Abstract
Tuberoinfundibular peptide of 39 residues (TIP39) is synthesized by two groups of neurons, one in the subparafascicular area at the caudal end of the thalamus and the other in the medial paralemniscal nucleus within the lateral brainstem. The subparafascicular TIP39 neurons project to a number of brain regions involved in emotional responses, and these regions contain a matching distribution of a receptor for TIP39, the parathyroid hormone 2 receptor (PTH2-R). We have now evaluated the involvement of TIP39 in anxiety-related behaviors using mice with targeted null mutation of the TIP39 gene (Tifp39). Tifp39−/− mice (TIP39-KO) did not significantly differ from wild-type (WT) littermates in the open field, light/dark exploration and elevated plus-maze assays under standard test conditions. However, the TIP39-KO engaged in more active defensive burying in the shock-probe test. In addition, when tested under high illumination or after restraint, TIP39-KO displayed significantly greater anxiety-like behavior in the elevated plus-maze than WT. In a Pavlovian fear-conditioning paradigm, TIP39-KO froze more than WT during training and during tone and context recall but showed normal fear extinction. Disruption of TIP39 projections to the medial prefrontal cortex, lateral septum, bed nucleus of the stria terminalis, hypothalamus and amygdala likely account for the fear- and anxiety-related phenotype of TIP39-KO. Current data support the hypothesis that TIP39 modulates anxiety-related behaviors following environmental provocation.
Keywords: Anxiety, conditioned fear, mouse knockout, neuropeptide, stress
Anxiety disorders are one of the most prevalent classes of psychiatric disorders, and stress-related symptoms are known to contribute to the morbidity of many diseases (Alonso et al. 2004). However, the etiology of these conditions remains poorly understood and existing anxiolytic treatments are beset by significant non-response rates, delayed onset of action and poor tolerability (Stein & Seedat 2004). The brain regions and neural circuits mediating anxiety in humans and anxiety-related behaviors in experimental animals are being defined in increasing detail (Davis & Shi 1999; Hariri & Holmes 2006). These brain regions are generally rich in neuropeptides. Based on their distributions and mode of action, neuropeptides have long been thought to contribute to emotional state, and some evidence supports the suggestion that they are preferentially recruited as neuromodulators under conditions of high neural activity, as might occur in disease or in response to stress (Hokfelt et al. 2003). For example, genetic mutation or pharmacological manipulation of corticotropin-releasing hormone (CRH), substance P, vasopressin, galanin and neuropeptide Y or their receptors affects stress and anxiety-like behaviors in rodents (Bale et al. 2000; Bannon et al. 2000; Coste et al. 2000; Heinrichs et al. 1997; Holmes et al. 2003; Inui et al. 1998; Kishimoto et al. 2000; Koster 1999; Miyakawa et al. 1994; Otto et al. 2001; Palmiter et al. 1998).
Tuberoinfundibular peptide of 39 residues (TIP39) was purified from hypothalamic tissue on the basis of its selective activation of the parathyroid hormone 2 receptor (PTH2-R) (Usdin et al. 1999). TIP39-containing cell bodies are principally localized within two brain regions, the subparafascicular area at the caudal end of the thalamus and the medial paralemniscal nucleus within the lateral brainstem (Dobolyi et al. 2002, 2003b). TIP39 containing fibers and PTH2-Rs are present at relatively high levels in rodent brain regions implicated in stress, fear and anxiety. Most notably, the subparafascicular TIP39 neurons project to PTH2-R-dense areas in the amygdala, lateral septum, bed nucleus of the stria terminalis, hypothalamus and medial prefrontal cortex (Dobolyi et al. 2003b; Faber et al. 2007). Thus, the anatomical distribution of the TIP39/PTH2-R system suggests that it is ideally positioned to modulate anxiety. In support of this hypothesis, direct central nervous system administration of TIP39 in rats decreases anxiety-like behavior in the elevated plus-maze (LaBuda et al. 2004) and stimulates adrenocorticotropin-releasing hormone (ACTH) release (Ward et al. 2001). Exogenous TIP39 also reduces vasopressin release following dehydration (Sugimura et al. 2003) and modulates responses to some types of pain (Dobolyi et al. 2002). Beyond these preliminary studies, however, the potential role of TIP39 in modulation of behavior has not been evaluated.
We recently developed a mouse line with targeted null mutation of Tifp39, the gene encoding TIP39 (Usdin et al. 2008). Homozygous Tifp39−/− mice have a defect in germ cell maturation, but no abnormalities outside the gonads have been observed. In males, the defect appears to be restricted to spermatogenic cells and serum testosterone levels are not significantly different from wild-type (WT) littermates. In the present study, we investigated the effect of targeted null mutation of Tifp39 on anxiety-related behaviors and Pavlovian fear conditioning and extinction, using freezing as an index of fear (LeDoux et al. 1984). Because the PTH2-R is expressed within the cardiovascular system (Usdin et al. 1996) and a vasodilatory effect of TIP39 in isolated rat heart has been reported following desensitization of the PTH1-R (Ross et al. 2007), we examined the effect of TIP39 on blood pressure, and compared baseline hemodynamic and cardiovascular parameters between WT and TIP39-KO mice to evaluate whether these could contribute to the behavioral phenotype. Because of the potential for recruitment of neuropeptides by environmental provocation, we also compared anxiety-related behaviors between WT and TIP39-KO mice under bright or dim illumination and with and without prior restraint.
Materials and methods
Generation and confirmation of TIP39 knockout
The generation and genotyping of mice in which the entire coding sequence of TIP39 was replaced with a lacZ/neomycin resistance cassette has recently been described (Usdin et al. 2008). Immunohistochemistry to confirm the absence of the previously described TIP39-containing fibers in the brain was performed as previously described (Dobolyi et al. 2003b) using an antibody to TIP39.
Mice used for the experiments reported here originated from F1H4 ES cells [a 129/SvEv-C57BL/6 F1 hybrid ES cell line (Valenzuela et al., 2003)] and were backcrossed two times into C57BL/6J. Mice were housed in groups of two to four with same-sex littermates in a temperature- and humidity-controlled vivarium under a 12-h light/dark cycle (lights on 0600 h). To avoid phenotypic effects resulting from genotypic differences in maternal behavior and early life environment (Holmes et al. 2005), WT and TIP39-KO were littermates generated from heterozygous (HET) × HET matings.
Testing was conducted in male mice aged between 7 and 9 weeks at the start of testing. Unless otherwise stated, mice were experimentally naive and acclimated to the test room for 1 h and apparatuses were cleaned with a 70% ethanol solution and dried between subjects. The experimenter remained blind to genotype during testing: subjects were identified by ear tags. The number of mice per genotype tested is given in the figure legends. All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and strictly followed the NIH guidelines ‘Using Animals in Intramural Research’. Every effort was made to minimize the pain and suffering of the animal subjects.
