Abstract
Systemic and intrathecal methotrexate (MTX) are integral components of acute lymphoblastic leukemia (ALL) therapy, but can be associated with neurotoxicity. We describe here the case of an adolescent male with T-cell ALL who developed recurrent episodes of subacute neurotoxicity characterized by slurred speech, emotional lability, and hemiparesis after intrathecal MTX administration. Serial magnetic resonance imaging with diffusion-weighted imaging showed recurrent areas of restricted diffusion within cerebral hemispheric white matter, which correlated chronologically with the administration of intrathecal therapy and severity of clinical symptoms. Resolution of diffusion abnormalities did not preclude further toxicity and a large lesion could cause persisting symptoms.
Keywords: methotrexate, neurotoxicity, leukemia, diffusion-weighted imaging, intrathecal therapy, magnetic resonance imaging
INTRODUCTION
Methotrexate (MTX) was among the first drugs discovered to have significant activity against leukemia [1] and remains an integral component of treatment for acute lymphoblastic leukemia (ALL). Intravenous (IV) and intrathecal (IT) MTX have largely replaced cranial irradiation for treatment and prophylaxis of central nervous system (CNS) leukemia, which can cause neuropsychologic side effects, growth retardation, and second malignancies [2]. However, MTX can be associated with acute, subacute, and chronic neurotoxicities ranging from asymptomatic white matter changes to severe CNS demyelination [3-6].
Subacute neurotoxicity usually develops 5−14 days after administering IT- or high-dose IV-MTX, or both, and manifests as headache, altered mental status, aphasia, weakness, hemiparesis, or seizures. Diffusion-weighted imaging has been useful to diagnose subacute neurotoxicity by detecting areas of myelinopathy before such lesions may become conspicuous with other conventional magnetic resonance imaging (MRI) sequences [7-9]. We report the serial neurologic and MRI findings of a patient with T-cell ALL who was imaged for recurrent neurotoxicity after IT-MTX.
CASE REPORT
The patient is a 16-year-old male diagnosed with T-cell ALL after presenting with a leukocyte count of 211.3×109/L (92% blasts). Cerebrospinal fluid showed 2/mm3 leukocyte with 80% blasts (CNS status 2). He was enrolled on the St. Jude Total XVI protocol and received induction chemotherapy with prednisone (40 mg/m2/day on days 1−28), vincristine (1.5 mg/m2 on days 1, 8, 15, and 22), daunorubicin (25 mg/m2 on days 1 and 8), polyethylene glycol (PEG)–asparaginase (3000 units/m2 on days 3 and 16), cyclophosphamide (1000 mg/m2 on day 22), cytarabine (75 mg/m2 on days 22−25 and 29−32), and thioguanine (60 mg/m2 per day on days 22−35). IT chemotherapy (MTX 12 mg, hydrocortisone 24 mg, and cytarabine 36 mg) was given twice weekly. Leucovorin (5 mg) was given at hours 24 and 30 after each IT. On day 15 of induction, bone marrow minimal residual disease was 1% and CSF was clear.
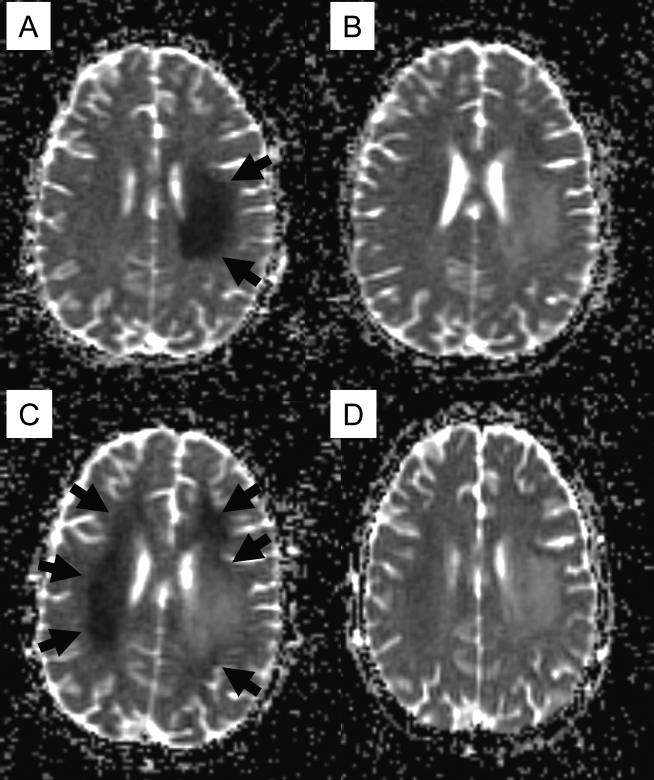
On day 20 of induction therapy (5 days after the fifth dose of IT therapy and 4 days after PEG–asparaginase), he complained of right-handed clumsiness, and the following morning noted marked weakness on his right side. On the neurologic exam, he was awake and alert with emotional lability. Speech was moderately dysarthric without aphasia. He had right facial weakness (forehead spared) and right hemiplegia. Sensation to touch, temperature, and vibration was intact. Conventional MRI showed subtle signal changes within left centrum semiovale, while an obviously abnormal area of restricted diffusion was demonstrated in diffusion-weighted imaging (Fig. 1A), and there was no evidence of cerebral thrombosis. Proton MR spectroscopy showed elevation of the choline peak within the lesion. The patient received aminophylline (5 mg/kg every 6 hours) and leucovorin (5 mg/m2 twice daily), and his symptoms improved over 7−10 days. He was able to walk with assistance and his speech became clearer. On follow-up MRI the area of restricted diffusion within the left hemisphere improved (Fig. 1B).
