Abstract
Three siblings with metachromatic leukodystrophy underwent umbilical cord blood transplantation at different stages of disease. Neuroimaging, nerve conduction studies, neurological and neuropsychological exams were used to measure outcome over two years. Post-transplant, the oldest sibling experienced disease progression. His two siblings had near or total resolution of signal abnormalities on neuroimaging. Their neuropsychological testing remained stable and nerve conduction studies have shown improvement. These results indicate pre-transplantation neurological exams may be the most significant predictor of outcome after transplant. To our knowledge, this report is the first to document neurological outcome of metachromatic leukodystrophy treated by umbilical cord blood transplantation.
Introduction
Metachromatic leukodystrophy (MLD) is an autosomal recessive lysosomal storage disease (LSD) due to a deficiency of arylsulfatase A (ASA). Absence of ASA results in accumulation of toxic sulfatides causing demyelination. Neurological findings may include spasticity, neuropathy, dementia, and seizures. Importantly, time of onset and severity correlate with residual ASA activity1, 2.
Many ASA gene mutations have been identified. Null mutations result in complete loss of enzyme activity, while residual mutations encode proteins with reduced enzyme activity1. Infantile MLD occurs in individuals with two null mutations. Adult MLD occurs in individuals with residual mutations. Juvenile MLD patients often possess a null and a residual mutation, resulting in intermediate enzyme activity. Pseudodeficiency (Pd) alleles exist that encode functional proteins with low activity when assayed in vitro3. Individuals who are compound heterozygotes for the Pd and null mutations have normal ASA activity in vivo, but low enzyme activity in vitro. Because this may lead to false positive diagnoses, the diagnosis of MLD requires the presence of urine sulfatides1.
Therapy for MLD was limited to supportive measures; however, recent interventions based upon unique features of lysosomal enzyme trafficking have shown promise. Lysosomal enzymes possess mannose-6-phosphate (M6P) residues, which bind M6P receptors (M6PR) located on endosomal or plasma membranes. Most of these enzymes are trafficked to the lysosome4, but some are released into the extracellular space where they can bind to adjacent cells, undergo endocytosis, and be sorted to lysosomes5, 6. This feature allows a normal cell to cross-correct nearby deficient cells. Intravenous enzyme replacement therapy (ERT) uses this characteristic to treat some LSDs7. Unfortunately the blood-brain barrier is believed to hinder the transport of large amounts of enzyme into the CNS7; nonetheless, recent studies in ASA-deficient mice have shown that ERT has been modestly effective in reducing sulphatide storage in the brain8. This data has led to the recent initiation of human clinical trials using human recombinant ASA. An interesting alternative to ERT uses transplanted hematopoietic cells to deliver enzyme to the CNS. These cells, once transplanted, can migrate into the CNS and subsequently rescue nearby cells9. Reports have shown bone marrow and umbilical cord blood transplantation (BMT and UCBT) are able to stabilize the neurodegeneration associated with some LSDs2, 9, 10.
This report documents our experience with three siblings diagnosed with juvenile MLD who underwent UCBT at different stages of disease. In this family, neurological exam at the time of UCBT was the best predictor of outcome, despite concomitant abnormal neuroimaging and NCS. Our results also include the stabilization or reversal of disease progression in two of these siblings two years after transplant. To our knowledge, this report is the first to document the neurological outcome of MLD patients treated with UCBT.
Results
The oldest brother was eight years-old at presentation with complaints of inattentiveness and episodic urinary incontinence for six months. His parents noted he was able to continue with many activities of daily living, but required occasional assistance. He had two healthy siblings, a six year-old brother and four year-old sister.
