Abstract
T cell exhaustion often occurs during chronic infections and prevents optimal viral control. The molecular pathways involved in T cell exhaustion, however, remain poorly understood. We demonstrate that exhausted CD8+ T cells are subject to complex layers of negative regulation due to co-expression of multiple inhibitory receptors. Exhausted CD8+ T cells expressed up to 7 inhibitory receptors. Co-expression of multiple distinct inhibitory receptors correlated with greater T cell exhaustion and more severe infection. Regulation of T cell exhaustion by diverse inhibitory pathways was non-redundant since blockade of PD-1 and LAG-3 simultaneously in vivo synergistically improved T cell responses and reduced viral load. Thus, CD8+ T cell responses during chronic viral infections are regulated by complex patterns of co-expressed inhibitory receptors.
Introduction
Following acute viral infection, memory CD8+ T cells can rapidly reactivate effector functions, have high proliferative potential and are maintained by antigen-independent homeostatic proliferation (refs. 1–3). These properties allow memory T cells to confer protective immunity. In contrast, during chronic viral infections, antigen-specific CD8+ T cells initially acquire effector functions, but become gradually less functional as the infection progresses. This loss of function, known as exhaustion, is hierarchical with properties such as proliferative potential and IL-2 production lost early, tumor necrosis factor (TNF) production persisting for somewhat longer and interferon-γ (IFN-γ) production lost only at the most extreme stages of exhaustion4. First described using lymphocytic choriomeningitis virus (LCMV)5, CD8+ T cell exhaustion during persisting infection is a common feature of many experimental models and chronic human infections such as HIV, HCV, and HBV4. T cell exhaustion is likely a major reason for ineffective viral control in these situations.
An important role for the PD-1:PD-L1 pathway has been reported for CD8+ T cell exhaustion during chronic viral infection6. PD-1, an inhibitory receptor in the CD28 superfamily7, was highly expressed by exhausted CD8+ T cells from chronically infected mice, but not by functional LCMV-specific CD8+ T cells from mice that had cleared the infection6. Blocking the PD-1:PD-L pathway in vivo increased virus-specific CD8+ T cell responses, enhanced per cell function and reduced viral load6. A role for the PD-1 pathway was subsequently demonstrated during HIV, HCV, and HBV infections8-15. These studies indicated that exhausted CD8+ T cells could be rejuvenated to enhance antiviral immunity. However, functional restoration by PD-1:PD-L blockade was incomplete and defects in CD8+ T cells remained following PD-1 pathway blockade6, suggesting a role for other negative regulatory pathways in CD8+ T cell exhaustion. Comparing global gene expression profiles of exhausted CD8+ T cells to functional virus-specific effector and memory CD8+ T cells revealed upregulation of a number of inhibitory receptor genes in addition to PD-1 (ref. 16). Also, Kaufmann et al, demonstrated that HIV-specific CD4+ T cells can co-express PD-1 and another inhibitory molecule CTLA-4 (ref. 17). The impact that upregulation of these additional inhibitory receptor genes has on CD8+ T cell dysfunction during chronic viral infection, however, is not known. It is also unclear whether these inhibitory receptors are expressed by distinct subpopulations of exhausted CD8+ T cells or whether there are exhausted CD8+ T cells that co-express multiple inhibitory receptors. Finally, there is little information about whether these potential inhibitory pathways perform different functions in regulating T cell exhaustion.
We addressed these questions using the mouse model of chronic LCMV infection. Our results indicate that exhausted CD8+ T cells co-expressed multiple inhibitory receptors and that the pattern of inhibitory receptor co-expression impacted the functional quality of these virus-specific CD8+ T cells during chronic infection. The severity of chronic infection correlated with the number and intensity of inhibitory receptors expressed. In addition, in vivo blockade of two inhibitory receptor pathways, PD-1 and LAG-3 together led to substantially greater reversal of T cell exhaustion and viral control compared to either blockade alone. These observations indicate that layers of negative regulation exist on exhausted CD8+ T cells due to co-expression of multiple inhibitory receptors and have implications for therapeutic interventions during chronic infections.
Results
Inhibitory receptors expressed by exhausted T cells
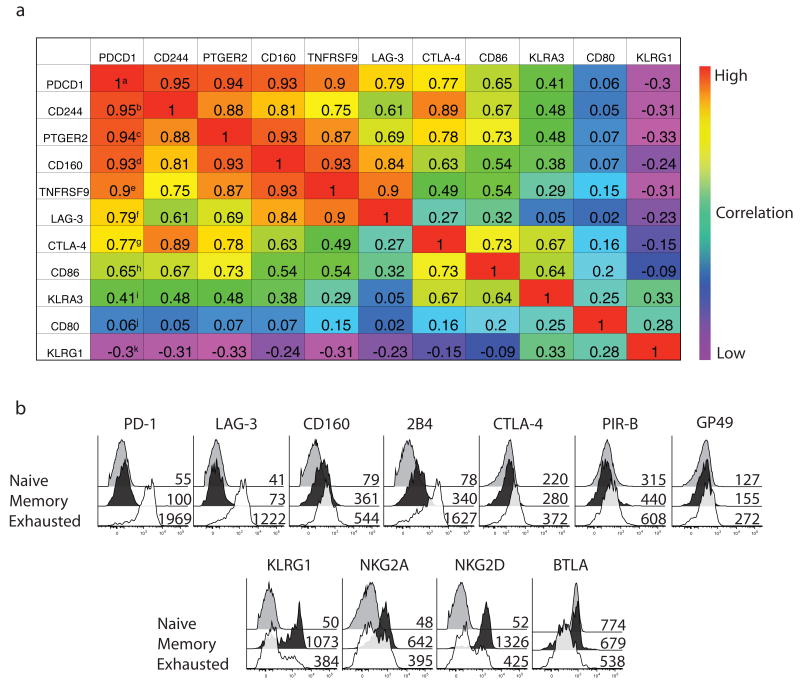
To determine which genes had the closest expression pattern to Pdcd1 (PD-1) in naive, effector, memory and exhausted CD8+ T cells, we performed a nearest neighbor analysis18 using previous gene expression data16. Within the top 100 neighbors of Pdcd1 we found several genes encoding surface receptors with inhibitory functions, including Cd244 (2B4)19, Cd160 (ref. 20)20, Ctla4 and Lag3 (ref. 21)21 (Supplementary Table 1 online) and the expression of several of these potential inhibitory genes was highly correlated with Pdcd1 expression (Fig. 1a). In contrast, the expression of other potential inhibitory receptors such as Klra3, Cd80 and Klrg1 was either neutrally or negatively correlated with Pdcd1 expression. Thus, at the population level, exhausted cells had coordinate upregulation of specific inhibitory receptor genes.
Figure 1. Memory and exhausted CD8+ T cells express multiple inhibitory receptors.
