Abstract
Introduction:
The patterns of failure and mechanisms of sarcoma-specific death are poorly characterized among the minority of patients with low grade soft tissue sarcoma (STS) who succumb to disease.
Methods:
Between 1982 and 2006, 2041 patients age ≥ 16 with low grade STS of all sites were treated with curative intent and prospectively followed at a single institution.
Results:
Among this cohort of 2041 patients, 181 (9%) died from disease (DOD). Overall, 105 patients (58%) died from locally recurrent disease (DOLR), and 59 (32%) died from distant disease (DODR). In 17 patients (9%), the mechanism of sarcoma-related death could not be verified. DOD occurred at a median of 62 months, while median disease-specific survival for the entire cohort was not reached. Median follow-up was 66 months (range 2 – 431). On multivariate analysis, DOD was associated with site, size, and less than R0 resection. For DOLR, site, size, positive margins, liposarcoma histology, and local recurrence (by definition) were significant factors. For DODR, site, histology, and positive margins were not significant factors, while size and local recurrence were. Of DOLR, 80% were retroperitoneal, 68% were liposarcoma, and only 2% were extremity. Conversely, of DODR, extremity (47%) and trunk (18%) were the most common sites, but histology was more variable (liposarcoma 35%, MFH 20%, fibrosarcoma 12%, extraskeletal myxoid chondrosarcoma 10%). High grade recurrence rates were comparable among DOLR (27%) and DODR (25%).
Conclusion:
Among patients with low grade STS, DOD occurs in approximately 9% of patients. Non-extremity site, larger size, and less than R0 resection are the most important risk factors for DOD, and distinct patterns of recurrence and death are predicted by primary tumor site.
Keywords: soft tissue sarcoma, low grade, sarcoma-specific death
Introduction
Histologic grade is a defining factor for establishing prognosis in patients with soft tissue sarcoma (STS).1-3 Several grading classifications have been described, including the three-tier National Cancer Institute (NCI) system4, the three-tier French Federation of Cancer Centres (FNCLCC) system5, 6, and the two-tier Memorial Sloan-Kettering Cancer Center (MSKCC) system1. Although no grading system is universally accepted, high grade histology, characterized by poor differentiation, cellular pleomorphism, coagulative necrosis, and mitoses3, has consistently emerged as a negative prognostic factor for patients with STS irrespective of which grading system is used6-11. Patients with high grade tumors are at significant risk for distant recurrence, and as many as 50 percent of these patients die of their disease12. Conversely, low grade STS confers an excellent prognosis with 5-year survival rates of 85% or greater.13, 14 An examination of the MSKCC post-operative nomogram for surgically-treated sarcoma patients demonstrates that differences in grade alone, using a two-tiered grading system, raise the risk of sarcoma-specific death from two- to five-fold depending on a patient's other clinicopathologic risk factors (http://www.mskcc.org/mskcc/html/6181.cfm).12, 15
A minority of patients with low-grade sarcoma succumb to sarcoma-related death. The risk factors and patterns of disease-specific death in this patient population remain poorly characterized. We sought to review the outcome of all low grade soft tissue sarcoma patients treated at a single institution to determine why patients with low grade STS die from disease.
Patients and Methods
From July 1982 through June 2006, 2265 patients with low grade STS of all sites underwent inpatient treatment at a single institution. These patients were prospectively entered and followed in a computerized database. Two-hundred twenty-two patients (9.8%) were excluded from the analysis for the following reasons: 148 patients (6.5%) had metastatic disease at presentation; 19 patients (0.8%) were younger than 16 years of age; and 55 patients (2.4%) did not undergo resection because of co-morbid conditions or locally advanced disease that was deemed unresectable. An additional 2 patients were retrospectively diagnosed with gastrointestinal stromal tumors (GIST) and were excluded. The remaining 2041 patients formed the basis of this study.
Following approval for this study by the Institutional Review Board, clinical, pathologic, and treatment data were reviewed and analyzed with respect to death from disease (DOD), death from locally recurrent disease (DOLR), and death from distant disease (DODR). Histologic grade was classified using a binary system (low versus high).1 Age was determined from the date of diagnosis of the primary tumor. Depth was categorized as either superficial or deep to the investing fascia. By convention, size of the primary tumor was divided into 3 groups: ≤ 5 cm, > 5 cm but ≤ 10 cm, and > 10 cm. Sites included extremity (upper at or distal to the shoulder/axilla, and lower at or distal to the buttock/groin), retroperitoneal, trunk (chest wall, back, and abdominal wall), thoracic, head and neck, visceral gastrointestinal, visceral genitourinary, visceral gynecologic, and skin.
