Abstract
Psychostimulant drug experience leads not only to long-lasting changes in behavior but also modifications in the activity and morphology of pyramidal neurons in the medial prefrontal cortex. The objective of the present study was to establish whether repeated treatment of rats with amphetamine is accompanied by changes in the pattern or types of synapses in the medial prefrontal cortex (mPFC) and, specifically, onto neurons that project to the lateral hypothalamus, where our earlier work has shown increased markers of neuronal activity after repeated amphetamine treatment (Morshedi and Meredith, 2008). Rats were treated with a behaviorally sensitizing regimen of amphetamine, following which synapses in the infralimbic and prelimbic cortices of the mPFC, were analyzed with unbiased stereology (physical disector and electron microscopy). All synapses were counted and their targets identified by standard methodological criteria. Repeated amphetamine administration was associated with a significant increase in the number of asymmetric axospinous synapses, no change in axodendritic or axosomatic contacts, and no change in the total number of synapses on corticolateral hypothalamic pyramidal neurons compared to vehicle-treated rats. Therefore, behavioral sensitization as a result of repeated exposure to amphetamine is accompanied by the increased formation of spine, but not dendritic, synapses onto pyramidal neurons in the medial prefrontal cortex.
Keywords: physical disector, synapse, psychostimulant, addiction, behavioral sensitization
INTRODUCTION
Persistent and enhanced changes in locomotion (behavioral sensitization) associated with the repeated administration of psychostimulants may stem from the reorganization and strengthening of specific synaptic circuits (Robinson and Kolb, 2004). Modifications to synaptic strength have been predicted for neurons in the medial prefrontal cortex (mPFC; Lu and Wolf, 1999; Peterson et al., 2000), possibly through alterations in glutamate receptor trafficking and expression, characteristic of synaptic plasticity (Lu and Wolf, 1999; Overton et al., 2000; Sun et al., 2005). Synaptic plasticity is also generally accompanied by changes in synapse morphology or arrangement (Ganeshina et al., 2004; Geinisman, 1993; Geinisman et al., 1993; Geinisman et al., 1996b; Toni et al., 1999; but see Luscher et al., 2000) so synaptic sprouting or remodeling may be characteristic of the sensitized state.
In 1989, Uranova and colleagues found that the repeated administration of amphetamine (AMPH) to rats led to a large increase in synaptic contacts made onto the dendrites of mPFC neurons and a large decrease in the synaptic contacts made onto spine necks. These findings were not supported by the work by Robinson and Kolb (1999), who demonstrated significant increases in both dendritic length and spine density after repeated AMPH administration, nor by Morrow et al. (2007), who showed an increase in the number of spine synapses following prenatal cocaine treatment.
The studies by Robinson and Kolb (1997, 1999) employed a well characterized model of behavioral sensitization that involved treatment followed by withdrawal from AMPH for several weeks. The morphological changes that they describe are assumed to be due to the drug's general reorganization of dopaminergic and perhaps glutamatergic inputs to the mPFC (Robinson and Kolb, 1997, 1999). However, their studies did not explore whether the structural changes translated into increased numbers or a reorganization of synapses nor did they examine the contribution that may be made by morphological change to any given neural circuit.
We have recently provided evidence that AMPH treatment will induce Fos in the mPFC pyramidal neurons that project to the lateral hypothalamus (LH), but not in those sending axons to other components in the reward circuitry (Morshedi and Meredith, 2008). Fos activation has been linked to dendritic spine plasticity (e.g. Chen and Hillman, 1992), and so we questioned whether the synaptic complement of these particular projection neurons would be altered by this repeated AMPH protocol. Thus, the object of the present study was to test, at the ultrastructural level, using the principles of unbiased stereology (Day et al., 2006; Geinisman et al., 1996a; Gundersen, 1977; Ingham et al., 1998; Morrow et al., 2007; Sterio, 1984), whether repeated AMPH administration would result in changes in the numerical density and absolute number of synaptic contacts in the prelimbic and infralimbic areas of the mPFC and whether such changes would preferentially occur at synapses on the mPFC-LH projection neurons in these regions.
