Angiogenesis not only depends on endothelial cell (EC) invasion and proliferation, but also requires pericyte coverage of vascular sprouts for vessel stabilization.1,2 These processes are coordinated by Vascular Endothelial Growth Factor (VEGF) and Platelet Derived Growth Factor (PDGF) through their cognate receptors on ECs and vascular smooth muscle cells (VSMCs), respectively.3,4 PDGF induces neovascularization by priming VSMCs/pericytes to release pro-angiogenic mediators.5–7 While VEGF directly stimulates EC proliferation and migration, its role in pericyte biology is less clear. Here, we define a role for VEGF as an inhibitor of neovascularization based on its capacity to disrupt VSMC function. Specifically, under conditions of PDGF-mediated angiogenesis, VEGF ablates pericyte coverage of nascent vascular sprouts leading to vessel destabilization. At the molecular level, VEGF-mediated activation of VEGF-R2 suppresses PDGF-Rβ signaling in VSMCs through the assembly of a previously undescribed receptor complex consisting of PDGF-Rβ and VEGF-R2. Inhibition of VEGF-R2 not only prevents assembly of this receptor complex but also restores angiogenesis in tissues exposed to both VEGF and PDGF. Finally, genetic deletion of tumor cell VEGF disrupts PDGF-Rβ/VEGF-R2 complex formation and increases tumor vessel maturation. These findings underscore the importance of VSMCs/pericytes in neovascularization8–9 and reveal a dichotomous role for VEGF and VEGF-R2 signaling as both a promoter of EC function and a negative regulator of VSMCs and vessel maturation.
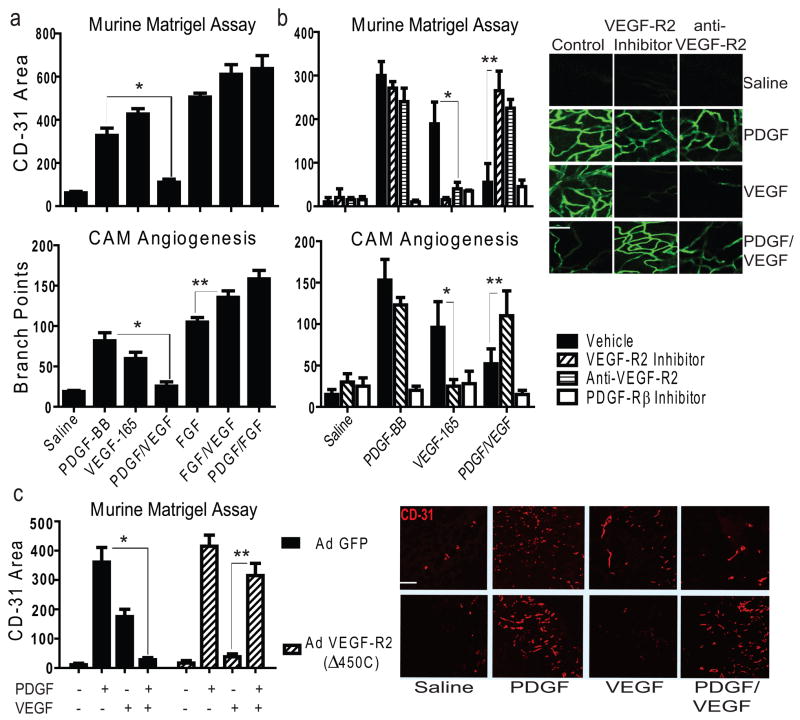
VEGF and PDGF contribute to angiogenesis and vessel stabilization by activating ECs and VSMCs respectively, suggesting that a combination of these factors might elicit a synergistic response. To directly test this in angiogenesis models, PDGF, VEGF or both were mixed with Matrigel and implanted subcutaneously into mice or topically applied to the CAM of 10-day old chick embryos. As expected PDGF-BB or VEGF, at an optimal angiogenic dose (Supplementary Fig. 1a–b), promotes robust neovascularization (Fig. 1a). Surprisingly, the combination of these cytokines results in complete suppression of angiogenesis (Fig. 1a). Inhibition of angiogenesis is not likely due to excessive proliferation of mural or stromal cells, since PDGF or VEGF each combine with bFGF to produce an increased angiogenic response (Fig. 1a).10 Thus, a specific antagonistic relationship exists between PDGF and VEGF during neovascularization.
