Abstract
Objective
To evaluate the efficacy and safety of miglustat, concomitant with enzyme replacement therapy (ERT), in patients with Gaucher’s disease type 3 (GD3).
Methods
This 24-month, phase II, open-label clinical trial of miglustat in GD3 was conducted in two phases. During the initial 12 months, patients were randomized 2:1 to receive miglustat or “no miglustat treatment.” The randomized phase was followed by an optional 12-month extension phase in which all patients received miglustat. All patients received ERT during the 24-month period. The primary efficacy end points were change from baseline to months 12 and 24 in vertical saccadic eye movement velocity as determined by the peak amplitude versus amplitude regression line slope. Secondary end points included changes in neurological and neuropsychological assessments, pulmonary function tests, liver and spleen organ volumes, hematological and clinical laboratory assessments, and safety evaluations.
Results
Thirty patients were enrolled, of whom 21 were randomized to miglustat and 9 to “no miglustat treatment.” Twenty-eight patients entered the 12-month extension phase. No significant between-group differences in vertical saccadic eye movement velocity or in the other neurological or neuropsychological evaluations were observed. Organ volumes and hematological parameters remained stable in both treatment groups, but improvement in pulmonary function and decrease of chitotriosidase levels were observed with miglustat compared with patients receiving ERT alone.
Interpretation
Miglustat does not appear to have significant benefits on the neurological manifestations of GD3. However, miglustat may have positive effects on systemic disease (pulmonary function and chitotriosidase activity) in addition to ERT in patients with GD3.
Currently, there is no effective treatment available for the neurological manifestations of patients with type 3 Gaucher’s disease (GD3). GD is characterized by the autosomal recessive inheritance of a functional deficiency of the lysosomal enzyme, glucocerebrosidase. Impaired activity of glucocerebrosidase leads to an accumulation of glucosylceramide in macrophages of various tissues, including the liver, spleen, bone and bone marrow, and lungs. Clinical manifestations of GD include organomegaly and hematological complications, with variable neurological involvement.1 With an estimated global prevalence of 1:200,000,2 GD is the most common of the lysosomal storage disorders.
GD is traditionally classified into three types. GD type 1 (GD1) represents 95% of cases2 and is currently classified as nonneuronopathic. The neuronopathic forms of GD can be either acute (GD2) or chronic (GD3). GD3 is characterized by systemic abnormalities and variable neurological impairment. The major neurological manifestation is early development of horizontal supranuclear gaze palsy.3 Other symptoms include cognitive impairment, myoclonic epilepsy, ataxia, and spasticity, which develop as the illness progresses.1,4 Enzyme replacement therapy (ERT) is approved in Europe for the management of nonneurological manifestations of the disease in GD3 patients. It does not appear to affect neurological or pulmonary involvement in GD.5–9
Miglustat is a substrate reduction therapy approved for the treatment of adult patients with mild-to-moderate GD1 for whom ERT is unsuitable or not a therapeutic option.10,11 Miglustat reversibly inhibits glucosylceramide synthase, the enzyme that catalyzes the first committed step in glycosphingolipid synthesis.12 In three pivotal studies in GD1 patients, miglustat showed beneficial effects on organomegaly, hemoglobin, and platelet levels.13–16
Miglustat has specific physicochemical properties that promote wide tissue distribution (as shown in animal models), and there is strong indirect evidence from preclinical studies that it crosses the blood–brain barrier.17 Miglustat has therefore been suggested as a potential therapy for GD3. A recently published case report has indicated that miglustat may improve neurological manifestations in GD3.18
Here, we report data from a clinical trial that evaluated the efficacy and safety of miglustat in patients with GD3.
Patients and Methods
Study Design and Objectives
This study was a 24-month, phase II, open-label clinical trial with an initial 12-month, randomized, controlled phase followed by an optional 12-month noncomparative extension phase conducted in patients with GD3. The primary objective was to evaluate miglustat as a treatment for GD3 by assessing changes in saccadic eye movement velocity and other disease manifestations, with particular emphasis on neurological parameters. The secondary objective was to assess the safety and tolerability of miglustat in GD3 patients, as well as its effect on visceral and systemic disease parameters.
Patients
Patients were recruited from two study centers: Center 1 in Bethesda, MD, and Center 2 in London. All patients had a diagnosis of GD confirmed biochemically. The diagnosis of GD3 had been confirmed clinically. Patients who had been stable on ERT for 6 months or longer or had undergone a successful bone marrow transplant ≥1 year before study entry were included. At Center 1, only patients 4 years or older were eligible, whereas at Center 2, there was no age restriction. All patients were required to have the ability to swallow a capsule of miglustat.
Patients undergoing concurrent therapy with other investigational agents and patients who were taking drugs or food supplements that may have interfered with gastrointestinal absorption or motility were excluded from the study. Patients who had suffered from clinically significant diarrhea (>3 liquid stools/day for >7 days) without definable cause within 3 months of a screening visit or who had a history of significant gastrointestinal disorders were also excluded. Other exclusion criteria included a current medical condition that rendered a patient unsuitable for the study, such as human immunodeficiency virus or hepatitis infection, and an adjusted creatinine clearance of less than 70 mL/min/1.73 m2.