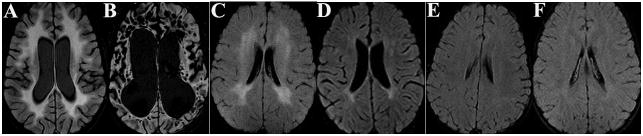
During examination, the child answered simple questions correctly (but slowly), had brisk patellar reflexes, and an left extensor plantar response. Subsequent brain MRI showed diffuse white matter signal abnormalities (WMSAs) consistent with MLD (Fig 1A). Positive urine sulfatide analysis confirmed this diagnosis. His siblings also had positive urine sulfatides, while their parents had negative urine sulfatides.
ASA analysis revealed the mother and all three children had low ASA activity (<5.6 nmol/hr/mg protein; normal range: 49–82). The father had normal ASA activity. Genetic analysis revealed that the mother was a compound heterozygote for the 635C>T (A212V) null mutation and one copy of a common Pd allele (resulting in her low in vitro ASA activity). The father was identified as a heterozygote carrier for the 1277C>T (P426L) residual mutation. All three children possessed A212V and P426L mutations.
Unrelated cord blood units were located through national registries. Cord blood typing was high resolution for DRB1 and intermediate resolution for class I antigens (A, B). Clinical details regarding matching, conditioning, engraftment and outcome are shown in Table 1.
Table 1.
Transplantation Data
| Patient age/Gender at UCBT | 8 years/Male | 6 years/Male | 4 years/Female |
|---|---|---|---|
| UCB-HLA match | 5/6 Mismatch at B | 4/6 Mismatch at A&B | 4/6 Mismatch at A&B |
| CD34+/kg | 5.1 x 105/kg | 6.0 x 105/kg | 1.5 x 106/kg |
| Conditioning regimen | IV Busulfan with pharmacokinetics, Cyclophosphamide, alemtuzumab | IV Busulfan with pharmacokinetics, Cyclophosphamide, thymoglobulin | IV Busulfan with pharmacokinetics, Cyclophosphamide, thymoglobulin |
| GVHD prophylaxis | Tacrolimus, methylprednisolone | Tacrolimus, methylprednisolone | Tacrolimus, methylprednisolone |
| Time until engraftment Neutrophil=N Platelet=P | N:Day +24 P:Day +30 | N:Day +18 P:Day +43 | N:Day +5 P:Day +41 |
| GVHD (overall) | Grade III (skin, gut) | None | None |
| Complications | CMV colitis Chronic sinusitis | BK viral infection Hemorrhagic cystitis | Right ankle infection Chronic sinusitis Hypothyroidism |
Neurological exams and brain MRI were performed before UCBT and approximately every six months afterwards. Nerve conduction studies (NCS) were similarly scheduled, but could not be obtained before transplantation. Neuropsychological analysis (NPA) was performed before UCBT and approximately two years later.
White blood cell ASA (WBC-ASA) activity was tested every six months after UCBT with all of the children having persistently normal activity after UCBT. Every child had initial NCS with mild diffuse sensorimotor polyneuropathy typical of demyelinating/dysmyelinating disorders (Table 2).
Table 2.
Nerve Conduction Studies
| Median Nerve Sensory Velocity (m/sec) | Median Nerve Motor Velocity (m/sec) | Median Nerve F-Wave Latency (msec) | F-Wave Latency /Ht (msec/cm) | |
|---|---|---|---|---|
| Normal Ranges 9,10 (Age Range) | 39.6–58.710 (4–8 years) | 47.1–65.59 (4–6 years) 50.6–64.0 (6–14 years) | 16.4–22.59 (4–6 years) 18.1–28.4 (6–14 years) | |
| Age (years) /Months Post-Transplant | Middle Sibling | |||
| 6/6 | 33 | 38 | 25.2 | 0.219 |
| 7/14 | 38 | 34 | 27.5 | 0.233 |
| 7.5/20 | 38 | 39 | 28.1 | 0.236 |
| 8/24 | 42 | 41 | 26.8 | 0.217 |
| Age (years) /Months Post-Transplant | Youngest Sibling | |||
| 4.5/6 | 39 | 39 | 22.1 | 0.216 |
| 5.5/14 | 46 | 45 | NR | |
| 6/20 | 46 | 45 | 21.6 | 0.199 |
| 6.5/24 | 49 | 45 | 21.9 | 0.197 |
Pre-transplant NPA testing of the oldest child noted extensive CNS dysfunction with a full-scale IQ score of 59 (2nd percentile). Two months after UBCT, he became non-verbal, did not follow commands, and developed spasticity. Two years after transplant, he developed migrating simple partial epilepsy that has been unresponsive to medication. His brain MRI showed late-stage leukodystrophy (see Fig 1B).