(a) Nearest neighbor analysis using gene expression profiles from16. Numbers indicate the pearson correlation coefficient with numbers closer to 1 representing better correlation with the pattern of PDCD1 expression. Superscripted letters indicate nearest (a) to furthest (b) neighbor for this set of genes. (b) The expression of eleven inhibitory receptors on H-2Dbgp33+ CD8+ splenocytes from LCMV Arm and clone 13 mice (d30p.i.) was examined by flow cytometry by single marker staining in 4 color panels. Naive CD8+ T cells were total CD44Lo CD8+ cells from LCMV Arm immune mice. Mean fluorescence intensity is displayed for each population. Stains are representative of 3-5 mice each from at least 3 independent experiments.
Expression of inhibitory and natural killer (NK) receptors was then examined by flow cytometry on naïve and LCMV specific memory and exhausted CD8 T cells. Different strains of LCMV were used to generate either an acute infection and functional memory CD8+ T cells or chronic infections and exhausted CD8+ T cells. Initially, we used LCMV Armstrong (Arm) that results in an acute infection to examine functional memory T cells and the clone 13 strain that causes a chronic infection and T cell exhaustion (22-24; and see below). Exhausted CD8+ T cells specific for the H-2Db-restricted gp33-41 epitope from clone 13 infected mice had increased expression of PD-1, LAG3, 2B4, CD160, CTLA-4, PIR-B and GP49, but decreased expression of KLRG1, NKG2A, NKG2D and BTLA, compared to the functional H-2Dbgp33 tetramer+ memory CD8+ T cells from LCMV Arm immune mice. Each of these upregulated receptors has been reported to negatively regulate the function of T cells or other hematopoietic cells19,20,25-27. Thus, multiple inhibitory receptors were expressed by exhausted CD8+ T cells, but not by memory or naive CD8+ T cells. In addition, the upregulation of inhibitory receptors by exhausted CD8+ T cells was selective since several inhibitory pathways (for example, NKG2A, KLRG1, BTLA) were not elevated.
Varying the severity of LCMV infection in mice
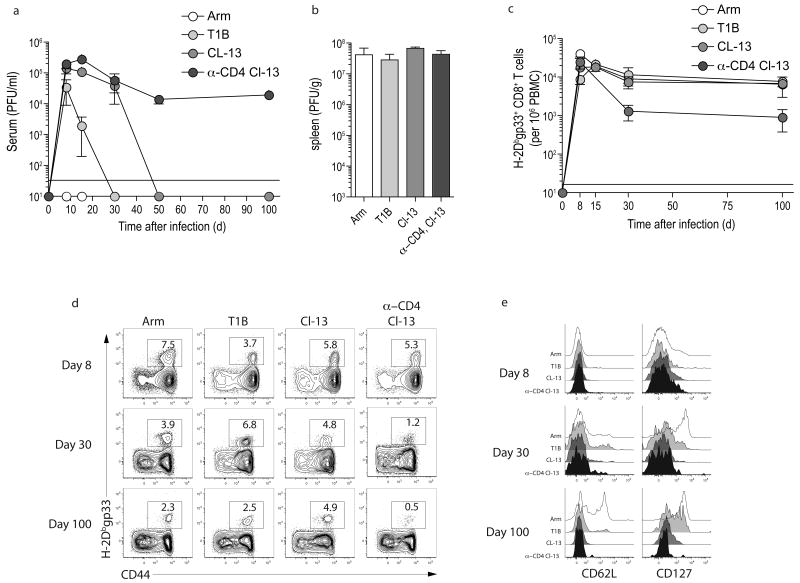
Next we examined how the severity of infection impacted virus-specific CD8+ T cell responses and expression of inhibitory receptors, using four types of LCMV infection in mice. In addition to the Arm and clone 13 infections mentioned above, we used LCMV T1B and infection with clone 13 initiated in the presence of transient depletion of CD4 T cells (anti-CD4 plus clone 13). We first defined the kinetics of viral replication using these four infections. Infection of adult C57BL/6 mice with LCMV Arm resulted in high viral replication on d3 p.i. and complete clearance by d8-10 p.i. (Fig. 2a,b and28). For T1B infection, systemic viral replication lasted ∼3–4 weeks, while for clone 13 viremia lasted ∼2–3 months (Fig. 2a). The anti-CD4 plus clone 13 infection resulted in lifelong viremia (Fig. 2a and5,23,29). Thus, using Arm, T1B, clone 13 and anti-CD4 plus clone 13 we could investigate how CD8+ T cell function and expression of inhibitory receptors compares during infections of different severity.
Figure 2. Four types of LCMV infection.
(a) C57BL/6 mice were infected with LCMV Arm, T1B, or clone 13. Also a group of mice was depleted of CD4 T cells with an injection of 200 μg of anti-CD4 (GK1.5) one day prior and one day after infection with LCMV clone 13 (anti-CD4 plus clone 13). Serum was collected from mice longitudinally and serum viral titers were determined by plaque assay. n=5 per time point. (b) Spleens were harvested from LCMV infected mice d3 p.i. and viral load was determined by plaque assay. n=4. (c,d) The frequency of H-2Dbgp33+ CD8+ T cells was monitored in the peripheral blood by flow cytometry and plotted over time. Numbers indicate the percent of CD8+ T cells that are tetramer+. (e) The expression of CD62L and CD127 was monitored on H-2Dbgp33+ CD8+ T cells by flow cytometry. Data from (d and e) represent n=5 per time point and are representative of 2 independent experiments.
All four types of LCMV infection generated robust LCMV specific CD8+ T cell responses that peaked ∼1–2 weeks p.i., contracted and then were maintained (Fig. 2c,d). Following Arm infection, virus-specific CD8+ T cells followed a typical pattern of functional memory CD8+ T cell differentiation30-32 including upregulation of CD127 and CD62L over time (Fig. 2e). During the three chronic viral infections, with the exception of some expression of CD127 at d100 post T1B infection, the expression of these markers of normal memory CD8+ T cell differentiation remained low for at least ∼100 days (Fig. 2e24,33,34).
