Abstract
Mental retardation (MR) is not a common feature observed in patients with classical ectodermal dysplasias (EDs). Several genes responsible for EDs and MR have been identified. However, the causation has yet to be identified in a significant number of patients with either ED or MR. Here, we have molecularly characterized a de novo balanced translocation t(1;6)(p22.1;p22.1) in a female patient who had mild features of ED with hypodontia, microcephaly, and cognitive impairment. Mapping of the translocation breakpoints in the patient revealed no obvious causative gene for either ED or MR. Whole genome array CGH analysis unveiled two novel submicroscopic deletions at 2q12.2 and 6q22.3, unrelated to the translocation in the patient. The 2q12.2 deletion contains a known ED gene, ectodysplasin-A receptor (EDAR), and the loss of one copy of this gene is considered to be responsible for the ectodermal phenotype in the patient. It is plausible that a potential autosomal MR gene deleted at 2q12.2 or 6q22.3 is likely the cause of the neurodevelopmental defects in the patient.
Keywords: mental retardation, ectodermal dysplasia, translocation, arrayCGH, EDAR
Introduction
Mental retardation (MR) is the most common developmental disability, affecting intellectual and adaptive functions in approximately 1–3% of the population. Yet, the underlying cause of MR is established in less than half the cases.1, 2 The finding of MR among almost all patients with autosomal microdeletions and the association of MR with submicroscopic alterations in the subtelomeric region of the autosomes indicates the ubiquitous distribution of autosomal genes that influence intelligence.3, 4 Thus far, a limited number of patients have been reported who have both MR and classical ectodermal dysplasia (ED). EDs are a heterogeneous group of developmental disorders that affect the secondary differentiation of ectodermally derived structures. The classical EDs include a combination of defects in hair, missing or abnormally shaped teeth, and a reduction in the number of sweat glands. An early diagnosis of ED is essential for the management of the disease in patients and is a prerequisite for reducing mortality that primarily results from hyperthermia and respiratory tract infections.
Several genes involved in ED have been identified in recent years. The most common form of ED, the X-linked EDA, is caused by defects in ectodysplasin-1.5, 6 This soluble tumor necrosis factor ligand binds to the EDA receptor, triggering a signaling cascade through NF-κB to activate target gene expression.7, 8, 9 The EDA signaling pathway has been well established, but work is now just beginning to identify genes that are the targets of this pathway.10, 11, 12, 13, 14, 15 The genes responsible for a large number of EDs and associated disorders have yet to be identified.
Chromosomal abnormalities including microduplications and microdeletions have been found in a significant number (10–15%) of individuals with MR and developmental disabilities (MR/DD).16 It is plausible that the microdeletions and microduplications harbor gene(s) responsible for the observed phenotypes in these patients. Furthermore, analysis of patients with submicroscopic deletions/duplications has facilitated identification of small regions of overlap linked to specific phenotypic characteristics and subsequent isolation of the causative genes. Recently, disruption of the Eu-HMTase1 gene has been shown to be associated with the 9q34 subtelomeric deletion syndrome, and a duplication of the HSD17B10 and HUWE1 genes have been found in association with X-linked MR.17, 18
We have analyzed a patient who has mild ectodermal defects and a (1;6) (p22.1;p22.1) balanced translocation.19 Although it was speculated that one of the gene(s) affected by the translocation results in the patient's clinical features, no known ED gene was found at either translocation breakpoint. However, utilizing array CGH we identified two submicroscopic deletions at 2q12.2 and 6q22.3, which included the deletion of the ectodysplasin-A receptor (EDAR) gene at 2q12.2.
Materials and methods
Case report
CMS8770 has previously been reported. 19 In brief, this female patient was born at 37 weeks to non-consanguineous parents after an uncomplicated pregnancy. She had speech delay, not saying any words at 24 months of age. Examination at 6.5 years of age revealed mild MR with a full scale intelligence quotient (IQ) of 73, a verbal IQ of 57, and a performance IQ of 62 on the WISC-III.19 Her head circumference was below the 2nd centile and height and weight were at the 25th centile. She showed esotropia and hyperopia. Ectodermal features included absent of lower incisors and some permanent teeth, thin sparse, straight, and blond scalp hair, sparse eye lashes, poor nail growth, and dry skin with decreased sweating. The patient's karyotype was determined to be 46,XX,t(1;6) (p22.1;p22.1). Parents were reported to be clinically normal and had normal karyotypes.