Figure 1. VEGF Inhibits PDGF-mediated Angiogenesis through VEGF-R2.
a, Upper: vessel growth into Matrigel as measured by CD-31+ area, *P<0.02. Bottom: branch points quantified on filter paper impregnated with saline or growth factors placed on embryonic day 10 Chick CAMs; *P<0.02, **p<0.05, results from one-way ANOVA. Further images are displayed in Supplementary Fig. 1d. b, Upper: vessel growth into Matrigel as in a, with the application of inhibitors; *P<0.001, **P<0.001. Bottom: branch points quantified on filter paper impregnated with saline or growth factor as in a, with the application of inhibitors; *P<0.02, **P<0.05 *results from two-way ANOVA. Right: confocal microscopy of Matrigel from mice injected intravenously with fluorescent Griffonia lectin; Scale bar, 100 μm c, CD-31+ area quantified by image analysis of Matrigel (right panel) impregnated with saline or growth factor implanted into mice infected with adenovirus expressing GFP only or GFP and truncated VEGF-R2 (Δ450C); Scale bar, 200 μm; *P<0.001, **P<0.001, results from one-way ANOVA. All error bars ± S.D; n>5 per group in all panels.
We next considered whether PDGF or VEGF receptors contribute to the antagonistic effects of combined VEGF/PDGF treatment on neovascularization. Following PDGF receptor blockade, suppression of angiogenesis is observed in the presence of either growth factor (Fig. 1b), consistent with a role for PDGF in the release of pro-angiogenic factors and the recruitment of VSMCs/pericytes to nascent EC sprouts.11–12 While combined PDGF/VEGF stimulation leads to the appearance of tenuous and small caliber vascular sprouts on CAMs at 48 hours, a complete absence of new vessel growth is evident on CAMs by 72 hours (Fig. 1b)(Supplementary Fig. 1c). Thus, combined PDGF/VEGF treatment initiates vessel sprouting but not a durable vascular response.
To assess the role of VEGF-R2 as a negative regulator of pericyte function, Matrigel and CAM tissues were treated with either a VEGF-R2 kinase inhibitor or anti-VEGF-R2 and stimulated with PDGF, VEGF or both. As expected, either inhibitor disrupts VEGF- but not PDGF-mediated angiogenesis in both models (Figure 1b).13 Interestingly, following PDGF/VEGF treatment, VEGF-R2 inhibition restores angiogenesis to a level achieved with either growth factor alone (Fig. 1b), suggesting that under conditions of VEGF/PDGF stimulation, VEGF-R2 negatively regulates neovascularization. To validate these observations, mice were injected with Matrigel impregnated with growth factors, as well as adenovirus expressing an inactive truncation mutant of the VEGF-R2 receptor. Gene delivery to the vascular compartment suppresses VEGF-R2 phosphorylation in these tissues (Supplementary Fig. 2a–b). Importantly, the VEGF-R2 truncation mutant restores the angiogenic response following PDGF/VEGF stimulation without affecting PDGF-mediated angiogenesis (Fig. 1c). These studies demonstrate that, in the presence of both VEGF and PDGF, VEGF-R2 can limit pro-angiogenic events.