Infection severity and inhibitory receptor expression
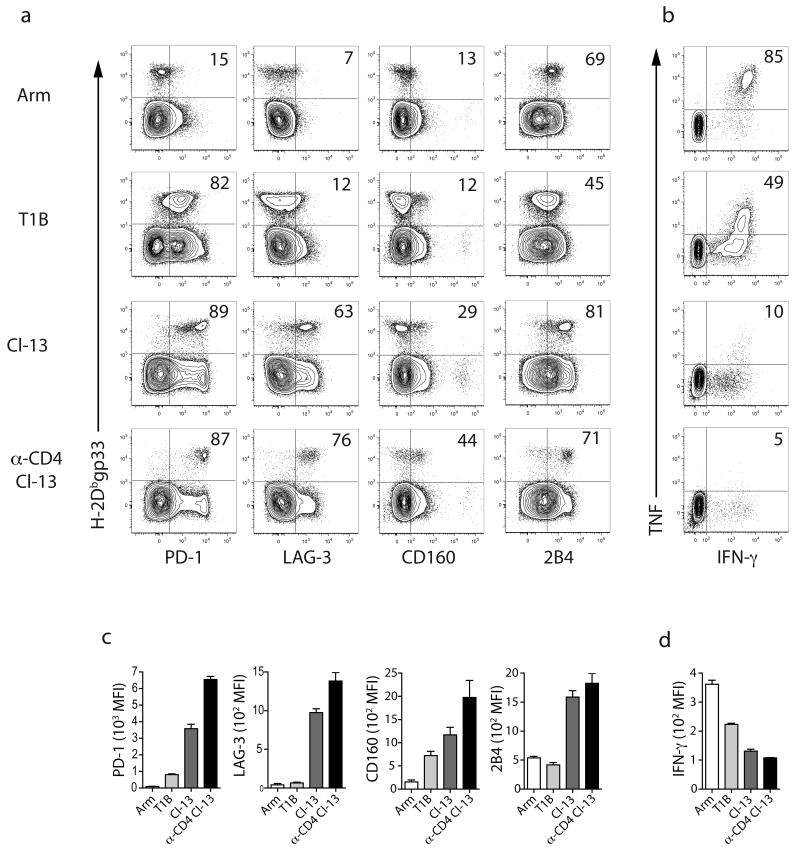
To begin assess what regulates expression of inhibitory receptors on exhausted CD8+ T cells, we examined the expression of PD-1, LAG-3, 2B4 and CD160 on virus-specific CD8+ T cells using the four infections described above. On d30 after LCMV Arm infection, H-2Dbgp33+ CD8+ T cells were largely PD-1lo, LAG-3lo, CD160lo, and 2B4Lo or 2B4Int (Fig. 3). The majority of virus-specific CD8+ T cells co-produced IFN-γ and TNF following peptide stimulation, an indicator of functional competence23,35. During T1B infection, the majority of H-2Dbgp33+ CD8+ T cells expressed PD-1, but expressed somewhat less 2B4 than was observed following Arm infection. LAG-3 or CD160 expression on these cells was low. Approximately half of the gp33-specific CD8+ T cells from T1B infected mice co-produced IFN-γ and TNF. During clone 13 infection, PD-1 and 2B4 were highly expressed. In this infection, H-2Dbgp33+ CD8+ T cells also expressed substantial amounts of LAG-3 and some cells expressed CD160. Only ∼10% of these gp33-specific CD8+ T cells co-produced IFN-γ and TNF. In the most severe infection, anti-CD4 plus clone 13, all four inhibitory receptors were expressed and these virus-specific CD8+ T cells were highly exhausted. In addition to a higher frequency of inhibitory receptor+ virus-specific CD8+ T cells, as the severity of infection increased, H-2Dbgp33+ CD8+ T cells exhibited substantially higher expression of each inhibitory receptor per cell and a lower amount of IFN-γ per cell (Fig. 3c,d). When PD-1Int and PD-1Hi subsets of exhausted CD8+ T cells36 were examined it was apparent that PD-1hi H-2Dbgp33+ exhausted CD8+ T cells expressed more 2B4, LAG-3, and CD160 than did their PD-1int counterparts (Supplementary Fig. 1 online). Thus, virus-specific CD8+ T cells expressing high amounts of PD-1 also co-expressed other inhibitory receptors.
Figure 3. Influence of the severity of infection on inhibitory receptor expression and CD8+ T cell function.
(a) The expression of four of the exhaustion associated inhibitory receptors was examined on H-2Dbgp33 CD8+ splenocytes from mice infected with Arm, T1B, clone 13 or anti-CD4 plus clone 13 on d30 p.i. by multiparameter flow cytometry. The numbers indicate the percent of tetramer+ CD8+ T cells expressing the indicated receptor. Similar inhibitory receptor expression profiles were also observed on H-2Dbgp276 tetramer+ CD8 T cells (data not shown) (b) ICS was performed on splenocytes from the four infection groups on d30 p.i using gp33-41 peptide. IFN-γ and TNF co-production was examined by flow cytometry. Numbers indicate the percent of IFN-γ+ CD8+ T cells also producing TNF. (c) The mean fluorescence intensity of inhibitory receptor expression was determined for each receptor during each infection. (d) MFI of IFN-γ for 3-5 mice per infection. All data are representative of three independent experiments with 3-5 mice per experiment.
Patterns of inhibitory receptor co-expression
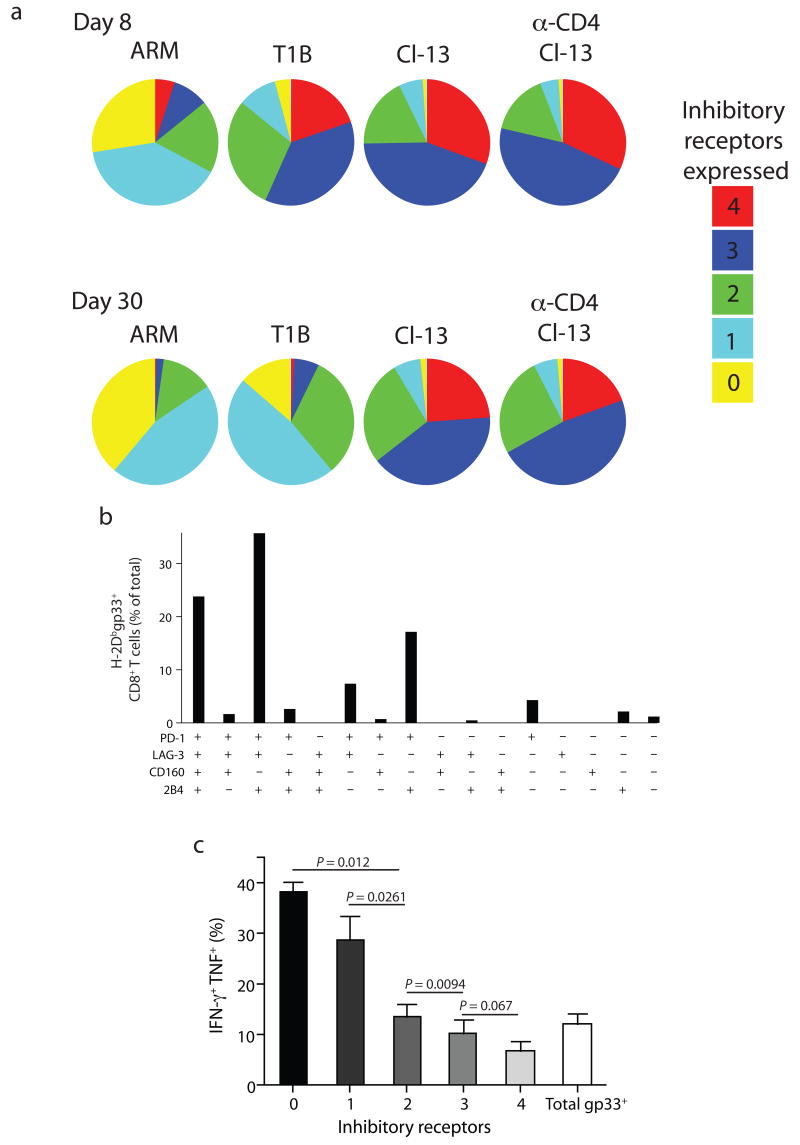
We next used multiparameter flow cytometry to analyze co-expression of PD-1, LAG-3, CD160 and 2B4. On day 8 p.i. a small proportion of H-2Dbgp33+ CD8+ T cells from Arm infected mice expressed more than one inhibitory receptor (Fig. 4a). Conversely, the majority of H-2Dbgp33+ CD8+ T cells from T1B, clone 13, and anti-CD4 plus clone 13 co-expressed three or four inhibitory receptors. At d30 p.i. the percentage of H-2Dbgp33+ CD8+ T cells from Arm immune mice that expressed two or more inhibitory receptors had decreased. During T1B infection, the initially high inhibitory receptor co-expression also decreased to some extent by d30 p.i. In contrast, the more severe clone 13 and anti-CD4 plus clone 13 infections resulted in a high co-expressing three to four inhibitory receptors at d8 p.i. and this high inhibitory receptor co-expression persisted at d30 p.i. Inhibitory receptor co-expression was similar for TCR transgenic P14 CD8+ T cells suggesting that TCR diversity was not the major determinant of the pattern of inhibitory receptor co-expression (Supplementary Fig. 2 online). Thus, during chronic LCMV infection there was substantial co-expression of multiple inhibitory receptors on the same virus-specific CD8+ T cells. Moreover, the extent of inhibitory receptor co-expression correlated with the severity of infection.