Molecular cytogenetics
Genomic contigs, clones, markers, mapping, and sequence information were obtained from the NCBI databases (http://www.ncbi.nlm.nih.gov/ge nome/guide/human). BAC and PAC clones were obtained from commercial resources. DNA was isolated using Qiagen Mini-Prep columns and was labeled by incorporation of digoxigenin-11-dUTP or biotin-16-dUTP (Boehringer Mannheim) by nick translation using DNA polymerase (Life Technologies). For in situ hybridization, metaphase chromosome spreads were obtained from lymphoblastoid cells using conventional methods, and fluorescence in situ hybridization (FISH) was performed essentially as described previously.20, 21 Commercially available chromosome-specific labeled centromeric alphoid probes were used to identify specific chromosomes.
Quantitative real-time RT-PCR
Total RNA was extracted from lymphoblastoid cell lines using the GenElute™ Mammalian Total RNA Purification Kit (Sigma), according to the manufacturer's protocols. Samples were treated with TURBO™ DNase (Ambion Inc.) using 2 units/50 μg RNA at 37°C for 30 min. RNA was subsequently purified using the RNeasy Mini Kit (Qiagen), following the manufacturer's protocols.
Quantitative Real-Time PCR was performed for two genes, ABCD3 and ZNF184, using the iScript™ One-Step RT-PCR Kit with SYBR® Green (BioRad) on an iCycler iQ™ Real-Time PCR detection System (BioRad). The primer pair sequences are provided upon request.
Whole genome array CGH and quantitative PCR
Genomic DNA was purified using the Zymo DNA Clean & Concentrator™ kit (ZymoResearch, Orange, CA, USA). DNA concentration and purity was determined with an ND-1000 Spectrophotometer (NanoDrop Technologies, Delaware). DNA was labeled, hybridized to the Affymetrix GeneChip® Human Mapping 250K Nsp Array, and washed according to the manufacturer's commercial protocol (Affymetrix). Data quality assessment was generated using the DM (Dynamic Modeling) calling algorithm within the Affymetrix GTYPE software package. Intensity information and copy number status for each probe set was extracted using Affymetrix Genotyping Console™ Version 2.0 software.
Genomic quantitative PCR was performed using iQ SYBR Green Supermix (Bio-Rad) on an iCycler iQ real-time PCR detection system (Bio-Rad), as described previously.21
Results
Delineation of translocation breakpoint regions at 1p22. 1 and 6p22.1 and analysis of candidate genes for ED and MR
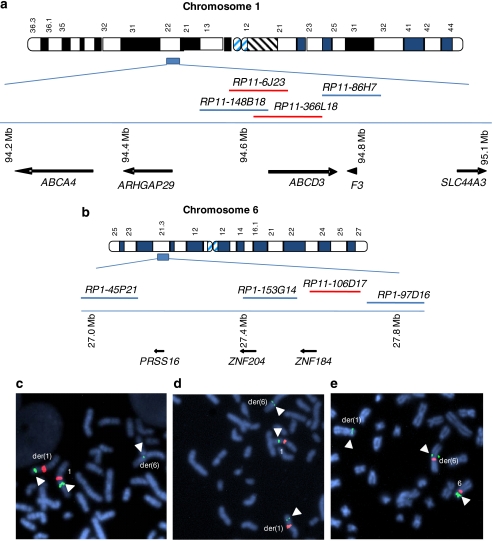
Using FISH, we localized the 1p22.1 breakpoint to within the clone RP11–366L18 (Figure 1a and c). Clones RP11–86H7, RP11–465K1, RP11–57H12, and RP11–146P11 mapped proximal and clone RP11–148B18 mapped distal to the 1p22.1 breakpoint. Subsequent analysis using the clone RP11–6J23 (Figure 1a and d), narrowed the 1p22 breakpoint to within a 60 kb region of overlap between clones RP11–6J23 and RP11–366L18 (Figure 1a). We mapped the 6p22.1 translocation breakpoint to a 140 kb region within clone RP11–106D17 (Figure 1b and e). Clones RP3–425P12, RP1–45P21, RP1–153G14 mapped distal and clones RP1–97D6, RP1–29K1, RP11–999A17, and RP11–150A6 mapped proximal to the 6p22 breakpoint.