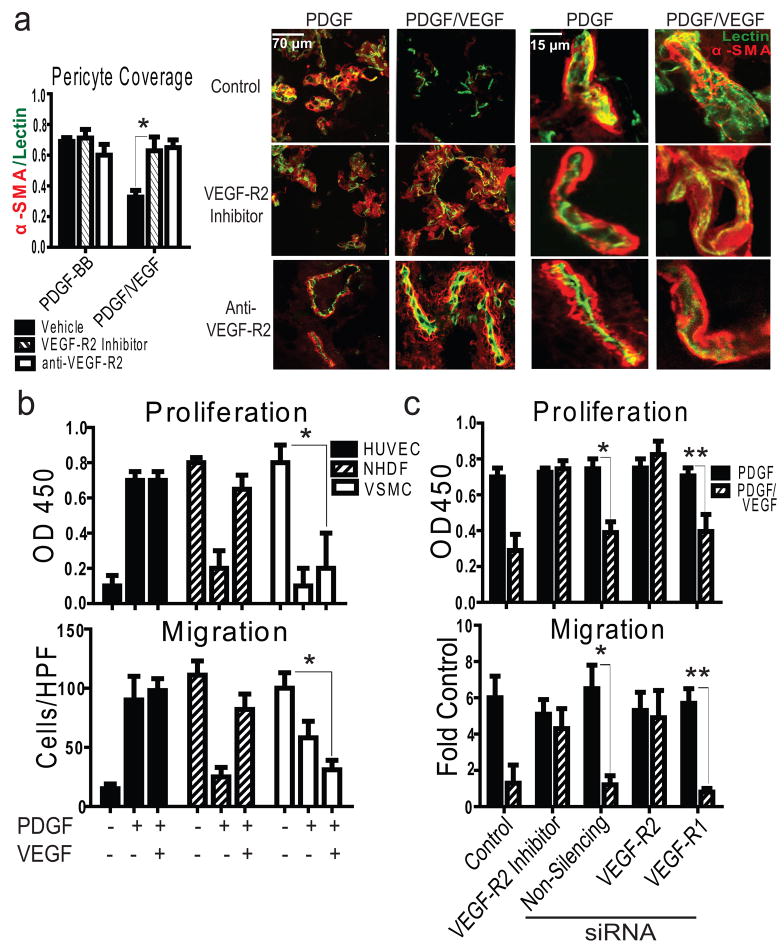
We next considered the effect of VEGF treatment on PDGF-stimulated pericyte recruitment to nascent vessels during angiogenesis. As expected, PDGF treatment alone produces a robust influx of alpha-smooth muscle actin expressing (α-SMA+; VSMC/pericyte marker) cells to the surface of patent blood vessels (Fig. 2a).5 However, stimulation of tissues with PDGF/VEGF results in a reduction in vascular pericyte coverage compared to tissues treated with PDGF alone. Importantly, pericyte coverage and neovascularization are restored following VEGF-R2 inhibition (Fig. 2a), suggesting that VSMC/pericyte VEGF-R2 negatively regulates this response.
Figure 2. VEGF Disrupts Pericyte Coverage/VSMC Activation through VEGF-R2.
a, Right: confocal microscopy images of reconstituted basement membrane impregnated with growth factors from mice perfused with fluorescent Griffonia lectin (green), stained for α-SMA (red). Left: pericyte coverage as assessed by α-SMA/lectin co-localization; *P<0.01, results from one-way ANOVA, n=6/group. b, Representative proliferation (upper, *P<0.001) and migration assays (lower, *P<0.001); *results from one-way ANOVA. c, Representative VSMC proliferation (upper, *P<0.01; **P<0.05) and migration (lower, *P<0.001, **P<0.001) assays performed after siRNA-mediated knock-down of VEGF receptors normalized to PBS control where indicated; results from one-way ANOVA, n>3 experiments. All error bars ± S.D.
To assess whether VSMC function could be influenced by the combination of PDGF and VEGF we examined the effects of these growth factors on proliferation and migration in primary human VSMCs in vitro. Although PDGF induces VSMC proliferation and migration, exposure to both PDGF and VEGF results in a complete suppression of these responses (Fig. 2b). In contrast, Human Umbilical Vein ECs (HUVECs), which express VEGFR-2 but not PDGF receptors and Normal Human Dermal Fibroblasts (NHDFs), which express PDGF but not VEGF receptors (Supplementary Fig. 3a), exhibit robust migration and proliferation in the presence of PDGF/VEGF. Similar results were obtained with a wide range of other ECs and VSMC/pericytes (Supplementary Fig. 4). Importantly, VSMCs pre-treated with a VEGF-R2-inhibitor or subjected to siRNA-mediated knockdown of VEGF-R2, but not VEGF-R1, demonstrate complete reversal of PDGF/VEGF-mediated suppression of cell migration and proliferation (Fig. 2c). This may be explained by the finding that VEGF significantly attenuates PDGF-induced pp60-Src and ERK signaling (Supplementary Fig. 5). Importantly, co-stimulation with bFGF and PDGF does not suppress PDGF-mediated VSMC chemotaxis (data not shown), further supporting a specific relationship between VEGF and PDGF.