General characterization
To test the effect of TIP39 on blood pressure, WT mice were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal), tracheotomized to facilitate breathing and polyethylene cannulas inserted into the carotid artery and jugular vein for blood pressure monitoring and intravenous drug injections, respectively (Batkai et al. 2004). The arterial cannula was connected to a pressure transducer and the signal was recorded using the IOX Data Acquisition System (Emka Technologies, France). Mean arterial blood pressure was calculated at baseline, 10, 30 and 60 min postinjection. Baseline left ventricular performance was characterized in a cohort of 4- to 5-month-old TIP39-KO and WT that had previously been exposed to the elevated plus-maze test. Mice were anesthetized with 2% isoflurane using 1-F microtip pressure–volume catheters (PVR 1045) and an ARIA pressure–volume conductance system (Millar Instruments, Houston, TX, USA) coupled to a Powerlab/4SP A/D converter (AD Instruments, Mountain View, CA, USA) (Mukhopadhyay et al. 2007; Pacher et al. 2003). Heart rate, left ventricular systolic pressure, left ventricular end diastolic pressure, maximum first derivative of ventricular pressure rise or decline with respect to time (+dP/dt, −dP/dt), stroke volume and cardiac output (CO) was calculated by PVAN 3.5 software (Millar Instruments). Cardiac index with CO normalized to body weight.
Mice were characterized in a functional observational battery (Boyce-Rustay et al. 2006). Motor co-ordination was assessed using the accelerating rotarod (Boyce-Rustay & Holmes 2005). The apparatus was a 3-cm-diameter dowel (Ugo Basile, Stoelting, Wood Dale, IL, USA) onto which the mouse was placed and then accelerated at a constant rate of 8 r.p.m./min up to 40 r.p.m. The latency to fall to the floor was automatically recorded by the apparatus, with a maximum cutoff latency of 5 min. Locomotor activity in the home cage was assessed in mice individually housed in a standard home cage placed within a photocell-based Photobeam Activity System (San Diego Instruments, San Diego, CA, USA) under normal vivarium conditions and left undisturbed for 72 h.
Exploration in the holeboard test was measured as previously described (File & Wardill 1975). Briefly, the mouse was placed in the center of a 45-cm2 arena with a Plexiglass floor containing 16 evenly spaced 3-cm diameter, 15-cm deep holes for 10 min. The number of head pokes into the holes was measured by the photocell-based Opto Varimex Minor activity meter (Columbus Instruments, Columbus, OH, USA).
Anxiety-related behaviors
Anxiety-like behavior was initially measured in a single cohort of mice using four well-validated tests (Cryan & Holmes 2005). One cohort was tested on three exploration-based assays – elevated plus-maze, novel open field, dark-light emergence test – in that order, with at least 1 week between tests. A second, naive, cohort was tested on the shock-probe burying test.
The elevated plus-maze test (Lister 1987) apparatus used in the original behavioral characterization consisted of two open arms (30 × 5 cm) and two closed arms (30 × 5 × 15 cm) extending from a central (5 × 5 cm) area. The walls were made from black Plexiglass and the floor from white textured plastic. A 0.5-cm raised lip around the perimeter of the open arms prevented mice from falling off the maze. The apparatus was elevated 62 cm off of the floor. The apparatus was placed in a room with black curtains encircling the apparatus and illuminated to 15 lux. The mouse was placed in the center facing an open arm and allowed to explore the apparatus for 6 min. Time spent in the open arms, and entries into the open and closed arms were measured by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA, USA). In addition, head-dipping (exploratory scanning over the sides of the maze) and stretch-attend postures (forward elongation of the head and shoulders followed by retraction to original position) were scored from videotape (Holmes et al. 2000).
The dark–light emergence test (Crawley 1981) apparatus consisted of a 40 × 40 × 30 cm white Plexiglass compartment illuminated to 400 lux and a 40 × 40 × 10 cm black Plexiglass compartment illuminated to 5 lux that were connected by a 7 × 12 cm aperture at floor level. The mouse was placed into the dark compartment facing away from the aperture and allowed to freely explore the apparatus for 10 min. Percent time spent in the dark compartment, latency to enter the light compartment, intercompartment transitions and scanning of the light compartment (defined as the head but less than all four paws in the light) were manually scored from videotape.
For the novel open field test, the mouse was placed in the center of a 40 × 40 × 30 cm open field (gray Plexiglass sides and white Plexiglass floor) illuminated to 600 lux for 30 min. Total distance traveled in the whole arena and time spent in the center (20 × 20 cm) was measured by the Ethovision videotracking system.
For shock-probe burying (Sluyter et al. 1996), the apparatus consisted of a Plexiglass cage (20 × 27 × 14 cm) covered with 3-cm deep standard cage substrate, with an electrified probe (4 cm long, 1 cm in diameter, 1 cm above bedding material) extending 4 cm into the cage from one side. Contact with the probe delivered a 2-mA shock. Following the delivery of the first shock, the duration of probe burying (defined as pushing or spraying substrate toward the probe with the forelimbs), the time spent in the probe zone (10 × 12 cm area surrounding the probe) and the number of shocks received over 5 min were scored manually from videotape.
Anxiety-related behavior under high illumination or following restraint
The elevated plus-maze apparatus used in the stress experiments consisted of two open arms (38 × 5 cm) and two closed arms (38 × 5 × 15 cm) extending from a central (5 × 5 cm) area. The walls were made from black Plexiglass and the floor of the closed arms from black plastic. The floor of the open arms consisted of a gray textured plastic. The apparatus was elevated 77 cm off the floor. The apparatus was placed in a room that contained various pieces of laboratory equipment stored on surrounding shelves. Mice were restrained in a plastic DecapiCone (Braintree Scientific, Braintree, MA, USA) for 30 min, allowed to recover in a housing cage for 5 min and then tested (along with undisturbed controls) on the elevated plus-maze as described above, except that the test was performed with a maze from a different manufacturer in a different room. A second cohort of mice was tested in the elevated plus-maze either under low illumination (15 lux) as above or high illumination (150 lux).
Stress-induced serum glucocorticoid levels
Mice were restrained in a plastic DecapiCone (Braintree Scientific) for 30 min. Following 5-min recovery in a housing cage, blood was collected from the submandibular vein located directly behind the cheek pouch using a GoldenRod animal lancet (MEDIpoint, Inc., Mineola, NY, USA). Serum corticosterone was determined using a radioimmunoassay kit from MP Biomedicals (Irvine, CA, USA; cat number 07120103).
Pavlovian fear conditioning
Learned fear was assessed via Pavlovian fear conditioning (Kim et al. 1992) in a 27 × 27 × 11 cm chamber with transparent walls and a metal rod floor. To provide a distinctive olfactory environment, the chamber was cleaned between subjects with a 69.5% water/29.5% ethanol/1% vanilla extract solution. After a 2-min acclimation period, the mouse received three pairings (1- to 2-min interval between pairings) between an auditory tone (30-second, 80-dB, 3-kHz tone) and footshock (2-second, 0.6-mA scrambled footshock) in which the shock was presented during the last 2 seconds of the tone. The presentation of stimuli was controlled by the San Diego Instruments Freeze Monitor system (San Diego Instruments).
Twenty-four hours after conditioning, memory for the tone (tone test) was tested in a novel context, in a different room from the training. The novel context was a 27 × 27 × 11 cm chamber with black/white-checkered walls and a solid-Plexiglass, opaque floor. The chamber was cleaned between subjects with a 70% ethanol/30% water solution. After a 2-min acclimation period, the tone was presented for 6 min. Twenty-four hours after the tone test, memory for the conditioned context (context test) was tested in the same room as the original training by placing the mouse in the training chamber for 5 min.