Histologic diagnosis was assigned by the published criteria of the World Health Organization Classification of Tumors of Soft Tissue and Bone.16 Twenty-eight different histologic types were observed in this cohort. Since current consensus opinion maintains that desmoid tumors are not true sarcomas because of their lack of metastatic potential,16 statistical analyses were performed both including and excluding patients with this diagnosis. Atypical lipomatous tumors were diagnosed in 112 patients. These patients were included in the analysis of well-differentiated liposarcomas.17
Margin status was determined either clinically (R2 for gross residual tumor left behind) or as part of the histopathologic assessment (R1 for microscopically positive margins, and R0 for microscopically negative margins). The date of recurrent disease was defined either by biopsy or by the radiographic detection of suspicious lesions when no biopsy was performed. Peritoneal recurrences of intra-abdominal and retroperitoneal sarcomas were considered local recurrences, while liver metastases were considered distant recurrences. Intra-thoracic recurrences of thoracic sarcomas were considered local if they occurred in the ipsilateral hemithorax and distant if they occurred in the contralateral hemithorax. Patients who did not die of disease were censored according to the date of their last follow-up.
Fisher's exact test was used to compare categorical variables across groups. The cumulative incidence of DOD, DOLR, and DODR was estimated using a competing risks method.18, 19 With this methodology, for each survival endpoint, death due to any cause other than the event of interest is treated as a competing risk. Follow-up was counted from the date of diagnosis to the date of death or date of last follow-up. Freedom from local recurrence was counted from the date of resection.
Associations of the examined clinical, pathologic, and treatment variables with the cumulative incidence of events were examined using the Gray test.20 To examine the association of cumulative incidence while adjusting for important prognostic factors, variables significant on univariate analysis at the 0.05 level were entered into a competing risk regression model.21 When examining the association between local recurrence and survival outcome, a landmark analysis was adopted,22 since a local recurrence could not be considered a baseline variable.
Results
Clinicopathologic and Treatment Characteristics
Between 1982 and 2006, 2041 patients aged 16 or greater underwent resection of low grade sarcoma with curative intent at a single institution. This represents approximately 35% of the total number of sarcoma patients treated during this time period. Table 1 depicts the clinicopathologic characteristics of the entire cohort of patients. The median age was 48 (range 16 to 93), 53% were female, and 82% presented with primary, localized disease. Extremity tumors were the most prevalent (46%), followed by retroperitoneal/intra-abdominal (16%), and trunk (15%). Visceral sarcomas, comprising 7.6% of the total (gynecologic 3.1%, gastrointestinal 3.1%, and genitourinary 1.4%), were grouped together for purposes of statistical analysis. GISTs were excluded from visceral sarcomas for the purposes of this analysis. Thoracic (8%), head and neck (6%), and skin (1%) were grouped as other sites.
TABLE 1.
Characteristics of 2041 Patients Age ≥ 16 Undergoing Resection of Low Grade Sarcoma with Curative Intent
| Characteristic | Number (N=2041) |
% | |
|---|---|---|---|
| Gender | Male | 952 | 47 |
| Female | 1089 | 53 | |
| Age at Diagnosis | Median 48 (16 – 93) |
||
| Presentation Status | Primary Disease | 1665 | 82 |
| Locally Recurrent | 376 | 18 | |
| Site | Extremity | 937 | 46 |
| Retroperitoneal/Intraabdominal | 328 | 16 | |
| Trunk | 315 | 15 | |
| Thoracic | 159 | 8 | |
| Visceral¶ | 154 | 8 | |
| Other† | 148 | 7 | |
| Histology | Liposarcoma | 731 | 36 |
| Desmoid/Fibromatosis¶¶ | 377 | 18 | |
| Dermatofibrosarcoma Protuberans (DFSP) | 191 | 9 | |
| Malignant Fibrous Histiocytoma | 181 | 9 | |
| Leiomyosarcoma | 128 | 6 | |
| Solitary Fibrous Tumor/Hemangiopericytoma | 113 | 5.5 | |
| Fibrosarcoma | 91 | 4.5 | |
| Chondrosarcoma†† | 34 | 2 | |
| Endometrial Stromal Sarcoma | 34 | 2 | |
| Other¶¶¶ | 163 | 8 | |
| Primary Tumor Size | ≤ 5 cm | 723 | 35 |
| 5-10 cm | 539 | 26 | |
| > 10 cm | 678 | 33 | |
| Unknown | 101 | 5 | |
| Depth | Deep | 1576 | 77 |
| Superficial | 448 | 22 | |
| Margin Status | R0 | 1391 | 68 |
| R1 | 463 | 23 | |
| R2 | 105 | 5 | |
| Unknown | 82 | 4 | |
| Radiotherapy | Primary Tumor | 292 | 14 |
| Recurrent Disease | 196 | 10 | |
| Chemotherapy | Primary Tumor | 65 | 3 |
| Recurrent Disease | 156 | 8 | |
| Disease Recurrence | Local | 667 | 33 |
| Distant | 171 | 8 | |
| Both | 120 | 6 | |
| Status at Last Follow- Up |
No evidence of disease | 1486 | 73 |
| Alive with disease | 135 | 7 | |
| Dead of other causes | 239 | 12 | |
| Dead of disease | 181 | 9 | |
Because of rounding, not all percentages sum to 100.