MATERIALS AND METHODS
Animals, drugs, and behavior
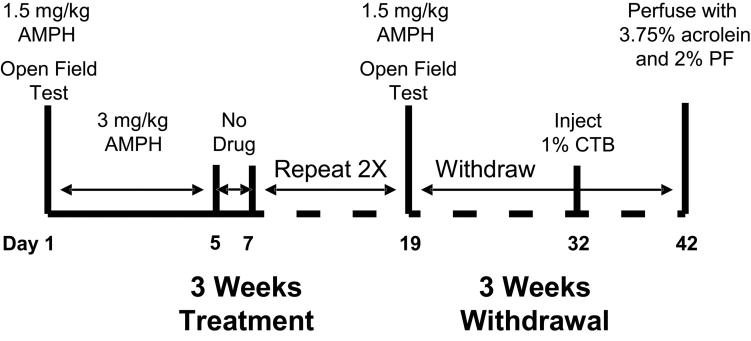
The protocols were conducted in accordance with the National Institutes of Health, “Guidelines for the Humane Care and Use of Laboratory Animals” (NIH Publications No. 80-23) and were approved by in-house Institutional Animal Care and Use Committees at the Rosalind Franklin University of Medicine and Science. Eight male Sprague-Dawley rats (Harlan, Indianapolis, IN), initially weighing 125-139 g, were housed 4 to a cage, on a 12 h light-dark cycle (lights on at 0700 h), with food and water available ad libitum. They were handled for 2 days prior to the start of experiments to minimize handling stress during treatment. Figure 1 illustrates the experimental design, which has been previously shown to produce robust locomotor sensitization (Morshedi and Meredith, 2007, 2008; Robinson and Kolb, 1999). One group of rats (n = 4) received repeated d-AMPH sulfate (AMPH in 0.9% saline, i.p.) injections: 3 mg/kg for 5 days, except for the first and last day when they received 1.5 mg/kg, followed by 2 drug-free days and repeated for 3 weeks, and the second group of rats was administered repeated saline injections (vehicle in the same volume) following the same procedure as that in the first group. On the first and last injection days (day 1 and day 19), locomotor activity in the open field was measured immediately after the injection of either AMPH or saline. On all other injection days, animals were returned to their home cage immediately after the AMPH or saline injection. All injections were administered at the same time each day. Ten days following the injection period, all rats were anesthetized (see below) and given stereotaxically-placed injections of the retrograde tracer, cholera toxin, subunit B (CTB), into the LH and were then euthanized a further 10 days later (Fig. 1).
Fig. 1.
Diagram of the AMPH treatment protocol. Vehicle-treated animals were handled and treated in the same manner. Abbreviations: AMPH, amphetamine; CTB cholera toxin subunit B; PF, paraformaldehyde (n = 4 per group)
Surgical and perfusion procedures
Rats were anesthetized for surgery with equithesin (3 mL/kg, i.p.: 1% sodium pentobarbital, 4.25% chloral hydrate in 10% ethanol) and secured in a Stoelting stereotaxic apparatus. A Hamilton syringe (25G; Hamilton Company, Reno, NV) filled with 1% CTB (#104, List Biological Labs, Campbell, CA) in double distilled H2O was positioned using stereotaxic coordinates taken from a rat brain atlas (Paxinos and Watson, 2005) and aimed at the LH (relative to bregma: anterior-posterior [AP]: −2.9 mm, medial-lateral [ML]: −1.5 mm; relative to skull: dorsal-ventral [DV]: −8.1 mm). CTB extensively fills dendrites and spines (Meredith, 1995) and, therefore, was selected as our retrograde tracer of choice for these ultrastructural studies. The tracer was pressure injected for 10 min at a rate of 10 nl/min (100 nl total volume) and the syringe left in place for 10 min to minimize leakage along the injection tract during removal.
At the conclusion of the experiment, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.1M phosphate buffered saline, followed by 50 ml of 3.75% acrolein in buffered 2% paraformaldehyde (PF), pH 7.4, and then by 200 ml of buffered 2% PF alone (Leranth and Pickel, 1989). All solutions were made at the same time and the perfusions were of uniform duration. The brains were removed, post-fixed for 30 min in buffered 2% PF, and cut sagittally to separate the hemispheres. The hemisphere ipsilateral to the injection site was then cut through the mPFC and the CTB injection site in the LH on a Vibratome (The Vibratome Co., St. Louis, MO) into coronal sections (30 μm) that were collected serially into 24-well cell culture plates filled with 0.1M phosphate buffer (PB).
Immunohistochemistry and processing for the electron microscope (EM)
In a pilot study using sample tissue, the concentrations of antisera, normal sera, and Triton X-100 (Tx) were titrated for each of the immunohistochemical reactions in order to determine the optimal concentrations that would preserve the ultrastructure for the EM.
The sections from the experimental animals were processed on the same day and the solutions for each antibody reaction were prepared as a single batch. Sections were incubated in 1% sodium borohydride in 0.1M PB for 30 min then rinsed extensively before being blocked for 1 h in 3% normal serum and 0.05% Tx, and then incubated for 72 h at 4°C in goat anti-CTB (1:5000; #703, List Biological Labs) with 3% normal serum and 0.05% Tx. They were then incubated for 90 min in biotinylated horse anti-goat IgG (1:200; Vector Laboratories) with 3% normal serum and 0.05% Tx at room temperature (RT), followed by 1 h in avidin-biotin complex (ABC). The sections were then reacted with 0.05% nickel-enhanced 3,3′-diaminobenzidine with 0.01% H2O2 (DAB-Ni) and rinsed thoroughly before being processed for the EM.