Figure 4. Concurrent expression of multiple inhibitory receptors increased with viral load and correlated with decreased functionality.
The simultaneous expression of multiple inhibitory receptors (PD-1, LAG-3, 2B4, and CD160) on H-2Dbgp33+ CD8+ splenocytes from the four infection groups was examined on d8 and d30 p.i. using Boolean gating analysis. (a) Individual populations were grouped according to the total number of inhibitory receptors expressed. (b) Relative abundance of each possible individual inhibitory receptor expression profile. (c) Intracellular cytokine staining with IFN-γ and TNF using gp33-41 peptide stimulation of exhausted CD8+ T cells (d30p.i. LCMV clone 13). ICS was done in conjunction with staining for 4 inhibitory receptors. Note that the expression of the inhibitory receptors examined here does not change during the 5 hr ICS stimulation (data not shown). After Boolean gating, the percentage of gp33 responding cells that produced both IFN-γ and TNF was determined and individual populations were grouped according to the total number of inhibitory receptors expressed. All data are representative of three independent experiments with 3-5 mice per experiment.
Multiparameter staining allowed us to determine which subpopulations of inhibitory receptor co-expressing exhausted CD8+ T cells were present during chronic infection. Eleven of the sixteen possible combinations were detected, but greater than 90% of the exhausted H-2Dbgp33+ CD8+ T cells from clone 13 infected mice fell into five subpopulations (Fig. 4b). Notably, during clone 13 (and anti-CD4 plus clone 13) infection, PD-1 was expressed by the vast majority of virus-specific CD8+ T cells followed by LAG-3 and 2B4 (Fig. 4b and Supplementary Fig. 3 online). CD160 was expressed by the lowest percentage of exhausted CD8+ T cells and was associated with co-expression of the other three molecules (Fig. 4b and Supplementary Fig. 3).
To determine whether the pattern of inhibitory receptor co-expression correlated with T cell function, co-staining for four inhibitory receptors was performed with intracellular cytokine staining (ICS) for IFN-γ and TNF following peptide stimulation. During clone 13 infection the degree of CD8+ T cell exhaustion correlated directly with the number of inhibitory receptors co-expressed (Fig. 4c). Thus, during chronic viral infections increased inhibitory receptor co-expression correlated with reduced T cell function.
In vitro blockade of inhibitory pathways
To begin to assess the role of inhibitory receptors on exhausted CD8+ T cells, we used three separate in vitro assays to investigate short-term (<18 h) changes in T cell properties in the presence of blocking antibodies: an in vitro killing assay37, an in vitro apoptosis assay36, and cytokine production by ICS (Supplementary Fig. 4 online). In vitro cytotoxicity was enhanced by anti-CD160, but not by anti-PD-L1, anti-LAG-3 or by blocking the 2B4 ligand, CD48. To examine T cell apoptosis and/or survival, exhausted CD8+ T cells were separated into PD-1int and PD-1hi subsets as described (ref. 36). Expression of active caspase 3 was reduced in the PD-1int subset in the presence of anti-PD-L1, but not anti-LAG-3 or anti-CD160. In contrast, anti-CD160, but not anti-PD-L1 or anti-LAG-3, improved survival of the PD-1hi subset. Moderate 2B4 expression can deliver a costimulatory signal, while high 2B4 expression can be inhibitory38. Blocking the 2B4 pathway using anti-CD48 decreased cytokine production by 2B4int LCMV specific CD8 T cells from chronically infected mice, but significantly increased IFN-γ production by 2B4hi exhausted CD8+ T cells. CD48 can also bind CD2 and deliver a costimulatory signal. Blockade of CD2 reduced IFN-γ production from both 2B4int and 2B4hi exhausted CD8+ T cells. These observations are consistent with a costimulatory role for CD48 binding to either CD2 or 2B4 on 2B4int virus-specific CD8+ T cells, but an inhibitory role of 2B4 via interactions with CD48 on 2B4hi exhausted CD8+ T cells. While future in vivo studies are warranted, these in vitro experiments suggest that the CD160 and 2B4 pathways can contribute to CD8+ T cell exhaustion.
