some attenuation in the effect of maintaining BMD on hip fracture (multivariable adjusted HR = 0.51; 95% CI = 0.40–0.67) in this subgroup, but it remained statistically significant. Similarly, women who maintained their BMD had a significantly lower risk of dying (Fig. 3; Table 5), but the magnitude of the association was slightly attenuated. Finally, there was some suggestion that accelerated loss was associated with an increased risk of mortality in this subgroup, but the p values were borderline significant and largely reflected a lower mortality rate in the expected group.
DISCUSSION
In this longitudinal study of >8000 women followed for up to 15 yr, 9% maintained their BMD until the end of the period or death. The mean age of study participants at the end of follow-up was 85 yr, indicating that these women maintained their BMD until old age and experienced a lower risk of fracture and mortality. The observation that some women do not lose bone mass with age showed that bone loss is not an inevitable consequence of aging. There is substantial heterogeneity in the manner that individuals' age.
Maintenance of BMD was associated with lower fracture rates, including hip fractures, the most serious consequence of osteoporosis.(23) It was also associated with lower mortality rates. Indeed, maintenance of BMD predicted fracture and mortality better than age alone and attenuated the effect of age on fractures and mortality. Thus, maintenance of BMD meets the criteria set forth that any marker of successful aging should be independent of age. Maintenance of BMD was associated with a lower risk of mobility disability, but this was not significant in the multivariable models. In the multivariable models, markers of poor health status, chronic disease (diabetes, hypertension), older age, smoking, and weight loss were independently associated with an increased risk of incident mobility disability. All of these factors were less common in the women who maintained their BMD. Thus, “maintenance” of BMD was no longer significant with these factors in the model.
Women who experienced accelerated bone loss were not at an increased risk of fracture, consistent with previous observations from SOF that rate of bone loss was a relatively weak predictor of fracture.(24) Accelerated bone loss was also not associated with an increased risk of mortality but was independently related to the development of walking disability. In the Rotterdam Study, development of osteoporosis played a significant etiologic role for the development of locomotor disability.(25) However, information on walking disability was assessed at the same time as BMD, unlike the fracture and mortality outcomes that were assessed every 4 mo over the follow-up period. Thus, it is difficult to tease out what came first: the accelerated bone loss or the walking disability.
Consistent with other studies using alternate definitions of successful aging,(15) modifiable behaviors were related to maintenance of BMD. Women who smoked at entry to the study were ∼50% less likely to maintain their BMD. Lower body weight at baseline was also associated with lower likelihood of maintaining their BMD, but a stronger association was observed with weight loss over the follow-up period. Most previous studies have focused on obesity and healthy aging and not weight loss. Weight loss late in life may be a marker of comorbid illness and has been linked with an increased risk of both fractures(26) and mortality.(27,28) Adjustment for weight change, however, did not substantially impact the association between BMD maintenance and outcomes.
A higher level of physical activity has been associated with successful aging, especially in studies that defined successful aging in terms of cardiovascular disease.(29) In our study, women who maintained BMD were more likely to walk for exercise and to have overall higher levels of physical activity, but these variables were not significant in the multivariate model, perhaps reflecting a correlation with physical performance.
Diabetes was associated with a lower likelihood of maintaining BMD, consistent with other reports on successful aging.(15,29) The incidence of diabetes is rising substantially, and diabetes is strongly linked to the development of cardiovascular disease. Because women with diabetes were less likely to maintain their BMD, our results are consistent with the higher fracture rates observed in diabetics.(30,31) The prevalence of other comorbidities such as hypertension and COPD were also lower in the maintainers in univariate analyses only. We did not observe significant differences in the prevalence of MI or stroke. This was surprising, given the strong association between cardiovascular disease and successful aging.(29) In SOF, we did not document or confirm cardiovascular events, and we had no measure of subclinical disease. This may have resulted in some misclassification of subjects.
A higher score on the mMMSE at baseline was related to maintenance of BMD independent of age and other covariates. Prior studies have found that higher BMD is associated with better cognitive function and lower risk of cognitive decline and dementia.(32,33) Together these findings suggest that factors that maintained cognitive function may also maintain BMD, such as serum estrogens(34,35) and low inflammatory markers.(36,37)
Estrogen use was associated with maintenance of BMD consistent with the well-established protective effect of estrogen in preserving BMD and preventing fractures. There are strong selection factors for estrogen use, with healthier women more likely to choose estrogen therapy(38) that can result in a spurious protective effect of estrogen use on mortality in observational studies. In theory, the higher proportion of estrogen users among women who maintained their BMD could have biased our finding of an association between this phenotype and reduced fractures and mortality. However, in secondary analyses, we excluded estrogen users, and our results were similar. Similarly, we further adjusted our models for use of other bone active agents including bisphosphonates, and this had little effect on our results.