Administration of Study Drug
Patients were randomized (2:1) to receive miglustat or “no miglustat treatment” for the first 12 months, followed by a 12-month extension where all patients received miglustat. Patients 12 years or older received the adult dosage of miglustat (200 mg three times a day), whereas patients younger than 12 years received a lower dosage adjusted to their body surface area. The investigator was permitted to reduce the dosage of miglustat for patients of any age if it was indicated clinically. All patients received ERT during the 24-month period.
Eye Movements
Patients had a general ophthalmological assessment before assessment of saccadic eye movements to exclude other causes of visual impairment. Horizontal (HSEM) and vertical saccadic eye movements (VSEM) were recorded at baseline, month 12, and month 24 using a video, infrared, or scleral search coil technique, and were sampled at 1kHz using REX (Real-time EXperimentation). Patients faced a screen and followed a target that jumped on the screen according to a pseudorandom sequence. The subjects sat with head in chin cup and forehead against a head rest, and faced a screen 1m away on which a red laser spot was back projected (this subtended about 0.5 degree). A mirror galvanometer moved the spot in a pseudorandom sequence from −15 to +15 degrees with target jumps of 2.5, 5, 7.5, 10, 12.5, 15, 20, 25, and 30 degrees in each direction. The target jumped approximately every 3 seconds with about 0.5-second variability. Subjects were instructed to follow the target. A minimum of 100 target jumps was recorded. A second similar recording was made after a break of at least 1 hour. The eye position and target data were sent in ASCII format to a masked observer who filtered and differentiated the data to obtain eye velocity. Saccades were detected using velocity criteria, and their characteristics (amplitude, duration, peak velocity) were determined. A plot of saccade peak amplitude (defined as saccade amplitude/saccade peak velocity) versus saccade amplitude was created for each session, and a regression line was fitted to the data using Matlab (version 7.04). The slope and intercept of the fitted line were the β and α used in this study.19 The local assessors were masked to the patient’s treatment status. Data for both sites were sent to a blinded central assessor for final evaluation (L.A.). That assessor was blinded to both treatment group and order of testing.
End Points
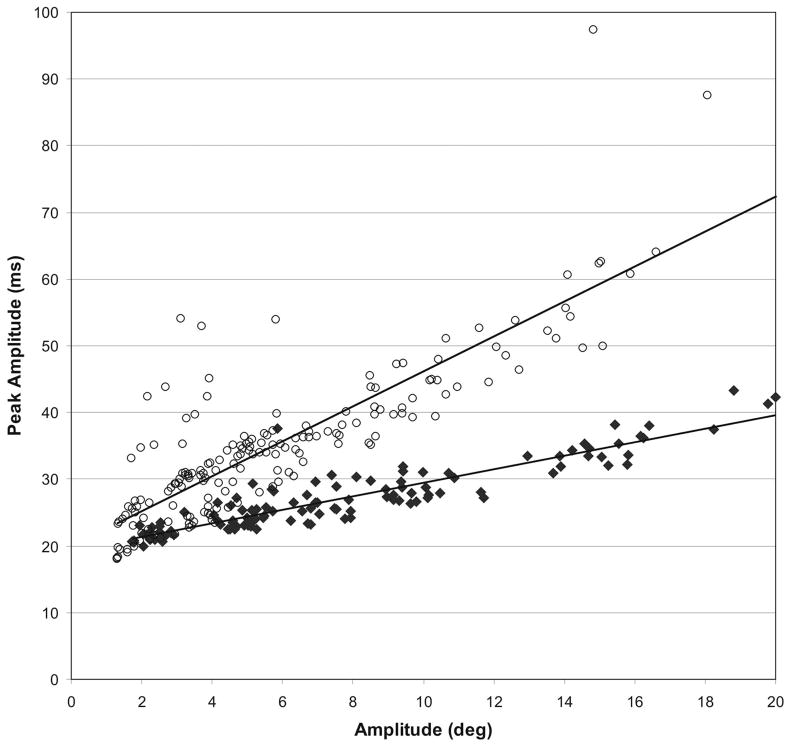
The primary efficacy end points were the change in VSEM velocity from baseline to month 12 and to month 24. The relation between peak amplitude (defined as peak amplitude/peak velocity) and amplitude was calculated by linear regression (Fig 1), showing that saccade peak amplitude is generally higher in GD3 patients compared with age-matched control subjects, at any given amplitude; that is, a reduction in the slope of the linear regression line (or a decrease of the slope, VSEM-α), represented an improvement in VSEM velocity. To formally confirm that vertical saccades are abnormal in patients with GD3, we compared the peak amplitude/amplitude slope of vertical downward saccades using the scleral search coil technique in 11 patients at the National Institutes of Health site (4.65 ± 3.93) with 10 age-matched control subjects (1.79 ± 0.49), and found them to be significantly different (p = 0.03).
Fig 1.