Simultaneous PD-L1 and LAG-3 blockade in vivo
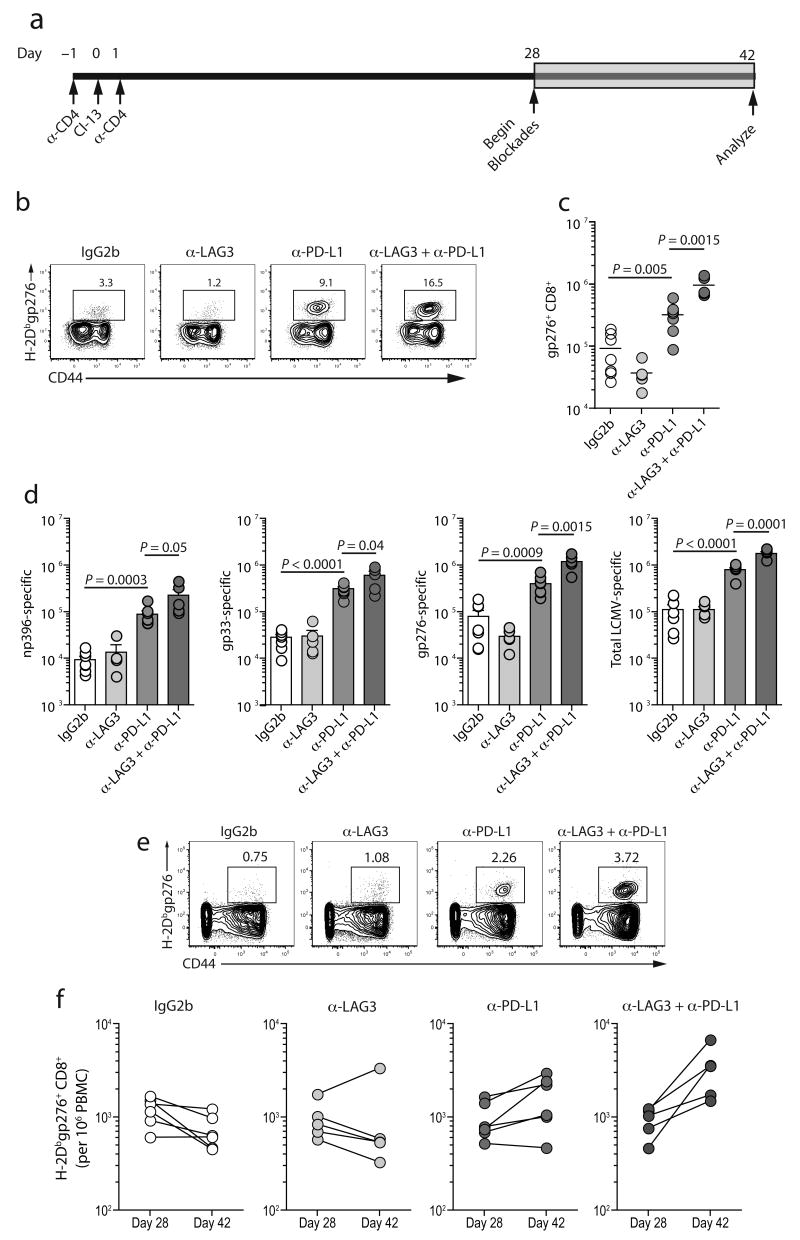
It is unclear if different inhibitory receptors have overlapping functions or whether these distinct pathways contribute independently to T cell dysfunction during chronic viral infection. To distinguish between these possibilities we performed blockade of two pathways simultaneously using blocking antibodies against PD-L1 and LAG-3 either alone or in combination in vivo during chronic LCMV infection (Fig. 5a).
Figure 5. PD-L1 plus LAG-3 blockade during chronic LCMV infection enhances antiviral CD8+ T cell responses.
(a) C57BL/6 mice were CD4 depleted and infected with LCMV clone 13. On D28 groups of mice were treated with either an isotype control antibody, anti-AG3, anti-PD-L1, or both anti-LAG3 and anti-PD-L1 every third day for two weeks (n=5-7 per group). (b) Staining with H-2Dbgp276 tetramer on CD8+ splenocytes after two weeks of treatment. Numbers indicate the percent of CD8+ T cells that were tetramer+. (c) Total number of H-2Dbgp276+ CD8+ T cells in the spleen after treatment. Note that similar results were observed for H-2Dbnp396, and H-2Dbgp33 tetramer staining (data not shown). (d) Total number of degranulating (CD107a+) and/or IFN-γ producing CD8+ splenocytes in response to peptide stimulation after two weeks of treatment. (e) Staining with H-2Dbgp276 tetramer on CD8+ PBMCs after two weeks of treatment. Numbers indicate the percent of CD8+ T cells that were tetramer+. (f) Frequency of H-2Dbgp276+ CD8+ T cells per 106 PBMCs before an after treatment. All data are representative of three independent experiments with 5-7 mice per experiment.
Two weeks of treatment with anti-LAG-3 alone did not improve the frequency or absolute number of H-2Dbgp276+ CD8+ T cells, while blocking the PD-1 pathway augmented the H-2Dbgp276 response (Fig. 5b,c). Despite the lack of impact of anti-LAG-3 alone on T cell numbers, combining LAG-3 blockade with PD-1:PD-L1 blockade resulted in an 81% increase in the frequency of H-2Dbgp276+ CD8+ splenocytes over the anti-PD-L1 treated group and a 5-fold improvement over the untreated group (Fig. 5b) with corresponding increases in absolute numbers (Fig. 5c). Dual blockade also significantly increased CD8+ T cell responses to np396, gp33, gp276, and a pooled peptide mixture containing 20 known LCMV epitopes compared to either single treatment or control groups when measured by ICS instead of tetramer staining (Fig. 5d).
We also measured the frequencies of LCMV-specific CD8+ T cells in the blood before and after treatment. The frequency of circulating H-2Dbgp276-tetramer+ CD8+ T cells remained essentially constant in the control and anti-LAG-3 only groups. PD-1:PD-L1 blockade alone increased responses ∼1.8-fold (P = 0.0456), while dual blockade of both LAG-3 and PD-1:PD-L led to a robust ∼3.7 fold increase (P = 0.0307) in the circulating pool of H-2Dbgp276+ CD8+ T cells. Thus, blocking the PD-1:PD-L pathway in vivo revealed a substantial role for the LAG-3 pathway in co-regulating CD8+ T cell exhaustion during chronic viral infection.
Increased function with LAG-3 and PD-L1 co-blockade
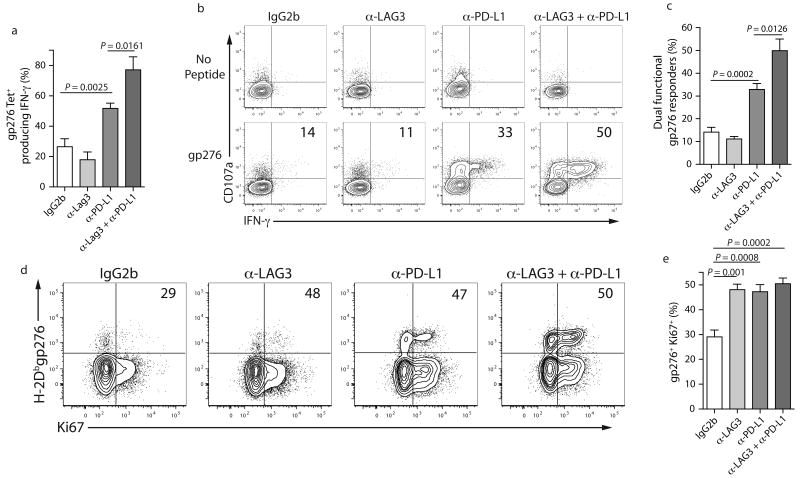
To determine if inhibitory receptor blockade increased the quality of the antiviral CD8 T cells, we examined per cell function. Treatment with anti-PD-L1 alone significantly increased the percentage of H-2Dbgp276 tetramer+ CD8+ T cells that could produce IFN-γ (Fig. 6a). While the anti-LAG-3 treatment alone had little impact on the severity of exhaustion, combined anti-LAG-3 and anti-PD-L1 blockade led to substantially greater per cell function compared to anti-PD-L1 alone, anti-LAG-3 alone or control treatment (Fig. 6a,b). Treatment with both anti-LAG-3 and anti-PD-L1 also significantly increased the percentage of dual functional (CD107+ and IFN-γ+) gp276-specific CD8+ T cells compared with the anti-PD-L1 or anti-LAG-3 alone treatment groups (Fig. 6b,c). While the percentage of IFN-γ and TNF dual producers was different between groups (Supplementary Fig. 5 online), the absolute number of dual cytokine producers and of dual CD107+ and IFN-γ+ virus-specific CD8+ T cells was substantially increased following in vivo blockade (data not shown). Thus, combining LAG-3 blockade with PD-1:PD-L1 blockade resulted in substantially better reversal of exhaustion compared to PD-1:PD-L1 blockade alone.