We included all women who had at least one BMD measure and used statistical techniques that allow for estimation of slopes based on the population level effect for women with only one BMD measure. Nevertheless, in secondary analyses, limiting our analytic cohort to women with at least two BMD results showed largely similar findings.
There are several strengths to our study. We studied a well-characterized cohort of >8000 women and followed them longitudinally over a median of 8 yr. Our follow-up rates were outstanding, with >95% of all follow-up contacts complete. We repeated measurements of BMD with a rigorous quality control program. All fractures and deaths were confirmed objectively. The study population was, however, limited to white women, so our results may not be generalizable to nonwhite women or men. Incident disability was defined as self-reported walking difficulty and was not objectively assessed. As noted earlier, incident disability was measured only at the clinic visits, unlike the information on fractures and deaths, collected every 4 mo. Finally, women were followed for up to 15 yr, until they died or were terminated from the study, but the number of women attending the last visit was a relatively small proportion of the entire cohort.
In conclusion, a small subset of women maintained their BMD over 15 yr of follow-up, suggesting that bone loss is not an inevitable consequence of aging. These women experienced lower rates of fracture, disability, and death, suggesting that maintenance of BMD may represent a clinical phenotype of successful aging. Genetic studies of osteoporosis could consider maintenance of BMD as a separate skeletal phenotype. Many of the factors that predicted maintenance of BMD were modifiable (e.g., smoking, physical function), and hence intervention efforts aimed at these modifiable behaviors may prevent fractures and premature death in older white women.
ACKNOWLEDGMENTS
We thank Dr Charles McCulloch for thoughtful advice in the statistical approach and review of our statistical methods. The Study of Osteoporotic Fractures (SOF) was supported by the National Institutes of Health (NIH) funding under the following grant numbers: AG05407, AR35582, AG05394, AR35584, and AR35583 for 20 yr. The competing renewal of SOF (2006) is supported by Public Health Service grants from the National Institute of Aging (NIA) under the following grant numbers: AG005407, AG027576, AG005394, and AG027574.
APPENDIX: INVESTIGATORS IN THE SOF RESEARCH GROUP
San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): SR Cummings (principal investigator), MC Nevitt (coinvestigator), DC Bauer (co-investigator), DM Black (coinvestigator), KL Stone (co-investigator), W Browner (coinvestigator), R Benard, T Blackwell, PM Cawthon, L Concepcion, M Dockrell, S Ewing, M Farrell, C Fox, R Fullman, SL Harrison, M Jaime-Chavez, W Liu, L Lui, L Palermo, N Parimi, M Rahorst, D Kriesel, C Schambach, R Scott, J Ziarno.
University of Maryland: MC Hochberg (principal investigator), R Nichols (clinic coordinator), S Link.
University of Minnesota: KE Ensrud (principal investigator), S Diem (co-investigator), M Homan (co-investigator), P Van Coevering (program coordinator), S Fillhouer (clinic director), N Nelson (clinic coordinator), K Moen (assistant program coordinator), F Imker-Witte, K Jacobson, M Slindee, R Gran, M Forseth, R Andrews, C Bowie, N Muehlbauer, S Luthi, K Atchison.
University of Pittsburgh: JA Cauley (principal investigator), LH Kuller (co-principal investigator), JM Zmuda (co-investigator), L Harper (project director), L Buck (clinic coordinator), M Danielson (project administrator), C Bashada, D Cusick, A Flaugh, M Gorecki, M Nasim, C Newman, N Watson.
The Kaiser Permanente Center for Health Research, Portland, Oregon: T Hillier (principal investigator), K Vesco (co-investigator), K Pedula (co-investigator), J Van Marter (project director), M Summer (clinic coordinator), A MacFarlane, J Rizzo, K Snider, J Wallace.
Footnotes
Dr Cauley has received research support from Merck & Company, Eli Lilly & Company, Pfizer Pharmaceuticals, and Novartis Pharmaceuticals. She has also received consulting fees from Novartis Pharmaceuticals. Dr Hochberg has received research support from the National Institutes of Health. He is a consultant for the following companies that have products related to osteoporosis and/or vertebral fractures: AMGEN, GlaxoSmithKline, Merck & Co., Novartis Pharma AG, Proctor & Gamble, Roche Laboratories, and Wyeth Pharmaceuticals. Dr Cummings receives research support from Amgen, Pfizer, Novartis, and Eli Lilly and Co. and consulting fees or honoraria from Eli Lilly and Co., Zelos, Merck and Co., Novartis, GlaxoSmithKline, Procter & Gamble, and Aventis. Drs Barnes, Ensrud, Hillier, Newman, Yaffe, and Zmuda and Ms Lui state that they have no conflicts of interest.