Figure 1.
Delineation of translocation breakpoint regions and candidate genes. Physically mapped BAC clones used in FISH analysis of chromosome 1 (a) and chromosome 6 (b) are indicated. Clones spanning the breakpoint are shown in red. Candidate genes from the region flanking the breakpoint regions are noted with arrows showing direction of transcription. FISH analysis of the 1p22.1 breakpoint spanning clones RP11–366L18 (green) (c) and clone RP11–6J23 (green) (d). Chromosome 1 centromeric control probes are shown in red. FISH analysis of the 6p22.1 breakpoint spanning clone RP11–106D17 (green) (e). Chromosome 6 centromeric control probes are shown in red.
Five known annotated genes, F3, SLC44A3, ABCD3, ABCA4, and ARHGAP29, were identified within 800 kb of the 1p22.1 translocation breakpoint region (Figure 1a). The chromosome 6 breakpoint region is in a relatively gene poor region. There are two known Zinc-finger genes, ZNF184 and ZNF204 near the breakpoint region (Figure 1b). A hypothetical gene LOC645950 and the PRSS16 gene are located telomeric to the ZNF204 (Figure 1b and data not shown). Attempts to amplify the putative LOC645950 gene using primers designed from the EST databases were unsuccessful.
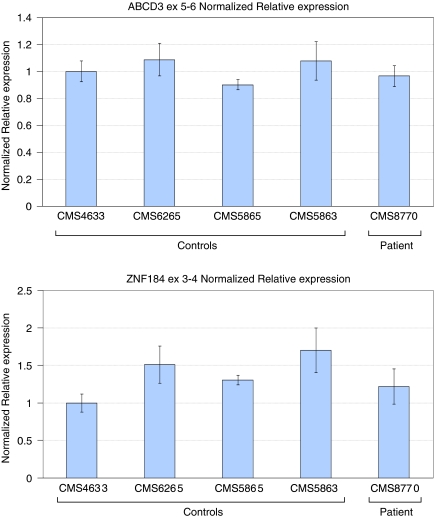
Candidate gene expression was measured by real-time RT-PCR. Four candidate genes could not be checked by this method because of no (ABCA4, ARHGAP29, and ZNF204) or limited (SLC44A3) expression in lymphoblastoid cell lines. The two genes that could be checked through real-time RT-PCR were ZNF184, the closest candidate gene to the chromosome 6 breakpoint region, and ABCD3, which partially overlaps with the telomeric side of the chromosome 1 breakpoint region. Real-time RT-PCR results for these genes are shown in Figure 2. Neither gene appears to have an altered transcript level in the patient as compared to controls.
Figure 2.
Normalized relative expression for ABCD3 and ZNF184. No significant difference in expression was seen between the patient, CMS8770, and four control individuals (CMS4633, CMS6265, CMS5865, and CMS5863) for either gene. Real-time RT-PCR was performed in triplicate. Data were analyzed using the iCycler iQ™ software to generate a standard curve for each gene and calculate the fluorescence generated from the experimental primer pairs relative to the reference gene RPII using the comparative Ct method.
To test for the presence of a chimeric transcript, we performed northern analysis on the patients RNA using a probe spanning exons 1–3 on ABCD3. The northern blot showed only the 4 kb band size of a normal ABCD3 transcript (data not shown).