Figure 6. Improved function of exhausted CD8+ T cells following dual LAG-3 plus PD-L1 blockade.
(a) Percent of H-2Dbgp276 CD8+ splenocytes that produced IFN-γ in response to gp276 peptide stimulation. (b,c) CD107a degranulation assay performed in conjunction with ICS for IFN-γ with gp276 peptide stimulation. The percentage of responding cells that both produced IFN-γ and degranulated is indicated. (d,e) Intracellular staining for Ki67 in H-2Dbgp276+ CD8+ splenocytes. The percentage of H-2Dbgp276+ CD8+ splenocytes expressing Ki67 is indicated. A similar trend was observed by BrdU incorporation (data not shown). All data are representative of three independent experiments with 5-7 mice per experiment.
Despite the lack of effect of anti-LAG-3 alone on antiviral T cell numbers or effector function, this treatment appeared to have an impact exhausted CD8+ T cells. Blocking LAG-3 alone, as well as PD-L1 alone or both LAG-3 and PD-L1 in vivo increased expression of PD-1 on virus-specific exhausted CD8+ T cells (Supplementary Fig. 6 online). Since TCR signals can increase PD-1 expression39, these results suggest that LAG-3, PD-L1, or LAG-3 plus PD-L1 blockade can modulate TCR signaling by persisting antigen. While these results do not exclude a role for PD-L1 and LAG-3 blockade operating through other cell types, they are consistent with a role for blockade of these inhibitory receptors acting directly on exhausted virus-specific CD8+ T cells.
We next asked whether the blockade treatments were associated with increased T cell division. While ∼30% of the H-2Dbgp276+ CD8+ T cells from control mice expressed Ki67, ∼50% of the virus-specific CD8+ T cells in all three treatment groups were Ki67+ (Fig. 6d,e). In contrast to the minimal impact of anti-LAG-3 alone on effector function, blocking LAG-3 alone, PD-1:PD-L1 alone or both together all had a similar positive impact on cell cycle for exhausted CD8+ T cells. However, despite apparently improved entry into cell cycle in all three treatment groups, substantially more virus-specific CD8+ T cells accumulated following anti-PD-L1 treatment and especially in the dual treated group compared to the anti-LAG-3 group.
Improved viral control by PD-L1 and LAG-3 co-blockade
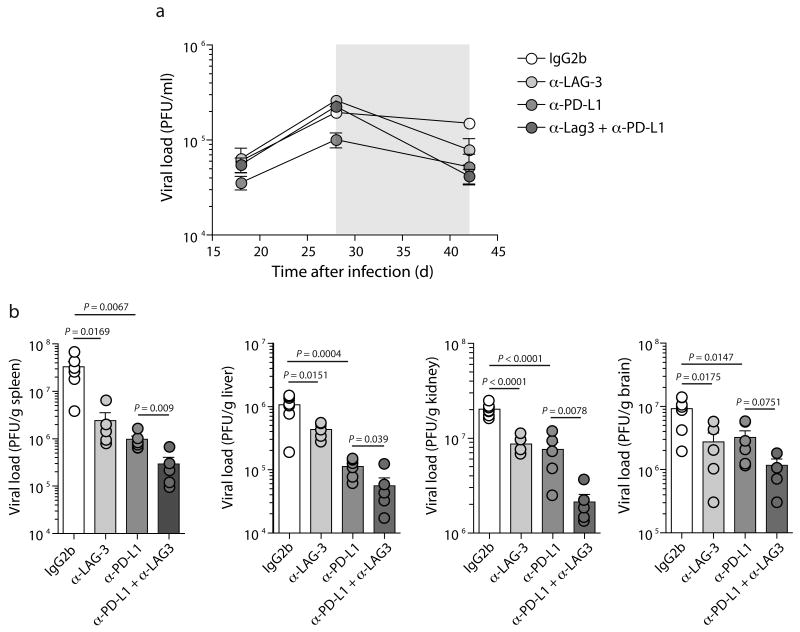
To determine whether the inhibitory receptor blockades impacted viral control, viral load in the blood was measured before and after treatment. While untreated mice had only slightly reduced viral load over this time (reduced ∼22%), viral load declined substantially more in all three groups of treated mice: decreased 70% for anti-LAG-3 alone, 48% for anti-PD-L1 alone, and 81% for the dual treated group (Fig. 7a). Treatment with anti-LAG-3 or anti-PD-L1 alone also resulted in significant reductions in viral load in the spleen, liver, and kidney (Fig. 7b). However, combined blockade of anti-LAG-3 plus anti-PD-L1 resulted in significantly greater reduction in viral load compared to either anti-LAG-3 or anti-PD-L1 treatment alone in multiple tissues. Thus, the combined anti-PD-L1 plus anti-LAG-3 blockade demonstrated substantial improvement in T cell responses and viral control achieved by blocking two inhibitory receptor pathways.
Figure 7. Increased viral control with dual PD-L1 plus LAG3 blockade.
(a) Longitudinal analysis of viral load in the serum. (b) Viral load in the spleen, liver, kidney, and brain. Data are representative of three independent experiments with 5-7 mice per experiment.
Discussion
We investigated the expression and co-expression of multiple inhibitory receptors by exhausted CD8+ T cells during chronic viral infection. Our results demonstrated: a) exhausted CD8+ T cells expressed up to 7 distinct inhibitory receptors; b) inhibitory receptors were co-expressed by the same exhausted CD8+ T cell and complex patterns of co-expression were apparent; c) the severity of viral infection impacted the diversity and amount of inhibitory receptors expressed; d) the inhibitory receptors LAG-3 and PD-1 operated as at least partially non-overlapping pathways negatively regulating T cell responses during chronic viral infection; and e) combined LAG-3 plus PD-L1 blockade synergized to enhance T cell responses and viral control during chronic LCMV infection. These observations indicate that multiple negative regulatory pathways can contribute to CD8+ T cell exhaustion and suggest that the pattern of inhibitory receptor co-expression is a useful correlate of the level of T cell exhaustion. Thus, reversal of T cell exhaustion could be improved by therapeutic targeting of multiple inhibitory receptor pathways simultaneously and approaches to fine tune T cell responses to persisting pathogens might be developed by targeting inhibitory receptors that regulate specific T cell properties.