Freezing was defined as the absence of any visible movement except that required for respiration and was scored from videotape at 5-second intervals by an observer blind to genotype. The number of observations scored as freezing was converted to a percentage [(number of freezing observations/total number of observations) × 100] for analysis.
Pavlovian fear extinction
Mice were tested for Pavlovian fear extinction as previously described (Wellman et al. 2007). Mice were fear conditioned as above and 24 h later placed in the novel context (as above). After a 2-min acclimation period, the tone was presented 40 times (5-second intertone-interval). To assess extinction recall 24 h later, mice were returned to the novel context and presented with the tone 20 times. Freezing during extinction was binned into four-trial blocks for analysis.
Shock sensitivity and nociception
To test for differences in shock sensitivity that might have contributed to genotypic differences in fear conditioning (and shock-probe burying), naive mice were place in the chambers used for fear conditioning and exposed to a 0.5-second footshock starting at 0.07 mA and increasing by 0.01 to 0.12 mA with 30 seconds between shocks. Reaction to the shock was assessed using a 4-point scoring system: 0, no vocalization or physical reaction; 1, lifting of feet; 2, vocalization; 3, jump.
For the hotplate test, mice were (singly) placed on a flat plate (Columbus Instruments) heated to 55°C. The latency to display a hindpaw shake or lick was timed by an observer, with a maximum response latency of 30 seconds. For the tail-flick test, mice were placed in a cylindrical restrainer with the tail placed into a groove into which an intense light beam was focused (Columbus Instruments). The latency to show a tail-flick response was timed by the apparatus, with a maximum response latency of 10 seconds. For the von Frey fiber test, the mouse was placed in an 11 × 11 × 12 cm Plexiglass compartment over a wire mesh floor. After a 15-min acclimation period, a succession of von Frey fibers were pressed into the left hindpaw until a response was observed (retraction, flick or lick in 7 of 10 applications of force). For the Hargreaves test, the mouse was placed into an 11 × 11 × 12 cm compartment of the Plantar Test Apparatus on top of a glass plate (IITC Life Science, Woodland Hills, CA, USA). After a 15-min acclimation period, a radiant heat light beam was focused onto a hindpaw from below. The latency to respond with a foot flick was timed by the apparatus, with a maximum response latency of 30 seconds to avoid possible tissue damage.
Statistical analysis
Genotype effects were analyzed with unpaired t-tests. Genotype × time (novel open field test, home cage activity), genotype × trial (accelerating rotarod), genotype × stress (elevated plus-maze) and genotype × trial block (fear extinction) were analyzed by two-factor analysis of variance (ANOVA), with repeated measures for time, trial, stress and trial block, respectively, followed by Newman–Keuls post hoc tests where appropriate. Statistical significance was set at P ≤ 0.05.
Results
General characterization
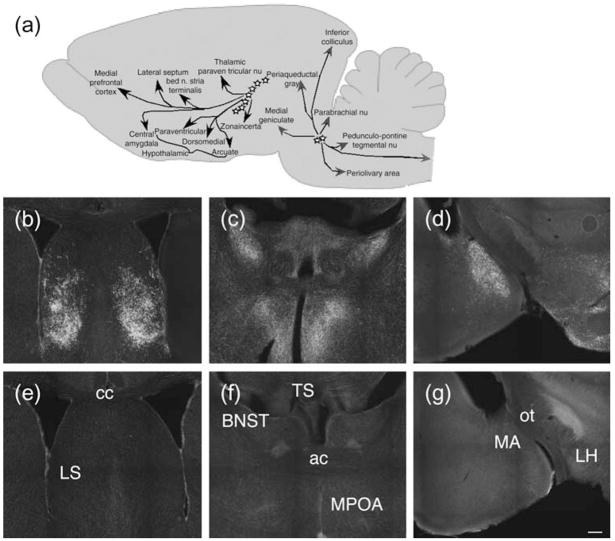
We recently described mice with replacement of the gene sequence encoding TIP39 (Usdin et al. 2008). Labeling with an antibody to TIP39 in WT and TIP39-KO confirmed that the limbic distribution of TIP39-like immunoreactivity that we have previously reported is absent in TIP39-KO (Fig. 1).
Figure 1. Absence of TIP39-like immunoreactivity in mutant mice.
(a) Cartoon illustrating location of TIP39 neurons and their major projections. (b–g) Antibody labeling in WT and TIP39-KO. Sections that include the lateral septum (b, e), bed nucleus of the stria terminalis and medial preoptic area (c, f), and parts of the amygdala (d, g) from WT (b–d) and age-matched TIP39-KO (e–g) were labeled with antibodies to TIP39. Note the absence of TIP39 labeling in sections from TIP39-KO (bottom row). LS, lateral septum; BNST, bed nucleus of the stria terminalis; TS, triangular septal nucleus; MPOA, medial preoptic nucleus; MA, medial amygdala; LH, lateral hypothalamus; cc, corpus callosum; ac, anterior commisure; ot, optic tract. Scale bar = 200 μm for all sections.
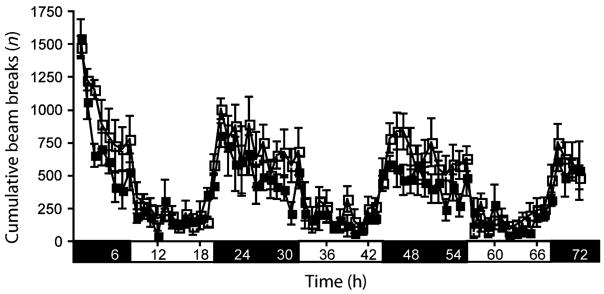
TIP39-KO and WT did not differ on a panel of cardiovascular parameters (Table 1), and TIP39 infusion into WT did not affect blood pressure (data not shown). TIP39-KO could not be distinguished from WT on casual observation or in a functional observational battery (Boyce-Rustay et al. 2006) for general health, reflexes and basic sensory and motor functions (Table 2). There were also no significant differences between the genotypes in home cage locomotor activity over 72 h of monitoring, exploration in a holeboard test or in motor coordination as assessed on an accelerating rotarod (Fig. 2, Table 2). Male TIP39-KO mated with females as demonstrated by the presence of copulatory plugs within 1 week in six of six WT females housed with male KO.
Table 1.
Normal measures of cardiovascular function in TIP39-KO
| WT | KO | |
|---|---|---|
| Left ventricular systolic pressure (mmHg) | 103.7 ± 3.9 | 102.9 ± 2.5 |
| End diastolic pressure (mmHg) | 5.3 ± 2.0 | 5.8 ± 1.4 |
| Mean arterial pressure (mmHg) | 81.3 ± 6.0 | 79.2 ± 1.7 |
| Heart rate (BPM) | 502.4 ± 15.1 | 510.9 ± 35.5 |
| Stroke volume (ml) | 23.0 ± 1.8 | 23.1 ± 1.1 |
| Cardiac output (ml/min) | 11.5 ± 0.09 | 11.9 ± 1.2 |
| Cardiac index (ml/min/kg) | 345.7 ± 26.5 | 387.0 ± 46.2 |
| Max dP/dt (mmHg/second) | 10022.4 ± 925.3 | 9973.7 ± 734.2 |
| Min dP/dt (mmHg/second) | −8680.1 ± 835.1 | −7955.1 ± 367.4 |
| Heart weight (mg) | 152.50 ± 11.1 | 152.00 ± 8.2 |
TIP39-KO and WT did not differ in cardiovascular parameters measured with PV catheters, as described in the methods. +dP/dt, −dP/dt: maximum first derivative of ventricular pressure rise or decline with respect to time. Data are mean ± SEM and n =6–7.