Sections were fixed in 2% osmium tetroxide (diluted in 0.1M PB) for 30 min at room temperature in the dark, dehydrated, and flat embedded in resin (25:15:55 Embed 812:Araldite 502:dodecenyl succinic anhydride; Electron Microscopy Sciences, Hatfield, PA) between two sheets of aclar fluorohalocarbon film. The embedding resin was allowed to polymerize at 60°C for 72 hours. Blocks were cut with an ultramicrotome (EM UC6, Leica Microsystems Inc., Bannockburn, IL) into ultrathin sections (silver, ∼60-70 nm), mounted on formvar-coated copper slot grids (Electron Microscopy Sciences), and stained with uranyl acetate and lead citrate prior to stereological analysis.
Quantitative analysis
We counted synapses using the physical disector in a specific area of the mPFC that was defined by unambiguous anatomical boundaries (Gabbott et al., 2005). We established the anterior boundary at +4.3 mm from bregma and the posterior boundary at +2.2 mm from bregma. Since the anterior-posterior extent of these mPFC regions measures 1500 μm, we collected 50 serial, thick sections (30 μm each) through each mPFC. A random number was generated for the first section of each mPFC, and then every 5th section was collected, so that 10 equally spaced sections were selected for analysis (Fig. 2; Day et al., 2006; Howard and Reed, 1998; Ingham et al., 1998; Ismail and Bedi, 2007). We then rotated the 10 sections so that their medial walls were aligned along the dorsoventral axis. Each section was then outlined on paper and a small reference point, referred to as a tangential reference point (Fig. 2), was placed at the top left-hand tip of the section (see Gabbott et al., 2005 for details). The dorsal boundary of the prelimbic area was then established by measuring a set distance ventrally from the tangential reference point on each section; the ventral boundary was likewise established according to set distances from the reference point on each section (Gabbott et al., 2005). Boundaries were also established for the adjacent series of 10 sections that were collected so that the volume of the mPFC could be estimated using the Cavalieri method. This set of sections was mounted onto poly-L-lysine slides from 0.05M Tris-HCl buffer, dried at 60°C overnight, stained with cresyl violet, dehydrated in an ascending series of alcohols, cleared in xylene, and coverslipped. The volume of the mPFC was estimated using a Nikon E400 microscope equipped with a motorized stage in 3 axes, a video camera, and StereoInvestigator software (MicroBrightField, Inc; Williston, VT). The mPFC (prelimbic and infralimbic regions) was outlined on each section and the reference volume was calculated as (Vref in μm3):
where a is the mean area of this part of the mPFC, t is the thickness of the Vibratome sections (30μm) and s is the total number of sections through the defined region of the mPFC (50 sections).
Fig. 2.
Diagram of coronal sections through the medial prefrontal cortex (mPFC) used for stereological analysis. Stereotaxic maps were reproduced, with permission from Elsevier Science, from the brain atlas of Paxinos and Watson (2005). The section farthest to the left is shown with the tangential reference point in the upper left corner. Measurements from this point, along the dorsoventral axis, were made to establish the dorsal boundary of the prelimbic cortex and the ventral boundary of the prelimbic or infralimbic cortices. The black boxes represent the 0.5 mm × 0.5 mm samples taken for estimation of the total number of synapses and synaptic density. The boxes overlie the prelimbic and infralimbic parts of the mPFC.
The physical disector is a 3-dimensional stereological technique that allows the counting of objects (synapses in this case) from a pair of disector sections (ultramicrotome sections in this study), which can be viewed simultaneously (see Braengaard and Gundersen, 1986 and Mouton, 2002 for reviews). The sections are a known distance apart and the distance between sections is 30% of the average projected height of the objects (synapses) that are to be counted (Howard and Reed, 1998; Ingham et al., 1998). The sampling strategy for the physical disector is generally referred to as a systematic uniform random sampling procedure (Braengaard and Gundersen, 1986; Howard and Reed, 1998). The analysis requires that reference and lookup sections are obtained from ultrathin sections cut from samples, taken randomly from the thick (30μm) sections for each animal (for details of this systematic random selection method, see Braendgaard and Gunderson, 1986). We used a random number generator to generate x and y coordinates, and a rotational value, to create a scaled square (equivalent to 0.5 mm × 0.5 mm) on paper. That square was first dropped onto an atlas image (Fig. 2) and then placed onto the corresponding area of the selected, embedded section. A piece of tissue, the size of the scaled square, was then cut out of the section, mounted on a block with resin, and resectioned with the ultramicrotome. As figure 2 illustrates, each sample had an equal chance of being in the reference volume, as required for disector analysis (Coggeshall, 1992). Ultrathin serial sections (∼10 per grid) were examined with a JEOL 1230 electron microscope (JEOL, Peabody, MA). All EM images were captured with a Hamamatsu ORCA HR CCD camera (AMT XR-60 imaging system, Danvers, MA) at 25,000X magnification. Physical disectors were collected using a systematic random sampling method, whereby each pair of micrographs was spaced 2 widths of the EM screen from the previous pair (Day et al., 2006; Hunter and Stewart, 1993; Ingham et al., 1998), and the analysis was performed on 15 pairs of electron micrographs per block.