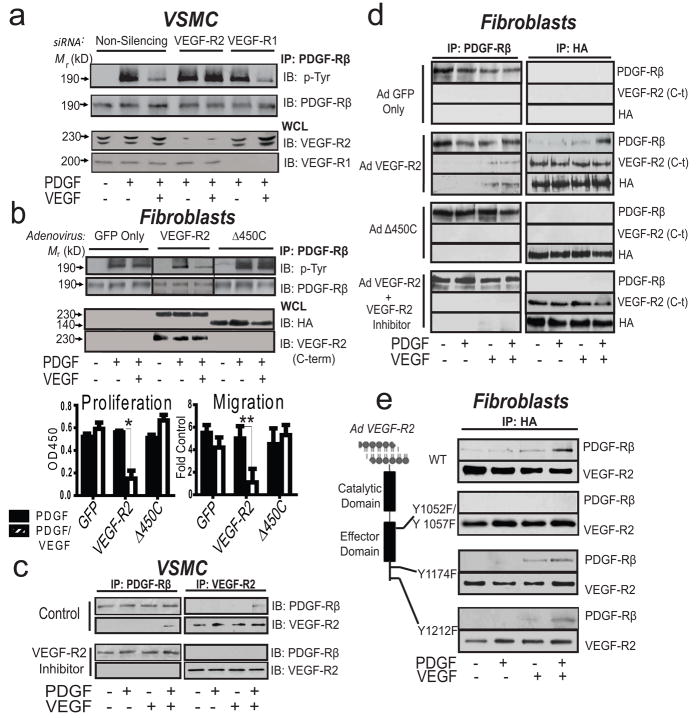
The antagonistic results of PDGF/VEGF treatment do not result from competition for receptor occupancy since treatment with a range of VEGF doses fails to suppress PDGF stimulation of PDGF-Rβ in NHDFs, which lack VEGF-R2 (data not shown). Thus, it appears that VEGF-ligation of VEGF-R2 in VSMC’s leads to inactivation of PDGF-Rβ, the dominant PDGF receptor on these cells. To test this possibility, VSMCs were exposed to varying concentrations of VEGF (in the presence or absence of PDGF) and the PDGF-Rβ phosphorylation (activation) status was evaluated. While VEGF alone fails to induce PDGF-Rβ phosphorylation, VEGF suppresses PDGF-mediated PDGF-Rβ tyrosine phosphorylation by fivefold, which can be reversed by PDGF titration (Supplementary Fig. 6a–b). Importantly, VEGF-121, which lacks a heparin-binding domain, also suppresses PDGF-mediated activation of PDGF-Rβ (Supplementary Fig. 6c). To evaluate the requirement of VEGF receptors for this process we subjected VSMCs to VEGF-R1 or VEGF-R2 siRNA-mediated knock-down and analyzed the effect of PDGF/VEGF co-stimulation on PDGF-Rβ activity. Knock-down of VEGF-R2 but not VEGF-R1 abrogates the VEGF-mediated inactivation of PDGF-Rβ, indicating that VEGF-R2 is essential for VEGF-mediated suppression of PDGF signaling in VSMCs (Fig. 3a). VEGF-R2 kinase inhibition also restores PDGF-Rβ phosphorylation in cells exposed to PDGF/VEGF, providing further evidence that VEGF-R2 negatively regulates PDGF-Rβ (Supplementary Fig. 7a). Thus, co-stimulation of VSMCs with PDGF/VEGF results in crosstalk between the cognate receptors for these ligands, leading to suppression of PDGF signaling.