Fig. 1.
Diffusion-weighted imaging (apparent diffusion coefficient map) of the brain at the onset of subacute methotrexate (MTX) neurotoxicity (A), resolution of the initial episode (B), recurrent neurotoxicity after rechallenge with intrathecal MTX (C) and resolution of recurrent episode (D). Areas of restricted diffusion are shown in arrows.
Because the patient showed improving neurologic and imaging findings, a sixth dose of IT therapy (MTX and hydrocortisone without cytarabine) with prophylactic aminophylline and leucovorin was administered 22 days after onset of the first neurologic symptoms. Three days after this administration, he presented with severe headache. His neurologic exam and another MRI were unchanged. Eight days after this IT therapy he developed transient left ptosis, worsening dysarthria, and intermittent left hemiparesis. These symptoms waxed and waned, and resolved over 72 hours. The onset of new focal neurological deficit prompted another MRI study, which showed a new area of restricted diffusion in the right centrum semiovale and bilateral frontal lobes as well as in the periphery of previously identified lesions in the left centrum semiovale (Fig. 1C).
Owing to these recurrent episodes, MTX was discontinued from the patient's therapy. At the end of induction, his bone marrow had no detectable minimal residual disease and spinal fluid was clear. Although his neurologic symptoms have improved, mild right hemiparesis persists. A repeat MRI, 2 months after the initial onset of neurotoxicity, showed stable bilateral leukoencephalopathy greater on the left side. Increased diffusion was seen in initial and recurrent lesions (Fig. 1D), and MR spectroscopy again showed marked elevation of choline.
DISCUSSION
Subacute neurotoxicity has been reported in 3−15% of patients after MTX administration and is associated with increased cumulative exposure, route of administration (IT and IV), age (older than 10 years) and a high methotrexate:leucovorin ratio [3,10,11]. Some patients have been successfully rechallenged with MTX after resolution of neurologic symptoms, but neurotoxicity can recur in 10−56% of patients [3,4,12].
The pathophysiology of MTX neurotoxicity remains unclear. MTX inhibits dihydrofolate reductase, which can increase adenosine and homocysteine [13]. Adenosine can dilate cerebral blood vessels, slow the release of neurotransmitters at presynaptic junctions, modify postsynaptic response, and slow the discharge rate of neurons [13]. The plasma homocysteine level of our patient was normal.
MTX neurotoxicity is often treated with aminophylline (2−5 mg/kg), a competitive antagonist of adenosine. Aminophylline completely resolved neurotoxic symptoms in 4 of 6 patients in one study [14]. MTX-related neurotoxicity is also reduced by administering leucovorin 24−36 hours after MTX [15]. We treated our patient with leucovorin after each intrathecal therapy and aminophylline before or after the sixth dose of IT-MTX but without a favorable response.
Diffusion-weighted imaging provides image contrast that depends on the molecular motion of water, which is normally anisotropic within white matter structures. Loss of anisotropy with restricted water diffusion may be encountered in various pathological conditions, including cytotoxic and intramyelinic edema. Cytotoxic edema secondary to acute ischemic brain lesions is caused by translocation of water into the intracellular compartment, where proton mobility is reduced [16]. Myelinotoxicity may manifest histologically with intramyelinic vacuolation with resultant apparent restriction of water diffusion. After restricted diffusion in left centrum semiovale and right hemiparesis improved, we administered another dose of IT-MTX. Unfortunately, this exacerbated the neurologic findings and MRI detected a recurrence of restricted diffusion with new involvement of the contralateral hemisphere, forcing us to discontinue MTX administration.