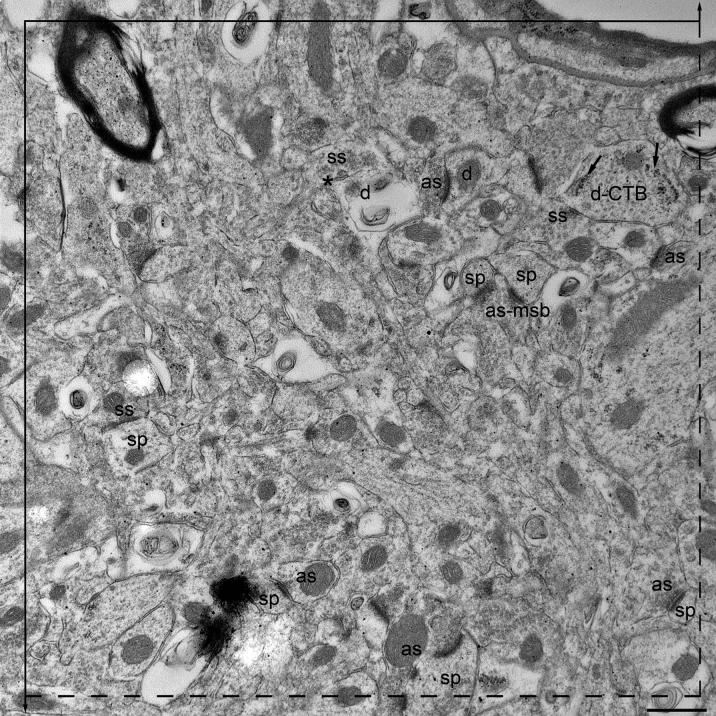
We analyzed 90 disectors for each animal per treatment group (total of 360 disectors per group). The absolute number of synapses was estimated using a disector height of 60 nm (Day et al., 2006; Ingham et al., 1998) and a rectangular unbiased counting frame (27.33 μm2), containing 2 inclusion (dashed) and 2 exclusion (solid, extending above and below the square to infinity) lines, which was placed over each electron micrograph in the pair (Figs. 3A-B). Synapses that fell within the counting frame or on the inclusion, but not on the exclusion, lines were counted. We counted “tops” of synapses in each pair of ultrathin sections. The synapse “top” was counted in the reference section when it did not appear in the “look-up” section and vice versa (Figs. 3A-B). We counted from 218-247 synapse “tops” per animal. All structures, such as myelinated fibers, cell somata, and blood vessels were included in the analysis, but regions obscured by folds or contamination, were avoided. Slides, sections, and grids were always coded so that the investigator was blind to the treatment groups.
Figure 3A-B.
A pair of electron micrographs, taken from serial sections through the mPFC, in which synapses within the unbiased counting frame are labeled. Synaptic contacts are labeled according to their membrane specialization (as, asymmetric; ss, symmetric) and their postsynaptic targets (sp, spine; d, dendrite). Asterisks (*) mark the tops of synapses, i.e., those found in the reference (upper panel) but not the look up (lower panel) section. Multisynaptic boutons (msb) and CTB (marked with arrows) in the postsynaptic target are both labeled. Each micrograph was used as both the reference (3A) and look up (3B) section. Scale bar, 500 nm.
A synapse was identified by the accumulation of at least 3 presynaptic vesicles and by the presence of a widened synaptic cleft with parallel, thickened pre- and post- synaptic membranes. Asymmetric synapses had postsynaptic densities 2.5-3 times thicker than the presynaptic densities, while the densities of symmetric synapses were equal. The associated postsynaptic targets were identified as soma, dendrite, spine, or unknown using recognized criteria (Peters et al., 1991). Somata were identified by the presence of a nucleus. Dendrites were identified by the presence of structures postsynaptic to the axon terminals, such as mitochondria, microtubules, and, with larger dendrites, rough endoplasmic reticulum. Dendritic spines often contained a spine apparatus and were usually smaller than dendrites, typically lacking mitochondria, microtubules, and rough endoplasmic reticulum. Boutons were classified as multisynaptic (MSB) if they contacted more than one completely separate, independent target, enclosed in its own plasma membrane.