Peak amplitude (saccade amplitude/saccade peak velocity) versus amplitude in a patient with type 3 Gaucher’s disease (GD3; open circles) and an age-matched control subject (filled diamonds). Regression lines through the data for the patient and for the control subject are also shown.
A reduction in the intercept value of the linear regression line (VSEM-β) could also represent an improvement in VSEM velocity; thus, change from baseline in VSEM-β was measured as a secondary efficacy end point, as were HSEMs (HSEM-α and -β).
Additional neurological end points in this study included a set of neuropsychological assessments (Purdue Peg Board test, Wechsler Scale, Benton visual retention test, Rey auditory verbal learning test, d2 test of attention, continuous performance test, and Trail Making Test) and a set of neurological assessments (assessing mental state, cranial nerves, motor skills, and other neurological symptoms). Brain auditory-evoked potentials were also assessed. Systemic end points included pulmonary function tests, liver and spleen organ volumes, hematological assessments (including hemoglobin and platelet counts), and clinical laboratory assessments (biochemical disease markers including chitotriosidase, hexosaminidase, and glucosylceramide). Safety assessments included recording of adverse events and tremor measurements (accelerometry and surface electromyographic (EMG)/clinical assessment), vital signs, and physical examinations.
Patients were assessed at baseline, months 12 and 24, or on early withdrawal from the study for all efficacy end points except for biomarker assessments, which were performed every 3 months during the 24-month treatment period.
Statistical Analyses
A sample size of 30 patients was selected on pragmatic grounds because limited longitudinal data for VSEM were available for GD3 patients at the time of study design. The sample was expected to provide 86% power to detect a difference of 0.0012 msec/degree between treatment groups on the primary efficacy end point, VSEM-α, at the two-sided 5% level of statistical significance. This calculation was based on VSEM data obtained from six GD3 patients evaluated before study initiation.
All efficacy evaluations for the 24-month period were performed on all randomized patients who entered the 12-month extension period and had at least one postbaseline efficacy assessment during the 12-month extension period. Patients randomized to “no miglustat treatment” who entered the 12-month extension period and received miglustat were included in the 12 months “no miglustat treatment” plus 12 months “miglustat” group. Patients randomized to “miglustat” who continued receiving miglustat during the 12-month extension period were included in the “24 months miglustat” group. Patients randomized to “no miglustat treatment” who entered the 12-month extension period but who did not receive miglustat were excluded from efficacy evaluations of the 12-month extension period.
Safety evaluations were performed for one single noncomparative cohort of patients, which included all randomized patients who received at least one dose of miglustat during the 24-month study period. Data from patients originally randomized to “no miglustat treatment” were included only from the date the first dose of miglustat was taken during the 12-month extension period.
For all efficacy end points, absolute values were calculated using standard descriptive statistical measures (mean and 95% confidence intervals). For the primary efficacy end point (VSEM-α), secondary eye movement end points (VSEM-β and HSEM), and organ volumes, treatment groups were also compared using an analysis of covariance model with terms for baseline, center, and treatment group.
Results
Patients and Treatment
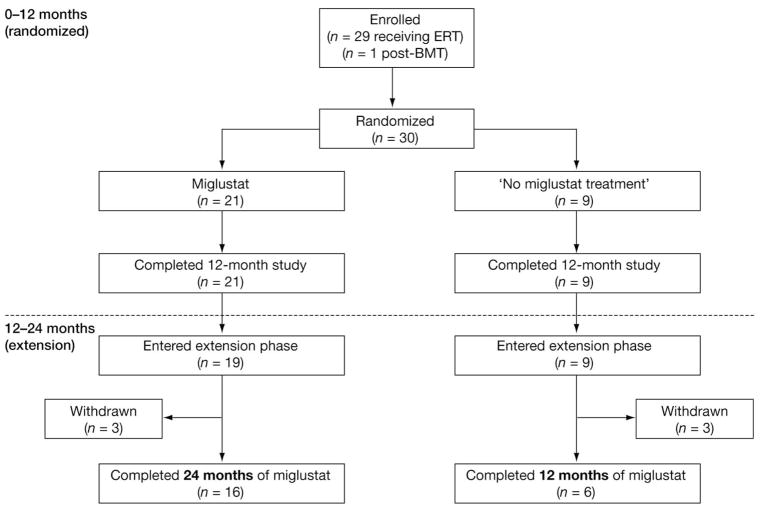
Thirty patients were enrolled in the study, of whom 29 were receiving ERT and 1 was not receiving ERT but had undergone 2 bone marrow transplants at 16 and 13 years before the study and were fully engrafted (Fig 2). All 30 patients randomized to receive either miglustat (n = 21) or no miglustat treatment (n = 9) completed the first 12 months of study treatment. Twenty-eight patients (93%) entered the 12-month extension, of whom 22 (73%) completed 12 months of extension treatment. Overall, the median (range) exposure to miglustat was 729 (6–802) days. Predose (trough) miglustat levels in cerebrospinal fluid ranged from 201 to 629 ng/ml (n = 4) or up to 60% of the trough level in plasma.
Fig 2.