Identification of two submicroscopic deletions, unrelated to the translocation, and deletion of the EDAR gene
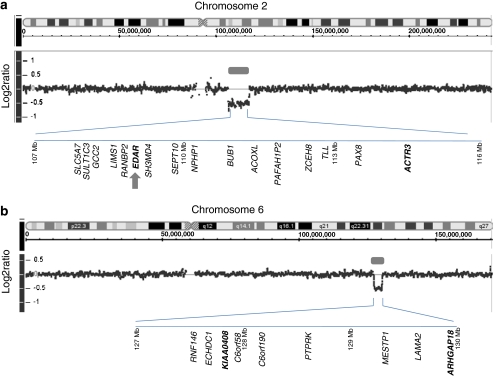
We analyzed DNA from cell lines for CMS8770 using an Human Mapping 250K Nsp Array and identified two submicroscopic deletions at 2q12.2q14.1 (106.5–117.3 Mb) and 6q22.3 (127.5–130 Mb) (Figure 3). We looked at the log2 ratio and corresponding negative Log10 P-values and determined the extent of the deletion in each case. We verified the deletions at 2q12.2q14.1 and 6q22.3 by real-time genomic PCR using DNA isolated from patient's blood (Figure 3 and data not shown). The deletion regions at 2q12.2 and 6q22.3 contain multiple annotated genes (Figure 3a and b). The 2q12.2 deletion contains a known ectodermal dysplasis gene, EDAR, and is therefore considered responsible for the ectodermal phenotype in this patient (Figure 3b).22 Furthermore, we confirmed the deletion of the EDAR gene using real-time genomic PCR (data not shown). The 6q22.3 deleted region contains several genes and could possible include a gene responsible for the neurodevelopmental defects in the patient (Figure 3b).
Figure 3.
Array CGH profiles of chromosome 2 (a) and chromosome 6 (b) in patient CMS8770 are shown. The location of deletion at 2q12.2 and 6q22.3 are indicated by gray bars. The log2 ratio of about −0.5 indicates the deletions are heterozygous. A representative gene content of the deletion is shown in each case. For a complete list of genes, see the gene content map of the corresponding region (NCBI). The deletions at 2q12.2q14.1 and 6q22.3 were further confirmed by real-time genomic PCR using DNA isolated from patient's blood. Genes used for real-time genomic PCR are indicated in bold types. The location of the EDAR gene on chromosome 2q12.2 is indicated by the gray arrow.
Discussion
This study has identified the translocation breakpoint critical regions in a patient with ED, mild MR, and a de novo t(1;6)(p22. 1;p22.1) translocation. The closest gene to the chromosome 1 breakpoint region is ABCD3. Real-time RT-PCR did not indicate changes in expression of this gene in lymphoblastoid cells from the patient and northern analysis failed to show the presence of a chimeric transcript resulting from the translocation. Taken together, these results suggest ABCD3 is not physically disrupted by the translocation.
There is no known gene present in the chromosome 6 translocation breakpoint region. This suggests that any deleterious effect of the translocation would likely be the result of a disruption in a distant transcriptional control site for genes flanking the translocation breakpoint region. As a result, expression of the target gene can be either upregulated or downregulated.
The two strong candidate genes that could be screened using mRNA derived from lymphoblastoid cell lines, ZNF184 and ABCD3, showed no change in expression compared to controls. The ABC family of transporters have been linked to such human diseases as retinopathies, cystic fibrosis, and cardiomyopathies.23 ABCD3 is a peroxisomal membrane protein involved in the biogenesis of peroxisomes and the transfer of long-chain acyl-CoA across peroxisomal membranes.24 The ABCD3 gene was found to be mutated in the biochemical disorder Zellweger syndrome; however, this association remains to be confirmed.25 Mutations in ABCA4 have been associated with a wide phenotypic spectrum.26 ABCA4 mutations are linked to Stargardt disease, the most common juvenile macular dystrophy, autosomal recessive cone-rod dystrophy, and retinitis pigmentosa.26, 27
There is no known ED gene located in either breakpoint region. Of all of the candidate genes identified in this study, ARHGAP29 is possibly the best candidate gene for the neurological findings in the patient if it is indeed affected by the translocation.28 ARHGAP29 is a GTPase which has been shown to regulate Rho activity.29 Rho proteins act as directors of cytoskeleton organization in dendrites, dentritic spines, and axons controlling the growth and development of these structures.28 Several members of this family of proteins have been implicated in MR.28 The PRSS16 gene encodes a serine protease similar to the PRSS12 autosomal MR gene30 and thus appears to be a good candidate. However, it has been reported to be expressed exclusively in the thymus.31
F3 is known to be integral member of the clotting pathway and is considered a poor candidate gene. Little is known about the other three candidate genes, ZNF184, ZNF204, and SLC44A3. Initial studies of ZNF184 expression show no difference in the patient and control, suggesting it is not affected by the translocation. SLC44A3 is a membrane protein that is predicted to act as a solute carrier and expressed at low levels in both skin and brain (UniGene Hs.483423).