Although T2-weighted images and fluid attenuated inversion recovery are often normal, diffusion-weighted imaging appears to be the most sensitive imaging modality at the onset of subacute MTX neurotoxicity [7,8]. In our patient, proton MR spectroscopy showed elevated choline peak within the lesion areas. Choline is believed to be an indicator of membrane turnover, and may be elevated in pathological conditions characterized by myelin breakdown [17]. Significant increase in choline has been reported at 20 weeks after high-dose IV-MTX treatment, suggesting that MR spectroscopy is another sensitive method to monitor the toxic effect of MTX in the brain [17]. Although most clinical symptoms and imaging abnormalities of MTX subacute encephalopathy are often transient [3,4,7,12], a persistent large area of T2 abnormality and an elevated choline levels suggest long-term and irreversible structural sequelae in this patient.
Our patient is currently on combination chemotherapy with IT hydrocortisone plus cytarabine (without IV- or IT-MTX). Whereas the efficacy of IT-MTX alone or in combination with hydrocortisone and cytarabine for the prophylaxis and treatment of CNS disease is well recognized [2], the efficacy of IT hydrocortisone plus cytarabine without MTX is unknown. Nevertheless, we have continued to omit IT-MTX to avoid the potential devastating neurotoxicity from its continuous use. For future CNS prophylaxis, IT liposomal cytarabine [18], IV thiotepa [19], or cranial irradiation may be considered after MRI and neurologic findings have stabilized.
ACKNOWLEDGEMENTS
We acknowledge the expertise of Dr. Vani Shanker in editorial review of the manuscript.
Supported in part by Cancer Center Core grant CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities. Dr. Ching-Hon Pui is an American Cancer Society Professor.
REFERENCES
- 1.FARBER S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood. 1949;4:160–167. [PubMed] [Google Scholar]
- 2.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Relling MV, Harrison PL, et al. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. 1998;12:1176–1181. doi: 10.1038/sj.leu.2401098. [DOI] [PubMed] [Google Scholar]
- 4.Walker RW, Allen JC, Rosen G, Caparros B. Transient cerebral dysfunction secondary to high-dose methotrexate. J Clin Oncol. 1986;4:1845–1850. doi: 10.1200/JCO.1986.4.12.1845. [DOI] [PubMed] [Google Scholar]
- 5.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26:1263–1269. [PMC free article] [PubMed] [Google Scholar]
- 6.Shuper A, Stark B, Kornreich L, et al. Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. J Child Neurol. 2000;15:573–580. doi: 10.1177/088307380001500902. [DOI] [PubMed] [Google Scholar]
- 7.Inaba H, Khan RB, Laningham FH, et al. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008;19:178–184. doi: 10.1093/annonc/mdm466. [DOI] [PubMed] [Google Scholar]
- 8.Rollins N, Winick N, Bash R, Booth T. Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcome. AJNR Am J Neuroradiol. 2004;25:1688–1695. [PMC free article] [PubMed] [Google Scholar]
- 9.Haykin ME, Gorman M, van Hoff J, et al. Diffusion-weighted MRI correlates of subacute methotrexate-related neurotoxicity. J Neurooncol. 2006;76:153–157. doi: 10.1007/s11060-005-4569-2. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney DH, Jr., Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 11.Dufourg MN, Landman-Parker J, Auclerc MF, et al. Age and high-dose methotrexate are associated to clinical acute encephalopathy in FRALLE 93 trial for acute lymphoblastic leukemia in children. Leukemia. 2007;21:238–247. doi: 10.1038/sj.leu.2404495. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe N, Takaue Y, Anzai T, Robertson R. Transient neurologic disturbances induced by high-dose methotrexate treatment. Cancer. 1985;56:1356–1360. doi: 10.1002/1097-0142(19850915)56:6<1356::aid-cncr2820560623>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernini JC, Fort DW, Griener JC, et al. Aminophylline for methotrexate-induced neurotoxicity. Lancet. 1995;345:544–547. doi: 10.1016/s0140-6736(95)90464-6. [DOI] [PubMed] [Google Scholar]
- 15.Winick NJ, Bowman WP, Kamen BA, et al. Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst. 1992;84:252–256. doi: 10.1093/jnci/84.4.252. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331–345. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 17.Chu WC, Chik KW, Chan YL, et al. White matter and cerebral metabolite changes in children undergoing treatment for acute lymphoblastic leukemia: longitudinal study with MR imaging and 1H MR spectroscopy. Radiology. 2003;229:659–669. doi: 10.1148/radiol.2293021550. [DOI] [PubMed] [Google Scholar]
- 18.Pui CH. Toward optimal use of intrathecal liposomal cytarabine. Leuk Lymphoma. 2007;48:1672–1673. doi: 10.1080/10428190701573687. [DOI] [PubMed] [Google Scholar]
- 19.Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006;24:3142–3149. doi: 10.1200/JCO.2005.03.3373. [DOI] [PubMed] [Google Scholar]