Patient disposition. ERT = enzyme replacement therapy; BMT = bone marrow transplantation.
Patient demographics are summarized in Table 1. There was a greater proportion of male patients in patients randomized to miglustat treatment than in those randomized to no miglustat treatment. The proportion of patients in the 2- to 11-year-old category was much greater than in the no miglustat treatment group.
Table 1.
Baseline Demographics by Treatment Group
| Demographics | Miglustat (n = 21) | No Miglustat Treatment (n = 9) |
|---|---|---|
| Male sex, n (%) | 10 (48) | 2 (22) |
| Mean age, yr (SD) | 10.4 (5.1) | 9.9 (4.0) |
| Age category | ||
| 2–11 yr, n (%) | 11 (52) | 8 (89) |
| 12–17 yr, n (%) | 7 (33) | 0 |
| ≥18 yr, n (%) | 3 (14) | 1 (11) |
| Mean weight, kg (SD) | 32.5 (13.7) | 35.4 (12.2) |
| Mean height, cm (SD) | 129.6 (21.0) | 137.2 (12.6) |
| Mean BSA, cm2 (SD) | 10,681 (3,101) | 11,511 (2,359) |
SD = standard deviation; BSA = body surface area.
All 30 patients had at least 1 current manifestation of GD at baseline. Seventeen patients (81%) in the miglustat group and all patients in the no miglustat treatment group had hepatomegaly (volume in milliliters > 25 × age in years) and splenomegaly (volume in milliliters > 2 × age in years). Nine patients (43%) in the miglustat group and 6 patients (67%) in the no miglustat treatment group had anemia, whereas 12 (57%) and 7 (78%) had thrombocytopenia (<150 × 103/ml), respectively. History of bone crisis was reported in four patients (19%) from the miglustat group and one (11%) from the no miglustat treatment group (Table 2). Apart from horizontal supranuclear gaze palsy, which was seen in all patients, baseline neurological manifestations were heterogeneous (see Table 2).
Table 2.
Frequency of Disease Historical Clinical Manifestations at Baseline
| Symptom | Patient Groups, n (&%)
|
||
|---|---|---|---|
| Miglustat (n = 21) | No Miglustat Treatment (n = 9) | Overall (n = 30) | |
| Horizontal supranuclear gaze palsy | 21 (100) | 9 (100) | 30 (100) |
| Hepatomegaly | 17 (81) | 9 (100) | 26 (87) |
| Splenomegaly | 17 (81) | 9 (100) | 26 (87) |
| Spinal alignment abnormalities | 13 (62) | 6 (67) | 19 (63) |
| Cognitive impairment | 12 (57) | 2 (22) | 14 (47) |
| Thrombocytopeniaa | 12 (57) | 7 (78) | 19 (63) |
| Dysarthria | 9 (43) | 2 (22) | 11 (37) |
| Anemia | 9 (43) | 6 (67) | 15 (50) |
| Seizures | 5 (24) | 1 (11) | 6 (20) |
| Ataxia | 5 (24) | 6 (67) | 11 (37) |
| Bone crisis | 4 (19) | 1 (11) | 5 (17) |
| Swallowing difficulties | 3 (14) | 2 (22) | 5 (17) |
| Pyramidal tract dysfunction | 3 (14) | 2 (22) | 5 (17) |
At the start of the study, there were three patients with mild thrombocytopenia at baseline: Patients 116 (144 × 109/L) and 117 (132 × 109/L) in the miglustat group, and Patient 113 (149 × 109/L) in the no miglustat treatment group.
Minor baseline electroneurography and myography (EMG) abnormalities such as low compound muscle action potential amplitudes in the left peroneal nerve were observed in three patients randomized to miglustat treatment.
The most common categories of nonneurological concomitant illnesses at baseline were respiratory/thoracic disorders consisting of asthma, rhinitis, sinusitis and interstitial lung disease (six patients in the miglustat group and one in the no miglustat treatment group), musculoskeletal and connective tissue disorders (four patients in the miglustat group and one in the no miglustat treatment group), ear and labyrinth disorders (four patients in the miglustat group and one in the no miglustat treatment group), and infections (five patients in the miglustat group and none in the no miglustat treatment group).
All patients reported the use of at least one concomitant therapy at baseline. The most frequently reported concomitant medications were ERT (imiglucerase; n = 29), salbutamol (n = 5), and penicillin (n = 5).
Neurological Outcomes
The results of the primary end point (VSEM-α) are summarized in Table 3. In general, no effect on VSEM-α was observed throughout the study. In the 24-month miglustat group, numerical increases were observed on VSEM-α up (1.87 [95% confidence interval (CI), 1.1–2.6] at baseline vs 2.22 [95% CI, 1.3–3.1] at month 24; see Table 3) and VSEM-α down (2.61 [95% CI, 1.8–3.5] at baseline vs 3.09 [95% CI 1.8–4.4] at month 24; see Table 3).
Table 3.