Before UCBT, the six year-old brother had a normal neurological exam. Brain MRI showed moderate diffuse WMSAs (Figure 1C). NPA showed mild inattentiveness and cognitive disturbance. He performed at a low-average level with a full-scale IQ score of 88 (21st percentile). After UBCT, his neurological exam remained relatively stable, although six months to a year afterwards he developed heel-cord tightness and decreased ankle deep tendon reflexes. Treatment with physical therapy and ankle braces has led to improvement of the contractures. Brain MRIs showed improvement with near resolution of WMSAs, associated with enlarged lateral ventricles (Figure 1D). Follow-up NPA remained at a low average to borderline level with a general-ability index in the eighth percentile (full scale IQ was not used due to a substantial variability among subtests). NCS revealed improvement of the median nerve sensory velocity from abnormal to normal over two years (Table 2). Median motor velocities remained stable and near 80% of the lower limit of normal (Table 2), as did sural sensory and peroneal motor nerve conduction (data not shown)11, 12. Median nerve F-wave values remained stable (and may be trending toward improvement).
The younger sister had a normal neurological exam and brain MRI (Fig 1E) before UCBT. NPA revealed high average-superior ability with a full-scale IQ score of 120 (91st percentile). After UCBT, her neurological exam remained normal. Six months after UCBT, her brain MRI developed mild periventricular WMSAs with a posterior prominence (data not shown), which resolved within the next six months and has remained stable (see Fig 1F). After UCBT, her NPA was interpreted in the high average-superior range even though her follow-up full scale IQ score was 98 (21st percentile; the score reduction was an artifact of the patient’s initially shy behavior during initial testing, later tests were scored in the high average to superior range). NCS revealed that median nerve sensory velocities improved from abnormal to normal for age over two years, while F-wave latency-to-height ratios remained stable or trended towards improvement (Table 2)11, 12. Median nerve motor conduction remained near 80% of the 3lower limit of normal (Table 2), as did sural sensory and peroneal motor nerve conduction (data not shown).
Discussion
Reports of hematopoietic cell transplantation (with or without mesenchymal stromal cells) for MLD have yielded a wide range of results13–20. Even with full engraftment, BMT often failed to alter the neurodegeneration9; however, numerous reports showed some stabilization of disease after transplantation9, 18–20. UCBT may be a better option than BMT because stored and cataloged UCB can be rapidly identified and transplanted, producing a shorter period between diagnosis and transplantation, an important characteristic to consider in neurodegenerative diseases. This feature was especially evident with the youngest sibling’s development of WMSAs six months after UBCT and their subsequent resolution. Unrelated UCB donors are also more likely to have normal enzyme activity than family members (often used in BMT) who could be heterozygote carriers with reduced enzyme activity and therefore reduce the efficacy of the transplant21.
Previous recommendations for the use of BMT to treat MLD required patients to have adequate neuropsychological function and independence in activities of daily living; however, no firm guidelines exist22. Some reports have advocated that only non-symptomatic patients undergo HCT9; however, in practice this categorization may be too vague to implement.