REFERENCES
- 1.Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 2.Depp CA, Glatt SJ, Jeste DV. Recent advances in research on successful or healthy aging. Curr Psychiatry Rep. 2007;9:7–13. doi: 10.1007/s11920-007-0003-0. [DOI] [PubMed] [Google Scholar]
- 3.Karasik D, Demissie S, Cupples LA, Kiel DP. Disentangling the genetic determinants of human aging: Biological age as an alternative to the use of survival measures. J Gerontol A Biol Sci Med Sci. 2005;60:574–587. doi: 10.1093/gerona/60.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowling A. Aspirations for older age in the 21st century: What is successful aging. Int J Aging Hum Dev. 2007;64:263–297. doi: 10.2190/L0K1-87W4-9R01-7127. [DOI] [PubMed] [Google Scholar]
- 5.Karasik D, Pavlovsky O, Batsevich V, Livshits G, Kobyliansky E. Use of the hand bones roentgenographs in the prediction of age in nine human populations. Anthropol Anz. 2000;58:199–214. [PubMed] [Google Scholar]
- 6.Karasik D, Hannan MT, Cupples LA, Felson DT, Kiel DP. Genetic contribution to biological aging: The Framingham Study. J Gerontol A Biol Sci Med Sci. 2004;59:218–226. doi: 10.1093/gerona/59.3.b218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 8.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: Longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 10.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 11.Magaziner J, Wehren L, Hawkes WG, Orwig D, Hebel JR, Fredman L, Stone K, Zimmerman S, Hochberg MC. Women with hip fracture have a greater rate of decline in bone mineral density than expected: Another significant consequence of a common geriatric problem. Osteoporos Int. 2006;17:971–977. doi: 10.1007/s00198-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 12.Orwoll ES, Bauer DC, Vogt TM, Fox KM. Axial bone mass in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1996;124:187–196. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ, III, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Determinants of bone loss from the femoral neck in women of different ages. J Bone Miner Res. 2000;15:24–31. doi: 10.1359/jbmr.2000.15.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med. 2005;28:298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, Block G, McHenry K, Baron RB. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol. 1987;126:796–802. doi: 10.1093/oxfordjournals.aje.a114716. [DOI] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR. Long-term risk of incident vertebral fractures. JAMA. 2007;298:2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 19.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 20.Koster A, Patel KV, Visser M, van Eijk JT, Kanaya AM, de Rekeneire N, Newman AB, Tylavsky FA, Kritchevsky SB, Harris TB. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56:636–643. doi: 10.1111/j.1532-5415.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Barnes DE, Cauley JA, Lui LY, Fink HA, McCulloch C, Stone KL, Yaffe K. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 23.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 24.Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, Ensrud KE, Hochberg MC, Cummings SR. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: The study of osteoporotic fractures. Arch Intern Med. 2007;167:155–160. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Odding E, Valkenburg HA, Stam HJ, Hofman A. Determinants of locomotor disability in people aged 55 years and over: The Rotterdam Study. Eur J Epidemiol. 2001;17:1033–1041. doi: 10.1023/a:1020006909285. [DOI] [PubMed] [Google Scholar]
- 26.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:857–863. [PubMed] [Google Scholar]
- 27.Nguyen ND, Center JR, Eisman JA, Nguyen TV. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22:1147–1154. doi: 10.1359/jbmr.070412. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Arnold AM, Naydeck BL, Fried LP, Burke GL, Enright P, Gottdiener J, Hirsch C, O'Leary D, Tracy R. “Successful aging”: Effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: A prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 31.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: The health, aging, and body composition study. Arch Intern Med. 2005;165:1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe K, Browner W, Cauley J, Launer L, Harris T. Association between bone mineral density and cognitive decline in older women. J Am Geriatr Soc. 1999;47:1176–1182. doi: 10.1111/j.1532-5415.1999.tb05196.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan ZS, Seshadri S, Beiser A, Zhang Y, Felson D, Hannan MT, Au R, Wolf PA, Kiel DP. Bone mineral density and the risk of Alzheimer disease. Arch Neurol. 2005;62:107–111. doi: 10.1001/archneur.62.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63:945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- 35.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 38.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women's Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]