Table 2.
TIP39-KO showed no physical, neurological or gross behavioral abnormalities as assessed in a functional observation battery
| WT | KO | |
|---|---|---|
| Physical characteristics (n = 16/genotype) | ||
| Body weight (g) | 29.9 ± 1.2 | 31.2 ± 1.4 |
| Missing whiskers (%) | 0 | 0 |
| Bald patches (%) | 0 | 0 |
| Piloerection | 0 | 0 |
| Splayed limbs (%) | 0 | 0 |
| Forepaw clutch (%) | 0 | 0 |
| Hindpaw clutch (%) | 0 | 0 |
| Empty cage observations (n = 16/genotype) | ||
| Wild running (%) | 0 | 0 |
| Spontaneous freezing (%) | 0 | 0 |
| Tremor (%) | 0 | 0 |
| Grooming (three-point scale) | 0.7 ± 0.2 | 0.7 ± 0.1 |
| Reactivity to handling (three-point scale) | 2.0 ± 0.3 | 2.1 ± 0.2 |
| Neurological reflexes (n = 16/genotype) | ||
| Righting reflex (%) | 100 | 100 |
| Eyeblink (%) | 100 | 100 |
| Ear twitch (%) | 100 | 100 |
| Whisker twitch (%) | 100 | 100 |
| Motor function (n = 8–10/genotype) | ||
| Wire hang latency (seconds) | 54.4 ± 4.4 | 54.1 ± 4.1 |
| Rotarod latency (seconds) | 77.5 ± 7.9 | 97.4 ± 11.9 |
| Fecal Boluses (n = 10/genotype) | ||
| Light-dark emergence (n) | 0.8 ± 0.4 | 2.8 ± 0.5* |
| Hole board (n = 16/genotype) | ||
| Head-dips | 64.1 ± 6.7 | 64.5 ± 7.1 |
Data are mean ± SEM or percent where indicated.
P < 0.05.
Figure 2. Home cage activity.
TIP39-KO did not differ in home cage locomotor activity from WT controls. WT, open squares; KO, solid squares. Data are mean ± SEM; n = 10/genotype.
Anxiety-related behaviors
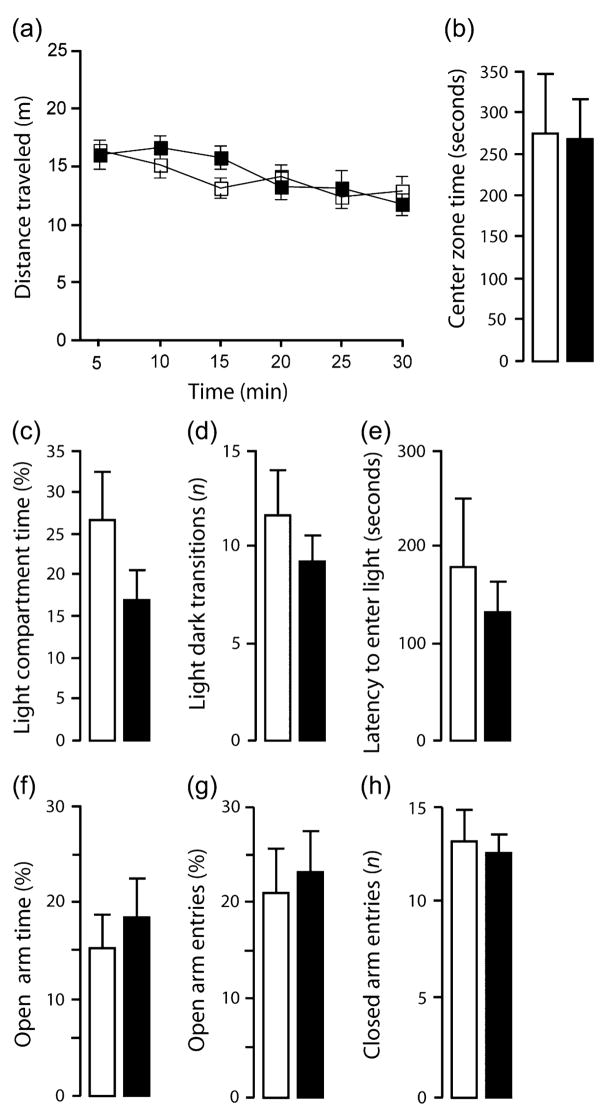
TIP39-KO were not different from WT in the novel open field, dark–light emergence and elevated plus-maze tests under standard test conditions, as measured by the main spatio-temporal measures (Rodgers et al. 1997) (Fig. 3a–h). However, TIP39-KO did differ from WT controls on several ethological parameters. In the elevated plus-maze, TIP39-KO displayed fewer head-dips (t = 2.48, df = 14, P < 0.05; Fig. 4a) and more stretched-attend postures (t = 2.47, df = 14, P < 0.05; Fig. 4b) in the open arms of the elevated plus-maze relative to WT. TIP39-KO also produced more fecal boluses in the dark-light emergence test than WT (Table 2).
Figure 3. Exploration-based anxiety tests.
In the novel open field test, total distance traveled (a) and time spent in the center (b) did not differ between genotypes. In the dark–light emergence task, time spent in the light compartment (c), number of transitions between the compartments (d) and the latency to enter the light compartment (e) were not significantly different between genotypes. In the elevated plus-maze test, TIP39-KO and WT showed similar percent open arm time (f), percent open arm entries (g) and number of closed arm entries (h). WT, open squares or bars; KO, solid squares or bars. Data are mean ± SEM; n = 10/genotype.
Figure 4. Ethological measures in the EPM.