We also examined the possibility that the ED or the MR seen in the patient is not the result of the translocation and is, in fact, the result of a separate cause. In several patients with apparently balanced translocations, additional chromosomal aberration(s) have been previously reported either at the breakpoint or at a remote location unrelated to the translocation.32 Whole genome array CGH analysis in this patient indeed revealed two unique submicroscopic deletions. The deletion at 2q12.2 included the EDAR gene. The EDAR gene has been previously reported to cause both dominant and recessive forms of EDs and thus is likely responsible for the ectodermal defects observed in patient CMS8770. We anticipate that the mild neurodevelopmental problems in the patient are likely due to a deletion of a second gene. A review of the deleted genes revealed several strong candidates for MR such as TLL and ACTR3 at 2q12.2 and ARHGAP18 at 6q22.3. However, the mild neurodevelopmental problems due of the combined haploinsufficiency of a number of genes remain a possibility. Identification of mutations or other overlapping deletions in additional MR patients affecting the candidate genes will help pinpoint the molecular cause of the neurodevelopment phenotype seen in this patient. Our finding also signifies the utility of whole genome scan in cases where no genes are found to be affected by the de novo translocation breakpoints.
Acknowledgments
We are grateful to the patient and the parents for participation in this study. We thank Cindy Skinner for assistance in the collection of patient material and Rachel Griggs for technical assistance. This study was approved by the Institutional Review Board of Self Regional Healthcare, the IRB of record for the GGC and was supported by a grant from NICHD (R01 HD39331) to AKS.
References
- Ropers HH. X-linked mental retardation: many genes for a complex disorder. Curr Opin Genet Dev. 2006;16:260–269. doi: 10.1016/j.gde.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Stevenson RE. Advances in X-linked mental retardation. Curr Opin Pediatr. 2005;17:720–724. doi: 10.1097/01.mop.0000184290.57525.fb. [DOI] [PubMed] [Google Scholar]
- Colleaux L, Rio M, Heuertz S, et al. A novel automated strategy for screening cryptic telomeric rearrangements in children with idiopathic mental retardation. Eur J Hum Genet. 2001;9:319–327. doi: 10.1038/sj.ejhg.5200591. [DOI] [PubMed] [Google Scholar]
- Anderlid BM, Schoumans J, Anneren G, et al. Subtelomeric rearrangements detected in patients with idiopathic mental retardation. Am J Med Genet. 2002;107:275–284. doi: 10.1002/ajmg.10029. [DOI] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Montonen O, Saarialho-Kere U, et al. Fine mapping of the EDA gene: a translocation breakpoint is associated with a CpG island that is transcribed. Am J Hum Genet. 1996;58:126–132. [PMC free article] [PubMed] [Google Scholar]
- Ezer S, Bayes M, Elomaa O, Schlessinger D, Kere J. Ectodysplasin is a collagenous trimeric type II membrane protein with a tumor necrosis factor-like domain and co-localizes with cytoskeletal structures at lateral and apical surfaces of cells. Hum Mol Genet. 1999;8:2079–2086. doi: 10.1093/hmg/8.11.2079. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Doffinger R, Smahi A, Bessia C, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–1057. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- Nishioka E, Tanaka T, Yoshida H, et al. Mucosal addressin cell adhesion molecule 1 plays an unexpected role in the development of mouse guard hair. J Invest Dermatol. 2002;119:632–638. doi: 10.1046/j.1523-1747.2002.01851.x. [DOI] [PubMed] [Google Scholar]
- Cui CY, Hashimoto T, Grivennikov SI, Piao Y, Nedospasov SA, Schlessinger D. Ectodysplasin regulates the lymphotoxin-beta pathway for hair differentiation. Proc Natl Acad Sci USA. 2006;103:9142–9147. doi: 10.1073/pnas.0509678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Durmowicz MC, Cui CY, Schlessinger D. The EDA gene is a target of, but does not regulate Wnt signaling. Gene. 2002;285:203–211. doi: 10.1016/s0378-1119(02)00407-9. [DOI] [PubMed] [Google Scholar]