Primary End Point (Vertical Saccadic Eye Movement-α) by Treatment Group
| 24 Months Miglustat | 12 Months No Miglustat Treatment + 12 Months Miglustat | ||||||
|---|---|---|---|---|---|---|---|
| Time Point | n | Mean Absolute Value | 95% CI | n | Mean Absolute Value | 95% CI | |
| Vertical Up | Baseline | 17 | 1.87 | 1.1–2.6 | 9 | 1.52 | 1.2–1.8 |
| Month 12 | 17 | 2.44 | 1.5–3.4 | 9 | 1.80 | 1.2–2.4 | |
| Month 24 | 14 | 2.22 | 1.3–3.1 | 6 | 1.34 | 1.2–1.5 | |
| Vertical Down | Baseline | 17 | 2.61 | 1.8–3.5 | 9 | 2.76 | 1.7–3.8 |
| Month 12 | 17 | 2.92 | 2.0–3.8 | 9 | 2.88 | 1.3–4.4 | |
| Month 24 | 13 | 3.09 | 1.8–4.4 | 5 | 2.48 | 1.2–3.8 | |
An increase in vertical saccadic-α up or down represents a worsening in vertical saccadic eye movement (VSEM) velocity. CI = confidence interval.
Analyses of secondary eye movement parameters did not show significant differences in VSEM-β, HSEM-α, or HSEM-β between the two treatment groups at any time point (data not shown). No statistically significant between-group differences were seen on the other secondary neurological evaluations (neurological examinations or cognitive tests) at the end of randomized therapy or at the end of the extension phase (data not shown).
Systemic Disease End Points
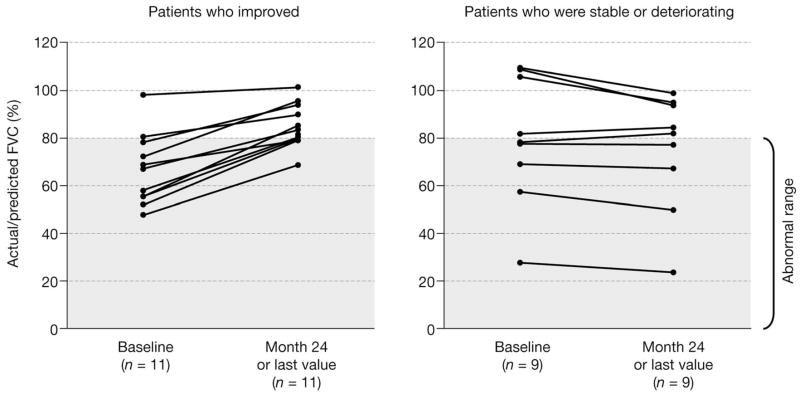
Changes in systemic disease parameters are summarized in Supplementary Table 1. During the randomized phase, pulmonary function testing indicated an improvement from baseline in percentage actual/predicted forced vital capacity in the miglustat group (from 75.1 [95% CI, 62.0–88.2] at baseline to 81.1 [95% CI, 65.7–96.5] at month 12) compared with the no treatment group (from 79.5 [95% CI, 61.3–97.6] at baseline to 81.3 [95% CI, 62.7–99.9] at month 12) (see Supplementary Table 1 and Fig. 3). Furthermore, during the extension phase, patients initially randomized to no miglustat treatment also showed an improvement with miglustat treatment (80.2 [95% CI, 62.0–98.3] for patients remaining in the study at month 12 vs 89.6 [95% CI, 80.8–98.5] at month 24). Patients with an abnormal forced vital capacity (<80%) at baseline showed the most improvement with miglustat treatment (Fig 3).
Fig 3.
Greater response to 24 months of miglustat treatment observed in patients with abnormal actual/predicted forced vital capacity (FVC).
Liver and spleen volumes remained stable in both treatment groups at all time points. Similarly, hemoglobin levels and platelet counts were stable throughout the 24-month study period, and no notable differences were observed between treatment groups (see Supplementary Table 1).
No cases of bone crisis were reported during the study. One patient randomized to the miglustat group had a tibia fracture while playing on a trampoline 15 months into the study. Another patient who had several fractures before entry into the study and was randomized to the no miglustat group had two events of severe femoral fracture: one reported at month 9 and one reported at month 12 of the study.