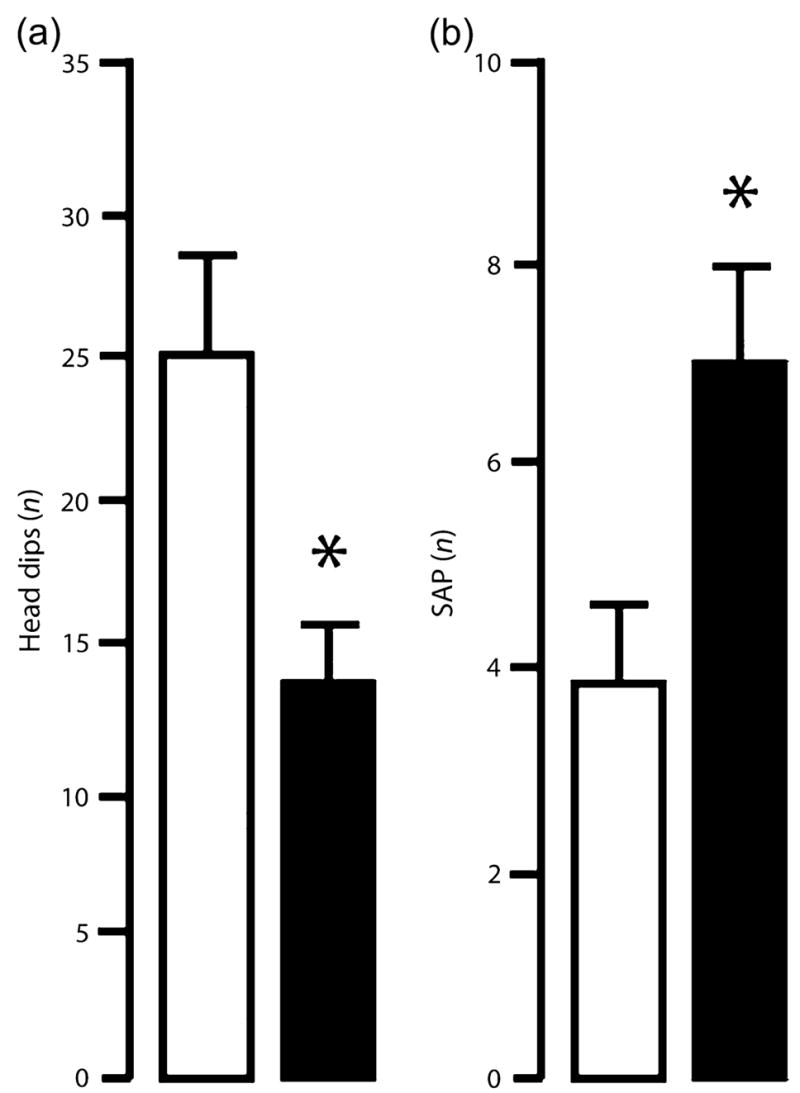
In the elevated plus-maze test, TIP39-KO displayed fewer head-dips (a) and an increased number of stretched-attend postures (SAP) (b) in the open arms relative to WT controls. WT, open bars, KO, solid bars. Data are mean ± SEM; n = 8/genotype. *P < 0.05
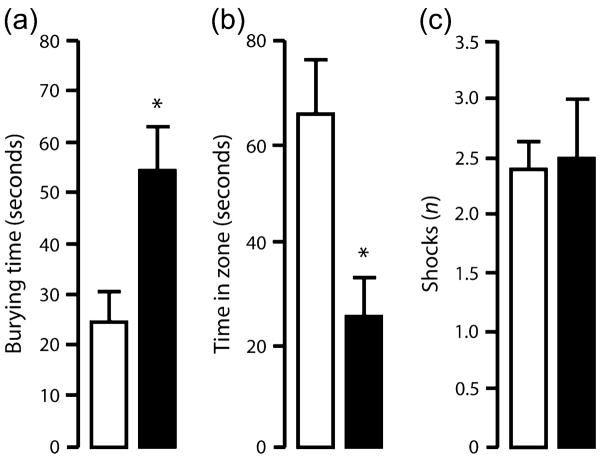
In the shock-probe defensive burying test, TIP39-KO spent more time engaged in defensive burying than WT (t = 2.72, df = 14, P < 0.05; Fig. 5a). TIP39-KO also spent significantly less time in the zone around the electrified probe than WT (t = 2.94, df = 14, P < 0.05; Fig. 5b). The number of shocks received did not differ between genotypes (Fig. 5c).
Figure 5. Shock-probe defensive burying test.
TIP39-KO spent more time engaged in defensive burying (a) and spent less time near the probe (b), but received a similar number of shocks (c), in the shock-probe defensive burying test when compared with WT. WT = open bars, KO = solid bars. Data are mean ± SEM; n = 10/genotype. *P < 0.05.
Anxiety-related behavior under high illumination or following restraint
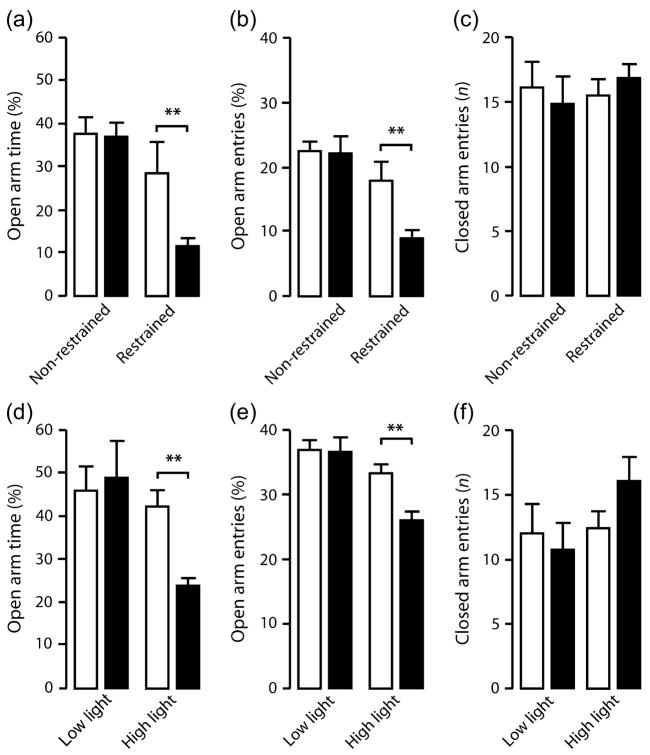
On comparison of WT and TIP39-KO mice with and without prior restraint in the elevated plus-maze, there was a significant interaction between genotype and restraint stress for percent open arm entries (F1,36 = 4.82, P < 0.5). There was a borderline significant genotype × stress interaction in percent open arm time (F1,36 = 4.08, P = 0.0508). Post hoc analysis showed that TIP39-KO spent significantly less time in the open arms (Fig. 6a) and made significantly fewer open arm entries (Fig. 6b) when compared with WT following restraint but not under non-stress conditions. There was not a significant effect of restraint on open arm entries of WT. The genotypes did not significantly differ in closed (Fig. 6c) or total (data not shown) arm entries.
Figure 6. Anxiety-related behavior in TIP39-KO mice under stress.
TIP39-KO spent less time in the open arms (a) and made fewer open arm entries (b), but did not differ in closed arm entries (c) when compared with WT in the elevated plus-maze following restraint stress (no restraint; WT, n = 10, KO, n = 8; restraint; WT, n = 8, KO, n = 14). TIP39-KO spent less time in the open arms (d) and made fewer open arm entries (e), while closed arm entries were not significantly changed (f) when compared with WT controls under high, but not low, illumination levels in the elevated plus-maze (low illumination; WT, n = 8/genotype; high illumination; WT, n = 9, KO, n = 8). WT, open bars; KO, solid bars. Data are mean ± SEM. **P < 0.01.