Estimation of mean synaptic numerical density (Nv syn): Synaptic number per μm3 was calculated using the following formula:
adapted from Sterio (1984) and de Groot and Bierman (1986), where Q-syn = synapses present in the reference section but not the lookup section (tops), h = height of the disector which is the distance (μm) between disector planes (section thickness), and A = sample area (μm2).
Estimation of ultrathin section thickness (Small's minimal fold Method; Small, 1968): At least 3 folds from each of the sections used for analysis were photographed at 25,000X magnification. Section thickness (h) was estimated as half the mean width of the measured folds.
Estimation of absolute number of synapses (N): The following formula was used:
where Nv syn is the mean synaptic density and Vref, the reference volume (see above for formulae for these).
Statistical Analysis
A Student's t-test was used to analyze differences in reference volumes and for all ultrastructural comparisons. The coefficient of error (CE), a measure of sampling error, was calculated for each ultrastructural analysis; it is generally accepted that the CE should ideally not exceed 0.1 (Sterio, 1984).
RESULTS
We calculated the CE for all classes of synapses and, in general, they were in the range of 0.05-0.1. However, the CE for the MSB was 0.3 for both the AMPH- and the vehicle-treated groups. These higher CEs represent the fact that MSBs were only infrequently encountered. Estimations of the Cavalieri volume revealed no difference in the mPFC volume between the AMPH- (3.15 ± 0.08 mm3) and vehicle-treated (3.08 ± 0.15 mm3) groups. We also estimated that the mPFC contains from 4.4-4.5 × 108 synapses, most of which were found to be asymmetric.
The effect of AMPH treatment on synaptic connectivity within the mPFC
We found that AMPH treatment did not affect the predominance of asymmetric synapses in the mPFC (∼73% of all synapses in the AMPH-treated group and 72% in the vehicle-treated group), the majority (74-80%) of which contacted spines. Symmetric synapses comprised approximately 25% and 28% of all synapses in the mPFC from the AMPH- and vehicle-treated groups, respectively.
There were 1.90 × 108 more synapses overall in the mPFC in the AMPH-treated than in the vehicle-treated group, but this 4% change was not significant (Table 1). When we analyzed the asymmetric synapses alone, we found that there were 2.42 × 108 more of these contacts after AMPH treatment, but this change of 8% was also not significant (Table 2). As for symmetric synapses, there were 0.52 × 108 fewer (4%) after AMPH treatment, another insignificant difference (Table 3).
Table 1.
Mean numerical density and absolute numbers of synapses in the mPFC.
| Vehicle-treated Mean ± SEM |
AMPH-treated Mean ± SEM |
|
|---|---|---|
| Total: | ||
| Numerical density (μm−3) | 1.42 ± 0.04 | 1.45 ± 0.08 |
| Absolute number × 108 | 43.56 ± 1.61 | 45.47 ± 1.35 |
Table 2.
Mean numerical density and absolute numbers of asymmetric synapses in the mPFC.
| Vehicle-treated Mean ± SEM |
AMPH-treated Mean ± SEM |
|
|---|---|---|
| Total: | ||
| Numerical density (μm−3) | 1.02 ± 0.03 | 1.07 ± 0.02 |
| Absolute number × 108 | 31.47 ± 2.01 | 33.39 ± 0.96 |
Table 3.
Mean numerical density and absolute numbers of symmetric synapses in the mPFC.
| Vehicle-treated Mean ± SEM |
AMPH-treated Mean ± SEM |
|
|---|---|---|
| Total: | ||
| Numerical density (μm−3) | 0.40 ± 0.03 | 0.37 ± 0.05 |
| Absolute number × 108 | 12.55 ± 0.72 | 11.58 ± 2.07 |
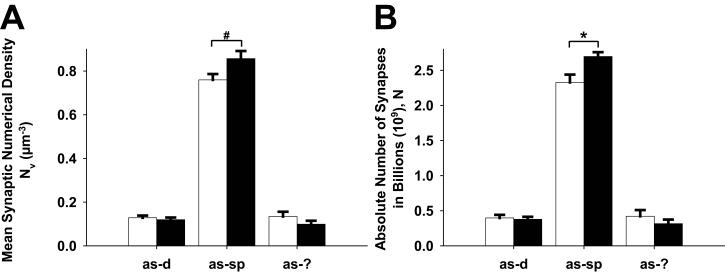
Interestingly, however, when synapses were classified on the basis of their postsynaptic targets, an important between-group difference emerged. There were 3.67 × 108 more axospinous synapses (16% increase) in the AMPH-treated compared to the vehicle-treated group, a difference that was significant, but there were fewer asymmetric synapses onto other targets (∼20% but not significant) in the AMPH-treated compared to vehicle-treated groups (Fig. 4). We also found a significant increase in symmetric synapses onto dendrites (44-50%) compared to spines (30-32%) in the mPFC (p < 0.05) in the AMPH-treated group. There was however no difference between groups in the number or density of symmetric synapses onto dendrites or spines, but there was a strong trend towards a decrease in symmetrical synapses onto cell bodies (0.24 × 108 fewer symmetric synapses after AMPH treatment; p < 0.08). Finally, there was no difference between the AMPH- and vehicle- treated groups in the number or density of MSBs in the mPFC.