In this family a normal neurological exam was the best indicator of prognosis. The oldest child had a mildly abnormal neurological exam at the time of transplant and continued to deteriorate neurologically. Whether the transplant or medical interventions associated with the transplant accelerated this progression cannot be determined. In contrast, the middle sibling of this family had a normal neurological exam on presentation, but could also have been viewed as being symptomatic with regards to his initial testing, and so a poor candidate for transplant. Importantly, after UCBT, he and his sister have had their disease stabilize or improve. Unfortunately, his neuropsychological status may have deteriorated slightly, although it is difficult to determine whether this deterioration occurred during the period directly after transplant while the transplanted cells were migrating into the brain or has been an ongoing process prior to and after transplantation.
Of note, the younger patient’s trend in improving NCS may serve as a biomarker of improved peripheral myelination after UCBT. The median nerve sensory conduction velocities have changed from abnormal to normal over two years, while motor values have been stable or may be improving (given the improved f-wave latency-to-height ratios). These findings may be the result of the reversal of pathology or may represent disease stabilization associated with normal developmental changes that were previously unable to occur in the setting of active disease. Sensory fibers may be the earliest type to respond following UCBT and perhaps are the most sensitive indicator of change; further observation with these patients and others is warranted. and caution should be used so that these values are not overemphasized when discussing treatment response with patients and their families. In addition, it is very interesting that the youngest child’s MRI data suggest a period of six months to a year for the transplant to start having a therapeutic effect.
Previous reports have shown there can be phenotypic variation in the onset and course of compound heterozygote patients possessing the P426L residual mutation and a null mutation2, 23. It may be that the course of each of these children’s disease reflects this variation and is not a solely based on their UCBT; however, one can estimate from the MRI and NCS data that all three children started developing pathology (e.g. WMSAs and polyneuropathy) at approximately the same age (4–5 years old). Behavioral patterns for age were also similar by parental report. We believe these similarities allow for a realistic comparison of these children’s responses after UBCT. Altogether, whether these are temporary improvements or permanent effects will require long-term monitoring. For these two younger siblings, UCBT has been beneficial in stopping progression of disease.
Acknowledgments
The authors would like to thank Kenneth H. Fischbeck and Barrington Burnett for critical review of the manuscript. They would also like to thank Joanne Molinari and Tracy Hayden for administrative assistance, as well as Thomas Flynn, Robert Zimmerman, and Larissa Bilaniuk for technical expertise and advice. T.M.P. was funded by NIH grant T32-HD043021-04
Footnotes
Statement of Conflict of Interest
None of the authors of this article have any potential conflict of interest or and potential conflict of interest regarding the work presented here. Tyler Mark Pierson was supported by NIH grant T32-HD043021-04 during the course of the study.
Contributor Information
Tyler Mark Pierson, Division of Neurology, Children’s Hospital of Philadelphia
Carsten G Bonnemann, Division of Neurology, Children’s Hospital of Philadelphia
Richard S Finkel, Division of Neurology, Children’s Hospital of Philadelphia
Nancy Bunin, Division of Oncology, Children’s Hospital of Philadelphia
Gihan I Tennekoon, Division of Neurology, Children’s Hospital of Philadelphia
References
- 1.Gieselmann V, Zlotogora J, Harris A, et al. Molecular genetics of metachromatic leukodystrophy. Hum Mutat. 1994;4:233–242. doi: 10.1002/humu.1380040402. [DOI] [PubMed] [Google Scholar]