In a separate experiment, there was a significant interaction between genotype and illumination level for percent open arm entries (F1,29 = 5.48, P < 0.01) and open arm time (F1,29 = 6.67, P < 0.05). Post hoc analysis showed that TIP39-KO spent significantly less time in the open arms (Fig. 6d) and made significantly fewer open arm entries (Fig. 6e) when compared with WT under high, but not low, illumination. WT behavior was not significantly affected by illumination condition. The genotypes did not significantly differ in closed (Fig. 6f) or total (data not shown) arm entries.
Stress-induced serum glucocorticoid levels
There was no significant difference between the genotypes in basal serum corticosterone level (WT = 87.9 ± 20.4 ng/ml, KO = 83.2 ± 13.4, n = 8, P > 0.05) or following 30 min restraint stress (WT = 439.4 ± 17.3 ng/ml, KO = 437.4 ± 13.0, n = 8, P > 0.05).
Pavlovian fear conditioning
In the fear condition experiment, there was a significant interaction between genotype and trial for freezing during training (F2,45 = 2.69, P < 0.05). Post hoc analysis showed that TIP39-KO froze significantly more than WT to the second but not first or third tone presentation (Fig. 7a). Freezing in TIP39-KO was also significantly higher than in WT during tone recall (t = 3.99, df = 33, P < 0.01; Fig. 7b) (but not during the pretone baseline period) and during context recall (t = 5.97, df = 33, P < 0.01; Fig. 7c).
Figure 7. Acquisition, expression and extinction of conditioned fear in TIP39-KO mice.
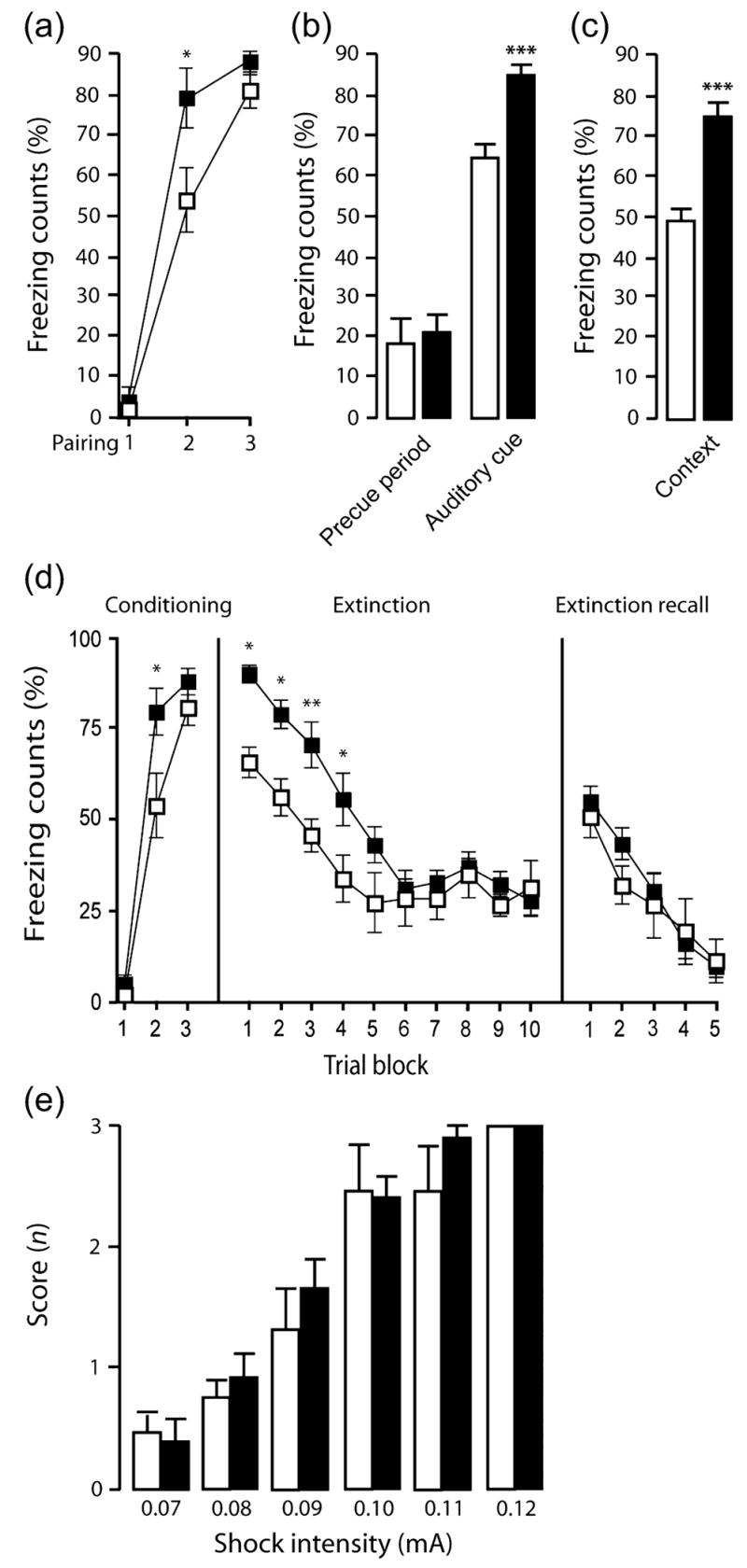
TIP39-KO mice froze more than WT controls during fear conditioning (a), during the tone test (b) and during the context test (c) (WT, n = 18; KO, n = 17). In an extinction test, TIP39-KO mice froze more during conditioning (d, first panel) and at the beginning of the tone test (d-second panel) than WT controls. Rates of within-session extinction (d, second panel) and extinction recall (d, third panel) were not different between genotypes (WT, n = 8; KO, n = 10). Shock sensitivity did not differ significantly between genotypes at any intensity measured (e) (n = 8/genotype). WT, open bars or squares; KO, solid bars or squares. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Pavlovian fear extinction
The difference during training was replicated in an separate experiment set up to evaluate extinction [Fig. 7d, left panel; (F2,45 = 3.40, P < 0.05) with significance again because of higher freezing in TIP39-KO than WT in the second but not first or third tone presentation]. During fear recall and extinction training, TIP39-KO showed higher initial freezing than WT to tone presentation but extinguished to WT levels with repeated tone presentations (effect of genotype: F1,15 = 8.49, P = 0.01; effect of trial block: F9,15 = 9.37, P < 0.01; genotype × trial interaction: F1,9 = 1.22, P = 0.17; Fig. 7d center panel). During extinction recall, freezing did not differ between genotypes, and all mice showed a significant reduction in freezing with repeated tone presentations (effect of genotype: F1,15 = 0.23, P = 0.64; effect of trial-block: F4,15 = 12.24, P < 0.01; genotype × trial interaction: F1,4 = 0.73, P = 0.79; Fig. 7d right panel).
Evaluation of nociception
TIP39-KO did not differ from WT in behavioral responses to shock at any intensity tested, which ranged from 0.07 to 1.2 mA (Fig. 7e). Genotypes also showed no significant differences in nociception, as assayed by either mechanical stimulation with von Frey fibers, or withdrawal latency to a thermal stimulus in the tail-flick, hotplate, or plantar withdrawal (Hargreaves) tests (Table 3).
Table 3.