Fig. 4.
Bar graphs showing the mean (A) asymmetric (as) synaptic density and (B) absolute number of asymmetric synapses in the mPFC following repeated AMPH or vehicle treatment. The results are separated by the postsynaptic targets (dendrite, d; spine, sp; unknown, ?); n = 4 per group; *p < 0.05, statistically significant increase in the total number of asymmetric axospinous synapse after repeated AMPH treatment.
The effect of AMPH treatment on synaptic connectivity of the mPFC-LH pyramidal neurons
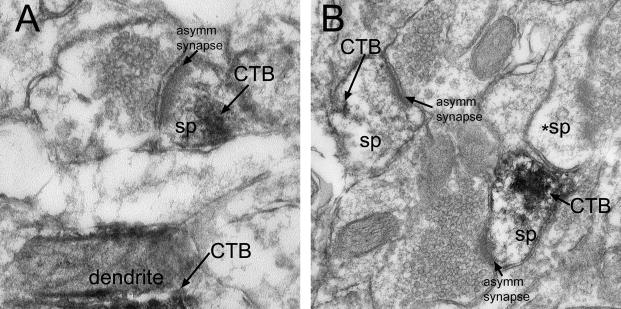
Histological examination of injection sites of CTB showed that they were central to the rostrocaudal extent and included much of the LH. They were placed lateral to the fornix and minimal or no spread of the CTB deposit occurred outside the LH. The CTB injection retrogradely labeled cells throughout the rostrocaudal extent of the mPFC, primarily in the deep layers, especially layer V of the infralimbic and prelimbic regions, as we have shown previously (Morshedi and Meredith, 2008). A few labeled cells were also found in the anterior cingulate, anterior insular, and dorsal peduncular cortices and were particularly sparse in the ventral and lateral orbital cortices. At the EM level, flocculent, DAB-Ni deposits were typically found on membranes in cell bodies, dendrites and spines of the pyramidal neurons in the PFC (Fig. 5A-B). These targets were readily identifiable, enabling us to evaluate any synaptic changes. However, no differences were found between AMPH- and vehicle- treated groups in the density or total number of synapses onto CTB-labeled neuronal structures, either overall or onto specific targets (Table 4).
Fig. 5 A-B.
Photomicrographs illustrating the flocculent DAB deposits in the spines of the mPFC after uptake and transport of CTB from the LH. Abbreviations: asymm, asymmetric; CTB, cholera toxin B subunit label; sp, spine; *sp, unlabeled spine. 60k final magnification.
Table 4.
Mean numerical density and absolute numbers of synapses onto CTB-labeled targets.
| Vehicle-treated Mean ± SEM |
AMPH-treated Mean ± SEM |
|
|---|---|---|
| Total Asymmetric | ||
| Numerical density (μm−3) | 0.27 ± 0.04 | 0.32 ± 0.04 |
| Absolute number × 108 | 8.22 ± 1.00 | 10.10 ± 0.93 |
| Total Symmetric | ||
| Numerical density (μm−3) | 0.18 ± 0.02 | 0.18 ± 0.04 |
| Absolute number × 108 | 5.51 ± 0.49 | 5.47 ± 1.28 |
| Asymmetric onto dendrites | ||
| Numerical density (μm−3) | 0.06 ± 0.004 | 0.06 ± 0.01 |
| Absolute number × 108 | 1.75 ± 0.17 | 1.77 ± 0.13 |
| Symmetric onto dendrites | ||
| Numerical density (μm−3) | 0.10 ± 0.01 | 0.12 ± 0.03 |
| Absolute number × 108 | 2.97 ± 0.25 | 3.56 ± 0.95 |
| Symmetric onto somata | ||
| Numerical density (μm−3) | 0.016 ± 0.002 | 0.008 ± 0.003ϕ |
| Absolute number × 108 | 0.50 ± 0.06 | 0.27 ± 0.10ϕ |
| Asymmetric onto spines | ||
| Numerical density (μm−3) | 0.20 ± 0.04 | 0.25 ± 0.03 |
| Absolute number × 108 | 5.92 ± 0.97 | 7.84 ± 0.76# |
| Symmetric onto spines | ||
| Numerical density (μm−3) | 0.10 ± 0.01 | 0.12 ± 0.03 |
| Absolute number × 109 | 1.18 ± 0.32 | 0.98 ± 0.23 |
p = 0.08
p = 0.10
DISCUSSION
Our results demonstrate for the first time that a behaviorally sensitizing, repeated AMPH treatment regimen significantly increases the number of asymmetrical (presumed excitatory) synapses made onto dendritic spines in the mPFC. However, there was no change in the number or density of asymmetrical or symmetrical contacts made onto dendrites in this part of the cortex. These data, therefore, point to newly formed spine synapses as being important adaptations underlying the behavioral changes associated with repeated AMPH exposure.