Biomarkers
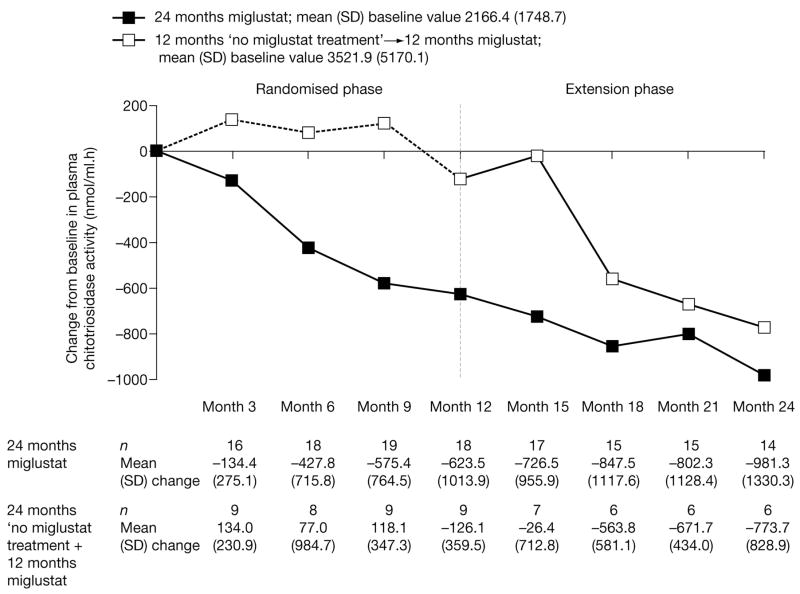
By month 6, a decrease from baseline in plasma chitotriosidase activity was already observed in the miglustat group (Fig 4). In the no miglustat treatment group, plasma chitotriosidase levels remained unchanged up to month 12. However, a reduction was seen 6 months after initiation of miglustat treatment, and this reduction was sustained up to month 24 (see Fig 4). There were no notable differences from baseline or between treatment groups in the other plasma markers (including hexosaminidase and glucosylceramide; data not shown).
Fig 4.
Changes from baseline in plasma chitotriosidase activity during miglustat treatment. Black squares represent 24 months miglustat with mean (standard deviation [SD]) baseline value of 2166.4 (1748.7); white squares represent 12 months no miglustat treatment plus 12 months miglustat with mean (SD) baseline value of 3521.9 (5170.1).
Safety and Tolerability
Twenty-nine patients were included in the safety analysis. One patient randomized to the miglustat treatment group did not receive the study drug and, therefore, was not included in the safety analysis. Miglustat was well tolerated in both adult and pediatric patients during this study. The most frequently reported adverse events were diarrhea (21 patients, 72%), tremor (11 patients, 38%), abdominal pain (10 patients, 34%), and cough (10 patients, 34%) (Table 4). These adverse events were generally mild and did not lead to study discontinuation.
Table 4.
Most Frequently Reported Adverse Events (Safety Population)
| Adverse Eventsa | Patients (N = 29), n (%) |
|---|---|
| Diarrhea | 21 (72) |
| Tremor | 11 (38) |
| Abdominal pain | 10 (34) |
| Cough | 10 (34) |
| Pyrexia | 8 (28) |
| Weight decreased | 7 (24) |
| Nasopharyngitis | 7 (24) |
| Vomiting | 6 (21) |
| Convulsions | 6 (21) |
| Headache | 6 (21) |
| Activated partial thromboplastin time prolonged | 6 (21) |
| Aspartate aminotransferase increased | 6 (21) |
aAdverse events that occurred in at least 20% of patients.
Ten patients (34%) received at least one medication for gastrointestinal adverse events during the first 13 weeks of the study, but the number of patients receiving such medication decreased over time, with only 5 (20%) taking these medications during the last 13 weeks of the study. Weight decrease was observed in a total of seven patients (24%), and was mild (5 to <10% decrease) in three patients and moderate (10 to <20% decrease) in four patients. There was no retardation of growth in pediatric or juvenile patients.
Four patients with normal baseline EMG experienced abnormalities during the study. One patient randomized to miglustat treatment had a confirmed polyneuropathy at the end of the study (month 24). Another patient, also randomized to miglustat therapy, experienced a subclinical polyneuropathy (ie, abnormalities on EMG consistent with a polyneuropathy without clinical signs and/or symptoms of polyneuropathy) and was withdrawn from the study by the investigator after 482 days of exposure to miglustat. Two patients had a subclinical mononeuropathy (one was from the no miglustat treatment group); both patients completed 24 months of treatment.
Six patients (21%) experienced at least one serious adverse event during the study. The most common serious adverse events were infections, experienced by three (10%) patients. These events were not considered to be related to miglustat treatment, and no deaths occurred during the study.
Discussion
This is the first randomized, controlled study that has evaluated the efficacy, safety, and tolerability of an oral drug treatment in patients with GD3. Saccadic eye movement velocity remained unchanged throughout the study period. Recently published data suggest that miglustat may improve neurological manifestations in GD3.18 However, this was a single case report. Given the natural history of the disease, it is not possible to rule out natural variation as an explanation for an apparent response in an individual patient. Miglustat may also improve and/or stabilize several clinically relevant neurological parameters in other neuronopathic lysosomal storage disorders such as Sandhoff’s disease and Niemann–Pick disease type C.20,21 However, this study did not show effects of miglustat therapy over 12 or 24 months on VSEM velocity, HSEM velocity, or other neurological or neuropsychological parameters.
A number of systemic disease parameters in this study showed improvement or stability during miglustat treatment. In particular, the improved pulmonary function seen in some patients may be important because ERT has been shown to have a suboptimal effect on pulmonary involvement in GD. In a study of the efficacy of ERT in GD3, 19 of 21 patients enrolled presented with interstitial lung disease, which was always unresponsive for up to 8 years of ERT.8 Similarly, in GD1 patients with pulmonary signs or symptoms, no normalization in pulmonary function or lung architecture was detected with ERT, despite a good response to treatment on other systemic manifestations.22 The incidence of pulmonary manifestations in GD is not well established,23 but they appear to be more frequent in patients with other severe disease manifestations.22 Pulmonary symptoms may therefore be of particular significance in GD3 patients, and miglustat may provide additional benefits to ERT in the treatment of these manifestations.