Normal nociception in TIP39-KO
| Test | WT | KO |
|---|---|---|
| von Frey (g) | 4.1 ± 1.1 | 4.5 ± 0.9 |
| Tail-flick latency (seconds) | 4.0 ± 0.7 | 3.9 ± 0.2 |
| Hotplate latency (seconds) | 8.0 ± 0.6 | 9.6 ± 0.9 |
| Hargreaves latency (seconds) | 4.7 ± 0.6 | 4.2 ± 0.4 |
TIP39-KO did not differ from WT in mechanical sensitivity tested with von Frey fibers or in thermal sensitivity evaluated in the tail-flick, hotplate and Hargreaves tests. Data are mean ± SEM and n = 10/genotype for each test.
Discussion
Initial characterization showed that male TIP39-KO developed normally and were not different from WT on a battery of tests that included evaluation of home cage activity, motor coordination, sensory functions and general health. (Note – female mice were not investigated in this study because of the potential confounds introduced by varying levels of ovarian hormones during the estrus cycle and the failure of ovarian development in female TIP39-KO.) Under standard test conditions, exploratory and anxiety-related behaviors in the hole-board, novel open field, elevated plus-maze and dark–light emergence tests were also largely similar between TIP39-KO and WT. Exceptions included increased risk-assessment (elevated plus-maze) behaviors and defecation (dark–light emergence) observed in TIP39-KO, which provided the suggestion of a modest increase in anxiety-like behavior.
A robust increase in anxiety-like behavior was seen in TIP39-KO in the shock-probe burying test. In this test, TIP39-KO showed more defensive burying following contact with the shock probe. This phenotype was not explained by increased sensitivity to pain because the genotypes did not differ in responsivity to footshock or in several assays of nociception. Therefore, the manifestation of a clear anxiety-like phenotype in the TIP39-KO in the shock-probe burying test can be argued to be because of an inherently greater anxiety-provoking nature of this task than the exploration-based assays in which TIP39-KO were largely normal.
This hypothesis was supported by the results of two experiments in which mice were tested on the elevated plus-maze under conditions designed to amplify the anxiety-provoking nature of the test – high illumination and prior restraint. Both these putative ‘stressors’ uncovered a significant increase in anxiety-like behavior in TIP39-KO. This was unrelated to changes in control measures of locomotor activity, demonstrating the anxioselectivity of the phenotype. Of note, while these stressors were adequate to reveal an anxiety-like phenotype in KO, they did not increase the anxiety-like behavior measured in WT. Although this was our a priori intent, it may seem surprising in light of previous evidence that similar manipulations heighten anxiety-like responses in rats (Bignante et al. 2008; Calvo et al. 1998; Garcia et al. 2005; Heinrichs et al. 1994; Hogg 1996; Martijena et al. 1997; Mechiel Korte & De Boer 2003). However, this could reflect a genuine species difference because we chose a 30-min restraint protocol based on literature, suggesting that either a longer period of restraint or repeated restraint is needed to induce significant anxiety-like responses in mice (Bignante et al. 2008, Chotiwat & Harris 2006; Dunn & Swiergiel 1999; Hata et al. 2001; Hsu et al. 2007; Schaefer et al. 2000). Furthermore, while high illumination levels are generally thought to increase elevated plus-maze anxiety-like responses in rats (Griebel et al. 1993; Hogg 1996), some investigators observe a threshold effect at relatively low illumination level (Garcia et al. 2005), and effects on mice are less well documented. A final consideration is that both the non-stressed TIP39-KO and WT showed relatively less anxiety-like behavior than in our initial experiments conducted under similar non-stressed conditions. This is likely because of the use of a different apparatus and test room, which we have subsequently found to produce less anxiety-like behavior regardless of mouse strain.
The abnormal anxiety-like phenotype of TIP39-KO extended to enhanced conditioned fear responses. The mutants exhibited increased freezing to a tone paired with shock during training and subsequently showed more freezing when re-exposed to either the tone or the conditioned context, indicating enhanced acquisition and/or enhanced expression of fear. This phenotype was replicated across two separate experiments. In contrast, fear extinction learning and recall were unaffected by loss of TIP39. The analysis of nociceptive responses we performed produced no indication that abnormal pain processing could account for genotype differences in conditioned fear. This is an important point to emphasize, given the presence of the PTH2-R in the dorsal horn of the spinal cord and primary afferent neurons (Dobolyi et al. 2003a, 2006) and previous observations that percutaneously or intrathecally delivered TIP39 has pro-nociceptive effects (Dobolyi et al. 2002).
Collectively, our data point to a specific increase in fear, and stress-related anxiety-like, behavior following loss of TIP39. This finding suggests that TIP39 normally acts to negatively modulate these behaviors. The distribution of TIP39 in the mouse brain is entirely consistent with this role. There is a high density and quite restricted distribution of TIP39-positive fibers and PTH2 receptors in regions known to mediate fear and anxiety, including parts of the amygdala, bed nucleus of the stria terminalis and lateral septum (Dobolyi et al. 2003b; Faber et al. 2007). In turn, the areas containing TIP39-positive neurons in the subparafascicular area receive inputs from these regions, suggesting a reciprocal circuit (Dobolyi et al. 2003b; Wang et al. 2006). Other neuropeptide systems, including CRH, neuropeptide Y, arginine vasopressin and galanin have been shown to affect rodent anxiety-like behaviors (Belzung et al. 2006; Griebel 1999; Karlsson & Holmes 2006). In general, however, the neurons synthesizing these peptides are more widely distributed than TIP39 neurons, and based on the available data, these peptides also have more varied actions. These observations suggest that TIP39 might play a relatively selective modulatory role in the brain with prominent effects on fear and anxiety.
That said, we cannot exclude the possibility that developmental compensations outside these neural circuits or in the periphery contribute to the phenotypic abnormalities currently seen in mice constitutively lacking TIP39. However, we did not detect a difference in testosterone level (Usdin et al. 2008), suggesting that gonadal hormone effects are unlikely to contribute to the behavioral differences. Moreover, although the distribution of TIP39 and the PTH2-R suggests potential effects on cardiovascular function, TIP39-KO showed normal baseline cardiac function. Finally, the projection pattern of TIP39 neurons, together with a pharmacological study in which TIP39 addition to hypothalamic cultures and injection into rat-brain-stimulated CRH and ACTH release (Ward et al. 2001), suggest that TIP39 affects the function of hypothalamic CRH neurons. However, we found that neither basal nor restraint-stress evoked corticosterone or ACTH (not shown) was affected by loss of TIP39. Thus, while it seems probable based on distribution that TIP39 influences hypothalamic and CRH function, this did not appear to be sufficiently robust to produce measurable differences under these conditions, and thus, it seems unlikely that hypothalamo-pituitary-adrenal axis abnormalities caused the observed stress-related behavioral differences in TIP39-KO. Nonetheless, generation of conditional TIP39-KO that circumvent developmental and peripheral issues may be ultimately required to fully elucidate the physiological role of TIP39.