Figure 3. An Inducible VEGF-R2/PDGF-Rβ Complex Forms in VSMC.
a, Immunoblot analysis of anti-PDGF-Rβ immunoprecipitated lysates from serum-starved VSMCs subjected to siRNA-mediated VEGF receptor knock-down. b, Upper: Immunoblot analysis of anti-PDGF-Rβ immunoprecipitated lysates from serum-starved NHDFs (VEGF-R2 null) infected with adenovirus. Immunoblots represent at least three similar experiments. Lower left: Representative proliferation (left) and migration (right) experiments with VEGF-R2 reconstituted NHDFs; *P<0.001, P<0.02, results from one-way ANOVA, n>3 separate experiments. c, Representative reciprocal co immunoprecipitation followed by immunoblot analysis with lysates derived from serum-starved VSMCs pre-treated with either control or VEGF-R2 inhibitor or d, serum-starved NHDFs transduced with truncated VEGF-R2 adenovirus or e, NHDFs transduced with VEGF-R2 catalytic site (Y1052F and Y1057F) and effector site mutants (Y1174F and Y1212F). IB, immunoblot; IP, immunoprecipitation; WCL, whole cell lysate; c-t, C-terminus. Immunoblots are representative of at least three similar experiments; mutant receptors are characterized in Supplementary Fig. 8.
To further define the underlying mechanism of VEGF-R2-dependent inhibition of PDGF-Rβ activity, VEGF-R2 or VEGF-R2(Δ450C) were expressed in NHDF cells, which lack VEGF-R2. VEGF induces phosphorylation of wild-type VEGF-R2 but not the truncated derivative (Supplementary Fig. 8a–b). NHDF cells transduced with VEGF-R2, or VEGF-R2(Δ450C) demonstrate equivalent phosphorylation of PDGF-Rβ in response to PDGF alone (Fig. 3b). However, when NHDFs expressing VEGF-R2 were stimulated with VEGF/PDGF, PDGF-Rβ exhibits decreased phosphorylation relative to cells expressing truncated VEGF-R2. This is associated with a concomitant reduction in proliferation and cell migration, relative to cells exposed to either cytokine alone, demonstrating that VEGF-R2 expression is sufficient to inhibit PDGF-dependent function in VSMCs. (Fig. 3b).
Given the functional crosstalk between VEGFR2 and PDGF-Rβ in VSMCs, we evaluated whether these receptors interact directly by performing reciprocal co-immunoprecipitation studies. Importantly, treatment of VSMCs with PDGF/VEGF results in the formation of a previously un-described VEGF-R2/PDGF-Rβ complex, whereas no interaction is detected following exposure of cells to either growth factor alone (Fig. 3c). The same biochemical interaction was not detected with PDGF-Rα (Supplementary Fig. 9a). Furthermore, pre-treatment of VSMCs with a VEGF-R2 inhibitor attenuates VEGF-R2/PDGF-Rβ complex formation, suggesting a requirement for VEGF-R2 activity. Similar findings are observed in the pericyte-like 10T1/2 cell line which is often used to approximate pericytes in vitro, and which have been found to exhibit growth suppression in co-culture with endothelial cells (Supplementary Fig. 9b).14,15 As a final biochemical confirmation, growth factor-dependent PDGF-Rβ/VEGF-R2 complex formation was identified in HEK-293 cells ectopically expressing both VEGF-R2 and PDGF-Rβ (Supplementary Fig. 9c). To confirm these findings by using a cell biological approach, we performed Proximity Ligation Analysis (PLA) which depicts close proximity of cellular molecules and demonstrates a 5-fold increase in the VEGF-R2/PDGF-Rβ interaction following PDGF/VEGF co-stimulation, relative to cells stimulated with either growth factor alone (Supplementary Fig. 10). To further characterize this interaction, NHDFs were transduced with full length or VEGF R2(Δ450C). As demonstrated in VSMCs, expression of full length VEGF-R2 but not the truncation mutant results in the formation of a VEGR-R2/PDGF-Rβ complex (following dual cytokine stimulation only) (Fig. 3d). These results demonstrate a requirement for the VEGF-R2 C-terminus in the VEGR-R2/PDGF-Rβ interaction.