The pattern of inhibitory receptor co-expression during the different LCMV infections suggests that the severity and/or type of infection can influence inhibitory receptor expression. These studies point to the pathogenesis of infection as an important parameter influencing inhibitory receptor expression and suggest a role for viral load. Future studies will be necessary to investigate the influence of inflammation and CD4 T cell help as well as to investigate the patterns of inhibitory receptor co-expression during other types of persisting infections. A number of studies have highlighted a relationship between the severity of infection and the extent of CD8+ T cell dysfunction6,8,23,40-42. The improved CD8+ T cell responses observed following inhibitory receptors blockade could benefit from both blockade of an inhibitory pathway and also from the enhanced control of infection and lower viral load. Inhibitory receptor blockade, therefore, could be a pivotal event that initially improves T cell function and viral control. The resulting lower viral load could then have a secondary positive effect on the severity of exhaustion. Given the potential for clinical applications it will be important to understand the contribution of continued inhibitory receptor blockade versus lower viral load to improved T cell responses.
The PD-1:PD-L1/PD-L2 pathway has received considerable attention for regulating T cell exhaustion. There has also been some debate, however, regarding precisely what T cell functions are regulated by PD-17,43. Our data provide a potential explanation for these apparently disparate results if the co-expression of other inhibitory receptors differed between T cell populations analyzed in different studies. For example, the greater effect of PD-1 pathway blockade alone on the PD-1Int CD8+ T cells36 could be due to the presence of multiple additional inhibitory receptors such as CD160 on the PD-1Hi subset of exhausted CD8 T cells. Thus, it may be necessary to consider the full repertoire of inhibitory receptors expressed when investigating the role of any individual inhibitory pathway.
The diversity of inhibitory receptors expressed by exhausted CD8+ T cells is remarkable. Among the set of inhibitory receptors examined here, exhausted CD8+ T cells expressed two inhibitory molecules in the CD28 superfamily (PD-1 and CTLA-4), but downregulated a third (BTLA). The PD-1:PD-L1 pathway is a major pathway regulating T cell exhaustion during a number of chronic viral infections 7. During chronic LCMV infection CTLA-4 does not appear to play a major role on exhausted CD8+ T cells6, but a role for CTLA-4 on HIV-specific CD4 T cells has been described17. LAG-3 binds to MHC class II, consistent with homology between LAG-3 and CD444. LAG-3 negatively regulates T cell activation and proliferation in several settings21,45, but its role during chronic viral infection was unknown. While a role for LAG-3 has been reported on Treg cells46, there is clearly a CD8+ T cell intrinsic role for LAG-3 in anti-tumor responses47. LAG-3 limits T cell proliferation and memory T cell homeostasis via cell cycle arrest25,48-50. The role of LAG-3 as a negative regulator of cell cycle progression of CD8+ T cells25,48-50 fits well with the increased Ki67 expression following LAG-3 blockade during chronic LCMV infection. The in vivo studies presented here are consistent with a CD8+ T cell intrinsic role for LAG-3, but also do not exclude a role for LAG-3 on other cell types. In addition to PD-1 and LAG-3 where in vivo blocking studies indicate a functional role in regulating dysfunction, exhausted CD8+ T cells also co-expressed a variety of other inhibitory receptors that might be future therapeutic targets. Exhausted CD8+ T cells expressed PirB and GP49, both of which have been implicated in negative regulation of mast cells, neutrophils, eosinophils, macrophages, and/or NK cells26,27. GP49 is interesting since GP49A is not inhibitory while GP49B is a negative regulator of Fc receptor signaling27,51. Because antibodies do not distinguish GP49A and GP49B, future studies will be necessary to further dissect this pathway. CD160 was also upregulated on exhausted CD8+ T cells and this molecule inhibits human CD4 T cell responses20. Our preliminary in vitro studies are consistent with an inhibitory role for CD160 on exhausted CD8+ T cells, but the exact role of this molecule on NK and T cells and during viral infection remains poorly understood. Lastly, 2B4/CD244 is a SLAM family member that can be either activating or inhibitory and has been most well characterized on T cells in the intestinal mucosa and NK cells19,52,53. Previous studies indicated that the amount of 2B4 per cell, and the expression of the downstream adaptor molecule SAP, are key determinants of whether 2B4 delivers an inhibitory versus activating signal53. Crosslinking 2B4 on 2B4Lo or 2B4Int cells delivers a positive signal38. However, when 2B4 expression is high and SAP expression is low, 2B4 is inhibitory38. SAP amounts are likely limiting in exhausted CD8+, but not effector CD8+ T cells based on gene expression profiles16. The high expression of 2B4 by exhausted CD8+ T cells and the effects of in vitro CD48 blockade are consistent with an inhibitory role for 2B4 on 2B4Hi exhausted CD8+ T cells. The diverse potential of these inhibitory receptors suggests that these pathways contribute in qualitatively different ways to T cell exhaustion. Indeed, the results from the dual PD-L1 plus LAG-3 blockade indicate that at least these two pathways impart distinct regulatory effects on exhausted CD8+ T cells.
A question that emerges from these and other studies is whether or not inhibitory receptors are the reason T cells become exhausted in the first place. Functional effector CD8 T cells upregulate PD-1 and LAG-3 during the first week of an acute viral infection6,16, however, and some of these cells form functional memory T cells once the infection is cleared and inhibitory receptor expression decreases. While prolonged expression of inhibitory receptors could have a role, the pathways involved in the induction of T cell exhaustion and the lineage fate decisions between T cell exhaustion versus T cell memory remain poorly understood. In previous studies ∼500 genes were identified that were differentially expressed in exhausted CD8 T cells compared to naïve, effector and memory T cells16 and it is likely that multiple pathways in addition to inhibitory receptors are involved in the early events controlling T cell exhaustion.
In summary, we have identified inhibitory receptor co-expression as a key feature of exhausted CD8+ T cells responding to chronic viral infection. Blockade of PD-1:PD-L revealed a novel role for co-regulation of exhausted CD8+ T cells by a second inhibitory receptor LAG-3 and the complex pattern of co-expression of these and other inhibitory receptors suggests a tunable array of inhibitory pathways operating on exhausted CD8+ T cells. Combination therapies for chronic viral infections have considerable potential and studies combining PD-1:PD-L or IL-10R blockade with therapeutic vaccination have shown promising results54,55. Our studies now demonstrate synergistic effects of blocking two inhibitory receptors together during chronic viral infection. Future studies should help define the intracellular targets of these inhibitory pathways and test the potential of therapeutic interventions that take advantage of combined targeting of multiple negative regulatory pathways during chronic viral infections.