In conclusion, this study has shown that TIP39, a neuropeptide localized in neural circuits subserving emotional processing, plays an important role in the modulation of fear- and anxiety-like behaviors, as demonstrated in a mouse with TIP39 deletion. The anxiety-like phenotype of TIP39-KO, which is selectively manifested under relatively strong provocation, provides a compelling example of the preferential recruitment of neuropeptides as modulators of anxiety under conditions of stress or pathology (Hokfelt et al. 2003; Holmes et al. 2003). While further investigation of its behavioral and physiological functions is required, current data suggest the possibility that the TIP39/PTH2-R system could contribute to the pathophysiology of anxiety disorders and be a novel therapeutic target for these and other stress-related neuropsychiatric diseases.
Acknowledgments
We thank Ildiko Racz and Sarah Irwin for help with preliminary experiments, Jim Pickel and the NIMH transgenic mouse core facility for help with generation of mice, and Heather Cameron and Jacqueline Crawley for the use of behavior testing equipment. This research was supported by the Intramural Program of the NIH, National Institute of Mental Health. M.W.S. and A.M. are employees of Regeneron and own stock in Regeneron.
Footnotes
The authors declare that there are no potential conflicts of interest.
References
- Alonso J, Angermeyer MC, Bernert S, et al. Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand. 2004;(Suppl):47–54. doi: 10.1111/j.1600-0047.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Jarai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol. 2004;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Yalcin I, Griebel G, Surget A, Leman S. Neuropeptides in psychiatric diseases: an overview with a particular focus on depression and anxiety disorders. CNS Neurol Disord Drug Targets. 2006;5:135–145. doi: 10.2174/187152706776359682. [DOI] [PubMed] [Google Scholar]
- Bignante EA, Rodriguez Manzanares PA, Mlewski EC, Bertotto ME, Bussolino DF, Paglini G, Molina VA. Involvement of septal Cdk5 in the emergence of excessive anxiety induced by stress. Eur Neuropsychopharmacol. 2008 doi: 10.1016/j.euroneuro.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res. 1998;800:227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Chotiwat C, Harris RB. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav. 2006;50:489–495. doi: 10.1016/j.yhbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Ueda H, Uchida H, Palkovits M, Usdin TB. Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc Natl Acad Sci U S A. 2002;99:1651–1656. doi: 10.1073/pnas.042416199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Bodnar I, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience. 2003a;122:1093–1105. doi: 10.1016/j.neuroscience.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. Expression and distribution of tuberoinfundibular peptide of 39 residues in the rat central nervous system. J Comp Neurol. 2003b;455:547–566. doi: 10.1002/cne.10515. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Wang J, Usdin TB. The distribution and neurochemistry of the parathyroid hormone 2 receptor in the rat hypothalamus. Neurochem Res. 2006;31:227–236. doi: 10.1007/s11064-005-9011-9. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 1999;845:14–20. doi: 10.1016/s0006-8993(99)01912-5. [DOI] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, Usdin TB. Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44:53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Cardenas FP, Morato S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:265–270. doi: 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Griebel G. Is there a future for neuropeptide receptor ligands in the treatment of anxiety disorders? Pharmacol Ther. 1999;82:1–61. doi: 10.1016/s0163-7258(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Martin JR, Misslin R. Some critical determinants of the behaviour of rats in the elevated plus-maze. Behav Processes. 1993;29:37–48. doi: 10.1016/0376-6357(93)90026-N. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hata T, Nishikawa H, Itoh E, Funakami Y. Anxiety-like behavior in elevated plus-maze tests in repeatedly cold-stressed mice. Jpn J Pharmacol. 2001;85:189–196. doi: 10.1254/jjp.85.189. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 1994;11:179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Bartfai T, Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol Behav. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Koenig EA, Gold E, Stephenson D, Yang RJ, Dreiling J, Sullivan T, Crawley JN. Phenotypic assessment of galanin overexpressing and galanin receptor R1 knockout mice in the tail suspension test for depression-related behavior. Psychopharmacology (Berl) 2005;178:276–285. doi: 10.1007/s00213-004-1997-1. [DOI] [PubMed] [Google Scholar]
- Hsu HR, Chen TY, Chan MH, Chen HH. Acute effects of nicotine on restraint stress-induced anxiety-like behavior, c-Fos expression, and corticosterone release in mice. Eur J Pharmacol. 2007;566:124–131. doi: 10.1016/j.ejphar.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Inui A, Okita M, Nakajima M, Momose K, Ueno N, Teranishi A, Miura M, Hirosue Y, Sano K, Sato M, Watanabe M, Sakai T, Watanabe T, Ishida K, Silver J, Baba S, Kasuga M. Anxiety-like behavior in transgenic mice with brain expression of neuropeptide Y. Proc Assoc Am Physicians. 1998;110:171–182. [PubMed] [Google Scholar]
- Karlsson RM, Holmes A. Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids. 2006;31:231–239. doi: 10.1007/s00726-006-0336-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Dobolyi A, Usdin TB. Tuberoinfundibular peptide of 39 residues produces anxiolytic and antidepressant actions. Neuroreport. 2004;15:881–885. doi: 10.1097/00001756-200404090-00030. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Martijena ID, Calvo N, Volosin M, Molina VA. Prior exposure to a brief restraint session facilitates the occurrence of fear in response to a conflict situation: behavioral and neurochemical correlates. Brain Res. 1997;752:136–142. doi: 10.1016/s0006-8993(96)01465-5. [DOI] [PubMed] [Google Scholar]
- Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Watanabe S, Niki H. Increased fearfulness of Fyn tyrosine kinase deficient mice. Behav Brain Res. 1994;27:179–182. doi: 10.1016/0169-328x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Ross G, Heinemann MP, Schlüter KD. Vasodilatory effect of tuberoinfundibular peptide (TIP39): requirement of receptor desensitizaion and its beneficial effect in the post-ischemic heart. Peptides. 2007;28:878–886. doi: 10.1016/j.peptides.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Wong ST, Wozniak DF, Muglia LM, Liauw JA, Zhuo M, Nardi A, Hartman RE, Vogt SK, Luedke CE, Storm DR, Muglia LJ. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Seedat S. Unresolved questions about treatment-resistant anxiety disorders. CNS Spectr. 2004;9:715. doi: 10.1017/s1092852900022355. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Murase T, Ishizaki S, Tachikawa K, Arima H, Miura Y, Usdin TB, Oiso Y. Centrally administered tuberoinfundibular peptide of 39 residues inhibits arginine vasopressin release in conscious rats. Endocrinology. 2003;144:2791–2796. doi: 10.1210/en.2002-0017. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Harta G, Mezey E. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology. 1996;137:4285–4297. doi: 10.1210/endo.137.10.8828488. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Paciga M, Riordan T, Kuo J, Parmelee A, Petukova G, Camerini-Otero RD, Mezey E. Tuberoinfundibular peptide of 39 residues is required for germ cell development. Endocrinology. 2008 doi: 10.1210/en.2008-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela DM, Murphy AJ, Frendewey D, et al. High--throughput engineering of the mouse genome coupled with high--resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A. Afferent connections of the subparafascicular area in rat. Neuroscience. 2006;138:197–220. doi: 10.1016/j.neuroscience.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Ward HL, Small CJ, Murphy KG, Kennedy AR, Ghatei MA, Bloom SR. The actions of tuberoinfundibular peptide on the hypothalamo-pituitary axes. Endocrinology. 2001;142:3451–3456. doi: 10.1210/endo.142.8.8308. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal cortico-limbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]