We next transduced NHDFs with VEGF-R2(Y1052/1057F), which fails to undergo auto-phosphorylation in response to VEGF (Supplementary Fig. 8e). Similar to the results observed after transduction of VEGF-R2(Δ450C), this catalytically inactive mutant fails to complex with PDGF-Rβ after dual growth factor treatment, demonstrating that VEGF-R2 activity is required for interaction with PDGF-Rβ (Fig. 3e). Importantly, complex formation is still observed when two VEGF-R2 effector sites (Y1174 and Y1212) are mutated.
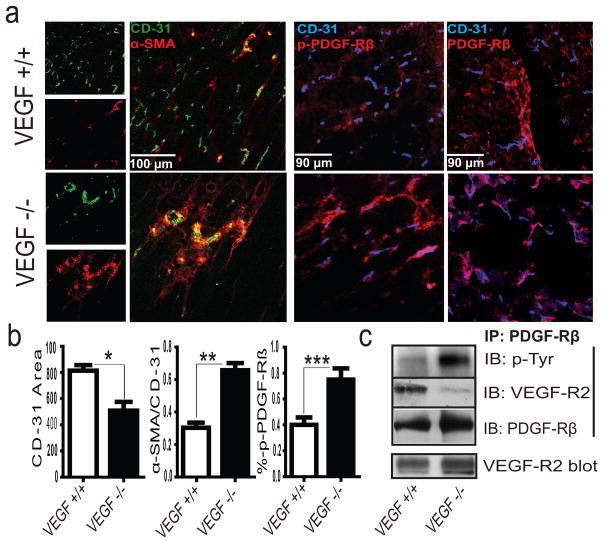
Tumors express high levels of VEGF and as a result develop tortuous, leaky and immature blood vessels.16–17 Anti-VEGF therapy results in vessel normalization characterized by increased pericyte coverage, tumor perfusion and chemotherapeutic sensitivity.18 To investigate VEGF’s role in tumor vessel normalization, we examined the vasculature of fibrosarcomas that either express or lack VEGF.19 Similar to results from anti-VEGF therapy, VEGF−/− tumors produce mature vessels with extensive pericyte coverage, whereas wild type (VEGF+/+) tumors develop an immature tumor vasculature with minimal pericyte coverage (Fig. 4a–b). Importantly, dual expression of PDGF-Rβ and VEGF-R2 appears limited to α-SMA+ perivascular cells (Fig. Supplementary Fig. 11b). Interestingly, the VEGF-R2/PDGF-Rβ complex is only detected in lysates of VEGF+/+ tumors, which feature an immature vasculature (Fig. 4c). By contrast, complex formation is attenuated in VEGF−/− tumors, concomitant with increased PDGF-Rβ phosphorylation (Fig. 4c) and decreased VEGF-R2 phosphorylation (Supplementary Fig. 11a). Next, we implanted human pancreatic tumor cells orthotopically into the tail of the mouse pancreas and allowed tumors to develop for two weeks. Analysis of immunoprecipitated protein from these tumors demonstrated PDGF-Rβ/VEGF-R2 complex formation (Supplementary Fig. 12a). While the tumor cells within these tissues express negligible levels of VEGF or PDGF receptors we did observe co-expression of VEGF-R2 and PDGF-Rβ on perivascular cells that co-stain with alpha-smooth muscle actin (Supplementary Fig. 12b–c). Taken together, these studies demonstrate that VEGF can induce the formation of a VEGF-R2/PDGF-Rβ complex implicated both in the suppression of pericyte function and in the regulation of tumor angiogenesis. Our findings support a mechanism where VEGF inhibits pericyte function and blood vessel maturation through the induction of a VEGF-R2/PDGF-Rβ complex, and may in part, explain the molecular basis of anti-VEGF therapy in cancer patients.