- Cui CY, Schlessinger D. EDA signaling and skin appendage development. Cell Cycle. 2006;5:2477–2483. doi: 10.4161/cc.5.21.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries BB, Winter R, Schinzel A, van Ravenswaaij-Arts C. Telomeres: a diagnosis at the end of the chromosomes. J Med Genet. 2003;40:385–398. doi: 10.1136/jmg.40.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, Smidt M, Banning MJ, et al. Disruption of the gene euchromatin histone methyl transferase1 (Eu-HMTase1) is associated with the 9q34 subtelomeric deletion syndrome. J Med Genet. 2005;42:299–306. doi: 10.1136/jmg.2004.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froyen G, Corbett M, Vandewalle J, et al. Submicroscopic duplications of the hydroxysteroid dehydrogenase HSD17B10 and the E3 ubiquitin ligase HUWE1 are associated with mental retardation. Am J Hum Genet. 2008;82:432–443. doi: 10.1016/j.ajhg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamoah A, Decker AB, Wiktor A, Van Dyke DL. Child with de novo t(1;6)(p22.1;p22.1) translocation and features of ectodermal dysplasia with hypodontia and developmental delay. Am J Med Genet A. 2003;118:82–85. doi: 10.1002/ajmg.a.10929. [DOI] [PubMed] [Google Scholar]
- Vervoort V, Beachmen M, Edwards P, et al. AGTR2 mutations in X-linked mental retardation. Science. 2002;296:2401–2403. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- Griggs BL, Ladd S, Saul RA, DuPont BR, Srivastava AK. Dedicator of cytokinesis 8 is disrupted in two patients with mental retardation and developmental disabilities. Genomics. 2008;91:195–202. doi: 10.1016/j.ygeno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- Schuierer MM, Langmann T. Molecular diagnosis of ATP-binding cassette transporter-related diseases. Expert Rev Mol Diagn. 2005;5:755–767. doi: 10.1586/14737159.5.5.755. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Aihara K, Takano T, et al. Characterization of the 70-kDa peroxisomal membrane protein, an ATP binding cassette transporter. J Biol Chem. 1999;274:11968–11976. doi: 10.1074/jbc.274.17.11968. [DOI] [PubMed] [Google Scholar]
- Paton BC, Heron SE, Nelson PV, Morris CP, Poulos A. Absence of mutations raises doubts about the role of the 70-kD peroxisomal membrane protein in zellweger syndrome. Am J Hum Genet. 1997;60:1535–1539. doi: 10.1016/S0002-9297(07)64247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde D, Riveiro-Alvarez R, Aguirre-Lamban J, et al. Spectrum of the ABCA4 gene mutations implicated in severe retinopathies in Spanish patients. Invest Ophthalmol Vis Sci. 2007;48:985–990. doi: 10.1167/iovs.06-0307. [DOI] [PubMed] [Google Scholar]
- Riveiro-Alvarez R, Valverde D, Lorda-Sanchez I, et al. Partial paternal uniparental disomy (UPD) of chromosome 1 in a patient with stargardt disease. Mol Vis. 2007;13:96–101. [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Rho GTPases: role in dendrite and axonal growth, mental retardation, and axonal regeneration. Neurology. 2007;68:1315–1318. doi: 10.1212/01.wnl.0000259588.97409.8f. [DOI] [PubMed] [Google Scholar]
- Saras J, Franzen P, Aspenstrom P, Hellman U, Gonez LJ, Heldin CH. A novel GTPase-activating protein for Rho interacts with a PDZ domain of the protein-tyrosine phosphatase PTPL1. J Biol Chem. 1997;272:24333–24338. doi: 10.1074/jbc.272.39.24333. [DOI] [PubMed] [Google Scholar]
- Molinari F, Rio M, Meskenaite V, et al. Truncating neurotrypsin mutation in autosomal recessive nonsyndromic mental retardation. Science. 2002;298:1779–1781. doi: 10.1126/science.1076521. [DOI] [PubMed] [Google Scholar]
- Cheunsuk S, Sparks R, Noveroske JK, et al. Expression, genomic structure and mapping of the thymus specific protease prss16: a candidate gene for insulin dependent diabetes mellitus susceptibility. J Autoimmun. 2002;18:311–316. doi: 10.1006/jaut.2002.0593. [DOI] [PubMed] [Google Scholar]
- Gribble SM, Prigmore E, Burford DC, et al. The complex nature of constitutional de novo apparently balanced translocations in patients presenting with abnormal phenotypes. J Med Genet. 2005;42:8–16. doi: 10.1136/jmg.2004.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]