Technical considerations
We began this study by suspecting that synaptic changes would be most pronounced on those neurons that target the LH, as we had shown previously that these neurons were Fos-activated by this treatment regimen (Morshedi and Meredith, 2007). However, we found no specific synaptic changes on neurons in this pathway. We believe this result was not a consequence of inadequate labeling, since CTB has been shown to label dendrites and spines extensively (see Meredith, 1995 for review), and the percentage of the total number of synapses onto CTB-labeled structures (33%) was consistent with the proportion of LH-projecting neurons in the mPFC (Gabbott et al., 2005).
The apparently anomalous finding that the number of axospinous synapses in the mPFC increases even though the total number of asymmetric plus symmetric or, asymmetric alone, synapses does not change can be explained by a closer examination of the data. The total synapse numbers obtained in our study included large, but nonsignificant, decreases in certain types and targets of synapses for the mPFC of the AMPH-treated group compared to controls. Thus, gains for one synapse/target subtype were offset by decreases in others when means were compared in statistical tests. This meant that the significant axospinous gain could only be appreciated if synapses onto each target were considered separately with statistical tests that compared the two groups.
Increased axospinous contacts associated with repeated AMPH treatment
The physiological function of prefrontal circuits is altered by many different experiences including drugs of abuse. We found an increase in only one type of synapse in the mPFC, those making excitatory synapses onto spines, which suggests a strengthening of these inputs to mPFC pyramidal neurons (Nasif et al., 2005; Onn and Grace, 2000). Our study reveals a 16% percent increase in such synapses, which correlates well with the 12-16% increase in the number of apical spines demonstrated by Robinson and Kolb (1997, 1999), using Golgi embedding and light microscopy but a similar treatment protocol. Nearly every dendritic spine in the cortex of adult rats is apposed by a synapse (Gray, 1959; Peters and Feldman, 1976), which would suggest that any new spines will be contacted by a new synapses. However, results from a recent in vivo imaging study of the barrel cortex in adult mice suggest that newly formed spines do not rapidly form new synapses. When newly formed spines do, in fact, take on a new synapse, the new contacts are most often derived through a remodeling of existing boutons resulting in the formation of new MSBs rather than by de novo sprouting (Knott et al., 2006). In contrast, our results provide little evidence for the remodeling of existing boutons, since the increase in axospinous synapses shown after AMPH treatment was not accompanied by an increase in MSBs. The reason for this discrepancy may be that MSBs are rare in this part of the cortex as they are in the CA1 of the hippocampus (Shepherd and Harris, 1998) or that synaptic remodeling requires different conditions (Knott et al., 2006).
We were surprised to find that neither the density nor number of axodendritic synapses changed after repeated AMPH treatment compared to controls, because neuronal reconstruction studies have suggested that there is a considerable increase in dendritic length of pyramidal neurons (Robinson and Kolb, 1999). One might, therefore, expect that the new dendritic surface area, created by the lengthening of dendrites, would accommodate new synapses. However, this may not be the case if dendrites added length merely to accommodate new spines. Certainly, in other studies, activity-dependent dendritic morphogenesis was most prominently expressed through an increase in filopodia-like protrusions or new spines (Maletic-Savatic et al., 1999). On the other hand, the increase in dendritic length could have accommodated new synapses but, perhaps over time, and due to repeated injections of a high dose of AMPH (present results and Robinson and Kolb, 1999), such dendrites undergo a certain degree of synaptic stripping ultimately yielding a synapse neutral condition. Synaptic stripping has been associated with the potent stimulus properties of methamphetamine (Brummelte et al., 2007) and may have occurred with this repeated regimen of AMPH.
Conclusions
We find that synaptic modifications to spines but not dendrites occur in response to a repeated AMPH paradigm known to produce robust behavioral sensitization (Morshedi and Meredith, 2007, 2008; Paulson et al., 1991). Such sensitization also changes the physiological activity of mPFC pyramidal cells (Onn and Grace, 2000) in a time course that parallels that of the synaptic changes (present results). Our work demonstrates that new spine synapses are important morphological adaptations underlying the behaviorally sensitized state with repeated AMPH administration, and they firmly support drug-induced synaptic plasticity but provide no evidence for new axodendritic synapses participating in that plasticity.