The significant decrease in chitotriosidase levels seen with miglustat in this study is consistent with Capablo and colleagues’18 recent case on the effects of miglustat in a GD3 patient, and suggests that miglustat may further decrease chitotriosidase activity in addition to ERT in GD3 patients. Chitotriosidase activity has become accepted as a marker of type and clinical severity in GD and of treatment response.24 The fact that there was a clear decrease in activity in GD3 patients treated with miglustat in addition of ERT is encouraging, and may suggest an additional and possibly different disease-modifying effect of miglustat therapy.
The tolerability profile of miglustat at 200 mg three times a day in this GD3 patient population was comparable with that reported in previous trials in GD1, where miglustat was administered at the approved dose of 100 mg three times a day.13,15 Consistent with previous data, gastrointestinal adverse events, particularly diarrhea and abdominal pain, were reported most frequently. In general, all adverse events were of mild or moderate severity and decreased in frequency over time. There were no cases of withdrawal from the study because of these adverse events, and gastrointestinal side effects were well controlled by medications such as loperamide. One confirmed polyneuropathy (requiring discontinuation of study drug) and one subclinical polyneuropathy were observed in patients randomized to miglustat. These neurological side effects should be interpreted with caution because the prevalence and incidence of peripheral nerve disease in GD3 is unknown and could also take into account recent observations from a prospective, observational study in 103 GD1 patients showing a prevalence rate of polyneuropathy of 10.7%.25
Patients with GD3 may exhibit a wide degree of variation for disease progression, and the severity of systemic disease and neurological deficits differs considerably between patients.4 Because of this wide variability of phenotypes, there is currently no recognized quantitative clinical end point for GD3 neurological symptoms. In addition, there is minimal information available on the natural history of GD3. We selected supranuclear gaze palsy (saccadic initiation failure) as our primary end point, because it was considered the only universal neurological manifestation in GD3 and is one of the criteria defining the condition, often being the earliest neurological sign.3,26,27 The difference in VSEM velocity in a GD3 patient compared with an age-matched control subject is shown in Figure 1. VSEM velocity was used as the primary efficacy measure in this study rather than the HSEM because it appears later and is virtually always milder; therefore, we hypothesized it could be more susceptible to changes after therapy. Also, some patients had unrecordable horizontal saccades.
The failure to detect treatment effects on saccadic eye movement velocity may have resulted from a number of factors. Vertical saccadic velocity deteriorates slowly, if at all (unpublished data). Our study might have been too short (especially the controlled phase) and might have lacked statistical power. The latter is likely in view of the marked variability of the saccadic eye movement defect. Other possibilities include a too small inhibitory effect on the synthesis of glucosylceramide of miglustat compared with its concentrations in the brain and the possible irreversible nature of the neurological defects in GD3.
Conclusions
This 24-month trial of miglustat, the first randomized, controlled study of a drug treatment in patients with GD3, did not show significant differences on the chosen neurological endpoints over 24 months. However, the data suggest that miglustat may have positive effects on systemic disease (pulmonary function and chitotriosidase activity) in addition to ERT in patients with GD3.
Supplementary Material
Acknowledgments
During the study, the sponsor changed from Oxford GlycoSciences, a wholly owned subsidiary of Celltech R&D and the original manufacturer of miglustat (OGT 918), to Actelion Pharmaceuticals (Allschwil, Switzerland; August 2004). This study was also supported by the intramural research program of the NIH (National Institute of Neurological Disorders and Stroke).