Methods
Mice, virus and infections
Four-week old C57BL/6 (B6) mice were used. Ly5.1+ P14 mice bearing the H-2Db-gp33 specific TCR were backcrossed 10+ generations to C57BL/6 and maintained in our animal colony. LCMV strains were propagated, titered and used as previously described23. B6 mice were infected with LCMV Armstrong (2 × 105p.f.u) i.p., LCMV T1B (2 × 106 p.f.u) i.v., or with LCMV clone 13 (2 × 106 p.f.u) i.v. In experiments where CD4-depleted mice were used, B6 mice were injected i.p. with 200 μg of anti-CD4 (GK1.5) on days −1 and +1 and infected with LCMV clone 13 on d0. P14 chimeric mice were generated by adoptively transferring 5 × 102 naive TCR transgenic T cells into naive B6 mice followed by LCMV Armstrong, T1B, or clone 13 infection. All mice were used in accordance with institutional IACUC guidelines.
Flow cytometry and ICS
Lymphocytes were isolated from the spleen and peripheral blood as previously described23. MHC class I peptide tetramers were made and used as described23. Antibodies were purchased from eBioscience, Biolegend, Invitrogen, Abcam, Accurate Chemical, R&D Systems, or BD Biosciences (Supplemental Table 2 online). Lymphocytes were stained and analyzed as previously described23. Intracellular cytokine staining (ICS) and the CD107a assay were performed as previously described6,23. Cells were stimulated with the indicated individual LCMV peptides or a pool of 20 LCMV epitopes (Supplementary Table 3 online56). Cells were analyzed on a LSR II flow cytometer (BD Immunocytometry Systems). Approximately 1 × 106 events were collected per sample. Data analysis was performed using FlowJo v8.2 (TreeStar). Doublets were removed by gating on a FSC-A vs. FSC-H plot. Dead cells were removed by gating on Live/Dead Aqua (Invitrogen) vs FSC-A. Cells were subjected to a lymphocyte gate by a FSC-A versus SSC plot. Then we sequentially gated on CD4−, CD8+, and H-2Dbgp33+ events versus. Positive and negative gates for each inhibitory receptor on H-2Dbgp33+ CD8+ T cells were drawn based on fluorescence minus one controls (FMO). We then used the Boolean gating function in FlowJo to assess each possible inhibitory receptor expression pattern. Co-expression patterns were analyzed using the SPICE program.
In vivo antibody blockade
Two hundred micrograms of rat anti-mouse PD-L1 (10F.9G2), rat IgG2b isotype control (BD biosciences) or rat anti-mouse LAG-3 (C9B7W) blocking antibody were administered intraperitoneally every third day for two weeks. The PD-L1 and LAG-3 antibodies do not deplete in vivo6,46. For PD-L1 and LAG-3 dual blockades 200 μg of each antibody was used. Control groups received 200 μg of isotype control antibody injection or, in some experiments, were untreated. No differences were observed between untreated and isotype-treated mice (data not shown).
Nearest Neighbor Analysis
Nearest neighbor analysis was performed essentially as previously described18 using the GeneNeighbor marker analysis algorithm to calculate the nearest neighbors in gene expression profile to PD-1. Gene expression profiles from naïve, effector, memory and exhausted CD8+ T cells used here have been previously published16.
Statistical Analyses
P values were calculated by pair-wise T tests and in some cases ANOVA where more than two groups were compared.
Supplementary Material
Acknowledgments
We thank E. Long (NIAID/NIH), V. Kumar (University of Chicago) and S. Reiner (University of Pennsylvania) for helpful comments and suggestions and B. Laidlaw for critically reading the manuscript. This work was supported by grants from NIH/NIAID (AI071309 to E.J.W and HHSN26620050030C to E.J.W. and G.J.F.) and the Bill and Melinda Gates Foundation Grand Challenge in Global Health (to G.J.F. and E.J.W.).
Footnotes
Author contributions: S.D.B. and E.J.W. designed the experiments. S.D.B. performed the experiments with assistance from H.S., T.Z., and A.P. Results were analyzed by S.D.B. and E.J.W. with input from W.N.H. and M.R.B. C.J.W, G.J.F and D.A.A.V. provided critical reagents and important intellectual input. The manuscript was written by S.D.B. and E.J.W.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and Cell-Fate Decisions in Effector and Memory CD8(+) T Cell Differentiation during Viral Infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007 doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 8.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 9.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JY, et al. PD-1 upregulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors, but not in long-term non-progressors. Blood. 2007 doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 12.Urbani S, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radziewicz H, et al. Liver infiltrating lymphocytes in chronic human HCV infection display an exhausted phenotype with high PD-1 and low CD127 expression. J Virol. 2006 doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boettler T, et al. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–40. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ, et al. Molecular SIgnature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann DE, et al. Upregulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007 doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 18.Golub TR, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–7. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 19.McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–94. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Cai G, et al. CD160 inhibits activation of human CD4(+) T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008 doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 21.Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–22. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed R, et al. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, et al. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970–9. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 26.Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–40. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojo S, Burshtyn DN, Long EO, Wagtmann N. Type I transmembrane receptor with inhibitory function in mouse mast cells and NK cells. J Immunol. 1997;158:9–12. [PubMed] [Google Scholar]
- 28.Lau L, Jamieson B, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nat. 1994;369:648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- 29.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and Functional Profiling of Memory CD8 T Cell Differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 31.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 32.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–41. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–9. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller MJ, et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–30. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 35.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by {alpha}PD-L1 blockade. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermans IF, et al. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods. 2004;285:25–40. doi: 10.1016/j.jim.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Chlewicki LK, et al. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–67. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 39.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostense S, et al. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31:677–86. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streeck H, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–7. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baixeras E, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–65. [PubMed] [Google Scholar]
- 46.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Grosso JF, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–95. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 49.Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 50.Byun HJ, et al. Proliferation of activated CD1d-restricted NKT cells is down-modulated by lymphocyte activation gene-3 signaling via cell cycle arrest in S phase. Cell Biol Int. 2007;31:257–62. doi: 10.1016/j.cellbi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Katz HR, et al. Mouse mast cell gp49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when coligated with the high-affinity Fc receptor for IgE. Proc Natl Acad Sci U S A. 1996;93:10809–14. doi: 10.1073/pnas.93.20.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laouar A, et al. Cutting Edge: Distinct NK receptor profiles are imprinted on CD8 T cells in the mucosa and periphery during the same antigen challenge: role of tissue-specific factors. J Immunol. 2007;178:652–6. doi: 10.4049/jimmunol.178.2.652. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–74. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 54.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–55. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks DG, et al. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–41. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotturi MF, et al. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J Virol. 2007;81:4928–40. doi: 10.1128/JVI.02632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.