ACKNOWLEDGEMENTS
We thank Ms. Figen Seiler, Jennifer Jackolin, and Elyse M. Sullivan for their technical assistance. We gratefully acknowledge the contribution of the Electron Microscopy Center at Rosalind Franklin University of Medicine and Science to this work. The work was supported by a USPHS grant from the NIH, DA016662.
REFERENCES
- Braendgaard H, Gundersen HJ. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Meth. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Neddens J, Teuchert-Noodt G. Alteration in the GABAergic network of the prefrontal cortex in a potential animal model of psychosis. J Neural Transm. 2007;114:539–547. doi: 10.1007/s00702-006-0613-4. [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Transient c-fos expression and dendritic spine plasticity in hippocampal granule cells. Brain Res. 1992;577:169–174. doi: 10.1016/0006-8993(92)90553-l. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- de Groot DM, Bierman EP. A critical evaluation of methods for estimating the numerical density of synapses. J Neurosci Methods. 1986;18:79–101. doi: 10.1016/0165-0270(86)90113-5. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004;125:615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Geinisman Y. Perforated axospinous synapses with multiple, completely partitioned transmission zones: probable structural intermediates in synaptic plasticity. Hippocampus. 1993;3:417–433. doi: 10.1002/hipo.450030404. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Gundersen HJ, van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996a;25:805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Persina IS, Beatty MA. Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. J Comp Neurol. 1996b;368:413–423. doi: 10.1002/(SICI)1096-9861(19960506)368:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Heller RE, Rossi M, Parshall RF. Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993;3:435–445. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 1977;111:219–223. [Google Scholar]
- Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. BIOS Scientific Publishers LTD; Oxford, UK: 1998. p. 246. [Google Scholar]
- Hunter A, Stewart MG. Long-term increases in the numerical density of synapses in the chick lobus parolfactorius after passive avoidance training. Brain Res. 1993;605:251–255. doi: 10.1016/0006-8993(93)91747-g. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail ZI, Bedi KS. Rats exposed to cocaine during late gestation and early postnatal life show deficits in hippocampal pyramidal and granule cells in later life. J Anat. 2007;210:749–760. doi: 10.1111/j.1469-7580.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrrcht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborsky L, editors. Tract tracing methods 2, recent progress. Plenum; New York: 1989. pp. 129–172. [Google Scholar]
- Lu W, Wolf ME. Repeated amphetamine administration alters AMPA receptor subunit expression in rat nucleus accumbens and medial prefrontal cortex. Synapse. 1999;32:119–131. doi: 10.1002/(SICI)1098-2396(199905)32:2<119::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dentritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Meredith GE. Recent developments in neuronal tract tracing methods. Cell Vis. 1995;2:184–195. [Google Scholar]
- Mouton PR. An Introduction for Bioscientists. The John Hopkins University Press; Baltimore, MD: 2002. Principles and Practices of Unbiased Stereology; p. 214. [Google Scholar]
- Morrow BA. Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse. 2007;61:862–865. doi: 10.1002/syn.20430. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Differential laminar effects of amphetamine on prefrontal parvalbumin interneurons. Neuroscience. 2007;149:617–624. doi: 10.1016/j.neuroscience.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Repeated amphetamine administration induces Fos in prefrontal cortical neurons that project to the lateral hypothalamus but not the nucleus accumbens or basolateral amygdala. Psychopharmacology (Berl) 2008;197:179–189. doi: 10.1007/s00213-007-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 2005;312:1305–1313. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Lokwan SJ, Berry MS, Clark D. D-Amphetamine potentiates muscimol-induced disinhibition of A10 dopaminergic neurons in the rat. J Neural Transm. 2000;107:1381–1391. doi: 10.1007/s007020070002. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th Edition Elsevier Academic Press; Amsterdam; Boston: 2005. [Google Scholar]
- Peters A, Feldman ML. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J Neurocytol. 1976;5:63–84. doi: 10.1007/BF01176183. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. Fine Structure of the Nervous System: Neurons and Their Supporting Cells. 3rd Edition Oxford University Press; New York, NY: 1991. [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–344. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Shephard GM, Harris KM. Three-dimensional structure and composition of CA3-CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV. Measurement of section thickness; Fourth European Regional Conference on Electron Microscopy; Rome, Italy. 1968. [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Klintzova AJ, Istomin VV, Haselhorst U, Schenk H. The effects of amphetamine on synaptic plasticity in rat's medial prefrontal cortex. J Hirnforsch. 1989;30:45–50. [PubMed] [Google Scholar]