Figure 4. VEGF Loss Attenuates VEGF-R2/PDGF-Rβ Complex Formation and Tumor Vessel Maturation.
a, Confocal microscopy of WT (VEGF+/+) and KO (VEGF−/−) fibrosarcomas demonstrating histological markers of vascular maturity and phosphorylated PDGF-Rβ (Y751) with b, image analysis; P<*0.01, **P<0.001, ***P<0.05, results from unpaired t-test, n>3 animals/group. c, anti-PDGF-Rβ immunoprecipitated tumor lysates derived from VEGF+/+ and VEGF−/− tumors.
Methods Summary
HUVECs, HMVECs, HAECs, and NHDFs were acquired from Clonetics. Human aortic VSMCs were isolated from three donors (Clonetics and Cascade Biologicals). The pericyte line C3 10T1/2 (clone 8) was acquired from ATCC. Murine lung endothelial cells (MLECs) were isolated by immunoselection using Dynal magnetic beads (Invitrogen). For in vitro studies, unless otherwise indicated, PDGF-BB, VEGF-165, and FGF-2 were used at 20 ng/mL, 40 ng/ml, and 50 ng/ml, respectively. 500 ng and 100 ng of growth factor was used for Matrigel and CAM experiments, respectively. Epitope-tagged mutant receptor constructs and adenoviruses were generated using standard cloning techniques and validated by DNA sequencing. The procedures for CAM,20 Matrigel,21 and murine fibrosarcoma19 experiments were performed with Institutional approval as previously reported or with slight modifications. Full Methods accompany this paper.
Supplementary Material
Acknowledgments
We thank Drs. Dwayne Stupack and David Schlaepfer for advice and review of the manuscript. Also we thank Dr. Alok Tomar for assistance with the PLA imaging; Dr. C. Heldin (Ludwig Institute for Cancer Research) for the PDGF-Rβ cDNA; Dr. Jay Desgrosellier for processing pancreatic tumor samples. This work was supported by NIH grants R37-CA50286 (D.A.C) and CA082515 (R.S.J.); and by a U.C. Academic Senate Grant (N.A.). C.S. was funded by the Deutsche Forschungsgemeinschaft (DFG STO 787/1-1) and L.M.A. received an IRACDA postdoctoral fellowship (NIH grant GM 68524).
Footnotes
Author Contributions
J.I.G. generated mutant receptors, performed biochemical, and cell imaging analyses, and prepared the manuscript. D.J.S. and E.M. assisted with experiments and manuscript preparation. S.B. performed angiogenesis assays. L.A. assisted with adenovirus production, cell isolation, and tumor staining. J.H. and L.S. performed RNA analysis and assisted with biochemical analyses. C.S. and R.J.S. generated, implanted, and harvested fibrosarcomas. D.A.C. and N.A. supervised and directed the project.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Andrae J, et al. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. The Biology of VEGF and its receptors. Nature Medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Inui H, et al. Differences in signal transduction between platelet-derived growth factor (PDGF) alpha and beta receptors in vascular smooth muscle cells. PDGF-BB is a potent mitogen, but PDGF-AA promotes only protein synthesis without activation of DNA synthesis. J Biol Chem. 1994;269:30546–52. [PubMed] [Google Scholar]
- 5.Vincent L, Rafii S. Vascular frontiers without borders: multifaceted roles of platelet-derived growth factor (PDGF) in supporting postnatal angiogenesis and lymphangiogenesis. Cancer Cell. 2004;6:307–9. doi: 10.1016/j.ccr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Pietras K, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jechlinger M, et al. Autocrine PDGFR signaling promotes mammary cancer metastases. J Clin Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song S, et al. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;11:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 10.Nissen LJ, et al. Angiogenic factors FGF-2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramsson A, Lindblom M, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sennino B, et al. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of PDGF-B by selective aptamer AX102. Cancer Res. 2007;67:7358–67. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KJ, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 14.Hirschi KK, et al. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Hirschi KK, Rohovsky SA, D’Amore PA, et al. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa S, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 18.Willett, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;2:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunstein J, et al. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 2004;23:2800–10. [PubMed] [Google Scholar]
- 20.Eliceiri B, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 21.Alavi, et al. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;6:2766–72. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.