- 2.Gieselmann V. Metachromatic leukodystrophy: genetics, pathogenesis and therapeutic options. Acta Paediatr Suppl. 2008;97:15–21. doi: 10.1111/j.1651-2227.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 3.Penzien JM, Kappler J, Herschkowitz N, et al. Compound heterozygosity for metachromatic leukodystrophy and arylsulfatase A pseudodeficiency alleles is not associated with progressive neurological disease. Am J Hum Genet. 1993;52:557–564. [PMC free article] [PubMed] [Google Scholar]
- 4.Geuze HJ, Slot JW, Strous GJ, et al. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985;101:2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratantoni JC, Hall CW, Neufeld EF. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968;162:570–572. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- 6.Willingham MC, Pastan IH, Sahagian GG, et al. Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981;78:6967–6971. doi: 10.1073/pnas.78.11.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarescu G, Hill S, Wiggs E, et al. The efficacy of enzyme replacement therapy in patients with chronic neuronopathic Gaucher’s disease. J Pediatr. 2001;138:539–547. doi: 10.1067/mpd.2001.112171. [DOI] [PubMed] [Google Scholar]
- 8.Matzner U, Herbst E, Hedayati KK, et al. Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet. 2005;14:1139–1152. doi: 10.1093/hmg/ddi126. [DOI] [PubMed] [Google Scholar]
- 9.Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immunopathol. 2004;26:119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- 10.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 11.Parano E, Uncini A, De Vivo DC, Lovelace RE. Electrophysiologic correlates of peripheral nervous system maturation in infancy and childhood. J Child Neurol. 1993;8:336–338. doi: 10.1177/088307389300800408. [DOI] [PubMed] [Google Scholar]
- 12.Rabben OK. Sensory nerve conduction studies in children. Age-related changes of conduction velocities. Neuropediatrics. 1995;26:26–32. doi: 10.1055/s-2007-979715. [DOI] [PubMed] [Google Scholar]
- 13.Bayever E, Ladisch S, Philippart M, et al. Bone-marrow transplantation for metachromatic leucodystrophy. Lancet. 1985;2:471–473. doi: 10.1016/s0140-6736(85)90402-7. [DOI] [PubMed] [Google Scholar]
- 14.Guffon N, Souillet G, Maire I, et al. Juvenile metachromatic leukodystrophy: neurological outcome two years after bone marrow transplantation. J Inherit Metab Dis. 1995;18:159–161. doi: 10.1007/BF00711755. [DOI] [PubMed] [Google Scholar]
- 15.Krivit W, Lockman LA, Watkins PA, et al. The future for treatment by bone marrow transplantation for adrenoleukodystrophy, metachromatic leukodystrophy, globoid cell leukodystrophy and Hurler syndrome. J Inherit Metab Dis. 1995;18:398–412. doi: 10.1007/BF00710052. [DOI] [PubMed] [Google Scholar]
- 16.Krivit W, Lipton ME, Lockman LA, et al. Prevention of deterioration in metachromatic leukodystrophy by bone marrow transplantation. Am J Med Sci. 1987;294:80–85. doi: 10.1097/00000441-198708000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Solders G, Celsing G, Hagenfeldt L, et al. Improved peripheral nerve conduction, EEG and verbal IQ after bone marrow transplantation for adult metachromatic leukodystrophy. Bone Marrow Transplant. 1998;22:1119–1122. doi: 10.1038/sj.bmt.1701485. [DOI] [PubMed] [Google Scholar]
- 18.Kidd D, Nelson J, Jones F, et al. Long-term stabilization after bone marrow transplantation in juvenile metachromatic leukodystrophy. Arch Neurol. 1998;55:98–99. doi: 10.1001/archneur.55.1.98. [DOI] [PubMed] [Google Scholar]
- 19.Navarro C, Fernandez JM, Dominguez C, et al. Late juvenile metachromatic leukodystrophy treated with bone marrow transplantation; a 4-year follow-up study. Neurology. 1996;46:254–256. doi: 10.1212/wnl.46.1.254. [DOI] [PubMed] [Google Scholar]
- 20.Malm G, Ringden O, Winiarski J, et al. Clinical outcome in four children with metachromatic leukodystrophy treated by bone marrow transplantation. Bone Marrow Transplant. 1996;17:1003–1008. [PubMed] [Google Scholar]
- 21.Martin PL, Carter SL, Kernan NA, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31:229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 23.Polten A, Fluharty AL, Fluharty CB, et al. Molecular basis of different forms of metachromatic leukodystrophy. N Engl J Med. 1991;324:18–22. doi: 10.1056/NEJM199101033240104. [DOI] [PubMed] [Google Scholar]