We are indebted to the patients and families who participated in the study.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Schiffmann R, Vellodi A. Neuronopathic Gaucher disease. In: Futerman T, Zimran A, editors. Gaucher disease. Boca Raton, FL: CRC Press Taylor & Francis; 2007. pp. 175–196. [Google Scholar]
- 2.Charrow J, Andersson HC, Kaplan P, et al. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 3.Patterson MC, Horowitz M, Abel RB, et al. Isolated horizontal supranuclear gaze palsy as a marker of severe systemic involvement in Gaucher’s disease. Neurology. 1993;43:1993–1997. doi: 10.1212/wnl.43.10.1993. [DOI] [PubMed] [Google Scholar]
- 4.Erikson A, Bembi B, Schiffmann R. Neuronopathic forms of Gaucher’s disease. Baillieres Clin Haematol. 1997;10:711–723. doi: 10.1016/s0950-3536(97)80035-2. [DOI] [PubMed] [Google Scholar]
- 5.Schiffmann R, Heyes MP, Aerts JM, et al. Prospective study of neurological responses to treatment with macrophage-targeted glucocerebrosidase in patients with type 3 Gaucher Disease. Ann Neurol. 1997;42:613–621. doi: 10.1002/ana.410420412. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PE, Harris CM, Vellodi A. Deterioration of the auditory brainstem response in children with type 3 Gaucher disease. Neurology. 2004;63:385–387. doi: 10.1212/01.wnl.0000130191.31669.48. [DOI] [PubMed] [Google Scholar]
- 7.Erikson A, Forsberg H, Nilsson M, et al. Ten years’ experience of enzyme infusion therapy of Norrbottnian (type 3) Gaucher disease. Acta Paediatr. 2006;95:312–317. doi: 10.1080/08035250500423804. [DOI] [PubMed] [Google Scholar]
- 8.Altarescu G, Hill S, Wiggs E, et al. The efficacy of enzyme replacement therapy in patients with type 3 Gaucher disease. J Pediatr. 2001;138:539–547. doi: 10.1067/mpd.2001.112171. [DOI] [PubMed] [Google Scholar]
- 9.Schiffmann R, Mankin H, Dambrosia JM, et al. Decreased bone density in splenectomized Gaucher patients receiving enzyme replacement therapy. Blood Cells Mol Dis. 2002;28:288–296. doi: 10.1006/bcmd.2002.0517. [DOI] [PubMed] [Google Scholar]
- 10.Miglustat summary of product characteristics. Available at: http://www.emea.europa.eu/humandocs/Humans/EPAR/zavesca/zavesca.htm Accessed September 5, 2008.
- 11.Miglustat package insert. Available at: http://www.fda.gov/cder/foi/label/2008/021348s005lbl.pdf. Accessed September 5, 2008.
- 12.Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–8365. [PubMed] [Google Scholar]
- 13.Cox T, Lachmann R, Hollak C, et al. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 14.Heitner R, Elstein D, Aerts J, et al. Low-dose N-butyldeoxynojirimycin (OGT 918) for type I Gaucher disease. Blood Cells Mol Dis. 2002;28:127–133. doi: 10.1006/bcmd.2002.0497. [DOI] [PubMed] [Google Scholar]
- 15.Pastores GM, Barnett NL, Kolodny EH. An open-label, non-comparative study of miglustat in type I Gaucher disease: efficacy and tolerability over 24 months of treatment. Clin Ther. 2005;27:1215–1227. doi: 10.1016/j.clinthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Elstein D, Hollak C, Aerts JM, et al. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type I Gaucher disease. J Inherit Metab Dis. 2004;27:757–766. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 17.Treiber A, Morand O, Clozel M, et al. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica. 2007;37:298–314. doi: 10.1080/00498250601094543. [DOI] [PubMed] [Google Scholar]
- 18.Capablo JL, Franco R, de Cabezón AS, et al. Neurologic improvement in a type 3 gaucher disease patient treated with imiglucerase/miglustat combination. Epilepsia. 2007;48:1406–1408. doi: 10.1111/j.1528-1167.2007.01074.x. [DOI] [PubMed] [Google Scholar]
- 19.Inchingolo P, Spanio M. On the identification and analysis of saccadic eye movements—a quantitative study of the processing procedures. IEEE Trans Biomed Eng. 1985;BME-32:683–695. doi: 10.1109/TBME.1985.325586. [DOI] [PubMed] [Google Scholar]
- 20.Patterson MC, Vecchio D, Prady H, et al. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 21.Lachmann RH, Wright N, Parker A, et al. Substrate reduction therapy in Sandhoff disease: evidence for improvement in nervous function in patients treated with miglustat. J Inherit Metab Dis. 2006;29(suppl 1):P10–P12. [Google Scholar]
- 22.Goitein O, Elstein D, Abrahamov A, et al. Lung involvement and enzyme replacement therapy in Gaucher’s disease. QJM. 2001;94:407–415. doi: 10.1093/qjmed/94.8.407. [DOI] [PubMed] [Google Scholar]
- 23.Miller A, Brown LK, Pastores GM, Desnick RJ. Pulmonary involvement in type 1 Gaucher disease: functional and exercise findings in patients with and without clinical interstitial lung disease. Clin Genet. 2003;63:368–376. doi: 10.1034/j.1399-0004.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 24.Vellodi A, Foo Y, Cole TJ. Evaluation of three biochemical markers in the monitoring of Gaucher disease. J Inherit Metab Dis. 2005;28:585–592. doi: 10.1007/s10545-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 25.Hollak C, Biegstraaten M, van Schaik IN, et al. Prevalence of polyneuropathy in adult type 1 Gaucher disease: a multinational prospective observational study. J Inherit Metab Dis. 2007;30(suppl 1):427-P. Abstract. [Google Scholar]
- 26.Harris CM, Taylor DS, Vellodi A. Ocular motor abnormalities in Gaucher disease. Neuropediatrics. 1999;30:289–293. doi: 10.1055/s-2007-973507. [DOI] [PubMed] [Google Scholar]
- 27.Accardo AP, Pensiero S, Perissutti P, et al. Saccadic analysis for early identification of neurological involvement in Gaucher disease. Ann NY Acad Sci. 2005;1039:503–507. doi: 10.1196/annals.1325.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.