Includes gynecologic 65/154 (42%), gastrointestinal 61/154 (40%), and genitourinary 28/154 (18%).
Includes head and neck 121/2041 (6%) and skin 27/2041 (1%).
Analyses performed excluding patients with desmoids/fibromatosis obtained similar results.
Includes extraskeletal myxoid chondrosarcoma 33/34 (97%), mesenchymal chondrosarcoma 1/34 (3%).
Comprises 19 histologic subtypes including cystosarcoma phyllodes, sarcoma NOS (not otherwise specified), malignant peripheral nerve sheath tumor, angiosarcoma, follicular dendritic cell tumor, synovial sarcoma, and malignant mesenchymoma among others.
Overall, 28 histologic subtypes were represented, with liposarcoma (36%) being the most frequent histologic diagnosis, followed by desmoid/fibromatosis (18%), dermatofibrosarcoma protuberans (DFSP—9%), malignant fibrous histiocytoma (MFH—9%), and leiomyosarcoma (6%). Given the sample size, the remaining 22% of cases (24 histologies) were grouped together for purposes of statistical analysis. Excluding patients with desmoid/fibromatosis, the distribution of histologic subtypes was liposarcoma (44%), DFSP (11.5%), MFH (11%), leiomyosarcoma (8%), and others (26%).
Primary tumor size was relatively evenly distributed among size categories (35% ≤ 5 cm, 26% 5 to 10 cm, and 33% > 10 cm). Seventy-seven percent of tumors were deep, and 68% of patients underwent R0 resection. Of 569 R1 and R2 resections, 198 (35%) and 172 (30%) were for extremity and retroperitoneal tumors, respectively. Notably, only 264 (46%) of R1 and R2 resections involved tumors > 10 cm.
Disease-Specific Survival and Characteristics of Patients Who Died of Disease
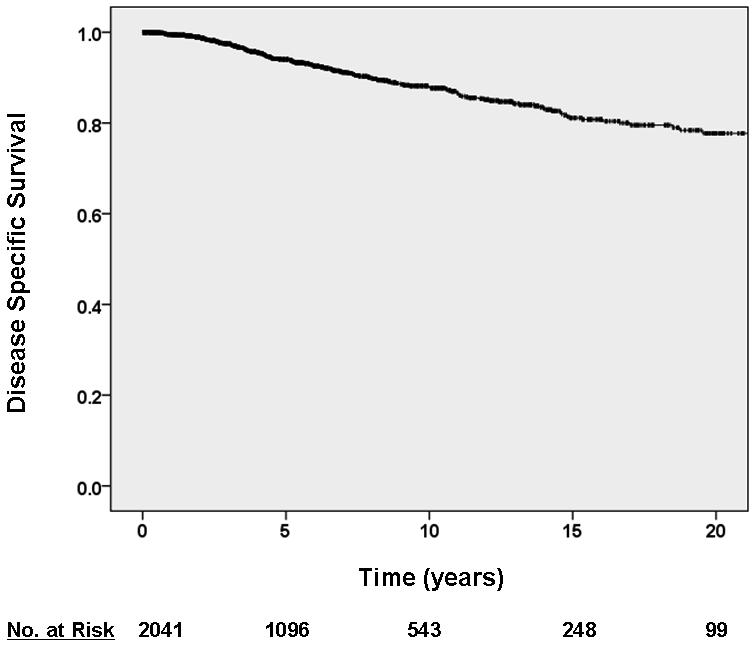
With a median follow-up of 66 months (range 0 – 431), the median overall survival (OS) was 243 months (95% confidence interval (CI) 224 – 263), and the median disease-specific survival (DSS) was not reached (Figure 1). Excluding patients with desmoids/fibromatosis, median OS was 226 months (95% CI 216 – 236), and median DSS was not reached. Four hundred twenty-one patients (21%) died during follow-up, of whom 181 patients died of disease (9%). An additional 135 patients (7%) were alive with disease at censoring. The clinicopathologic characteristics of the 181 patients who died of disease following resection with curative intent are depicted in Table 2. There were negligible differences when the 7 patients with desmoid/fibromatosis who DOD (all from local causes) were excluded.
Figure 1.
Kaplan-Meier curve depicting disease-specific survival for entire cohort of low grade sarcoma patients ≥ 16 resected with curative intent.
TABLE 2.
Characteristics of 181 Patients Age ≥ 16 Who Died from Low Grade Sarcoma Following Resection with Curative Intent
| Characteristic | Number (N=182) |
% | |
|---|---|---|---|
| Gender | Male | 98 | 54 |
| Female | 83 | 46 | |
| Age at Diagnosis | Median 57 (16 – 90) |
||
| Site | Extremity | 32 | 18 |
| Retroperitoneal/Intraabdominal | 97 | 54 | |
| Trunk | 19 | 10 | |
| Thoracic | 8 | 4 | |
| Visceral¶ | 14 | 8 | |
| Head and Neck | 10 | 6 | |
| Skin | 1 | 0.5 | |
| Histology | Liposarcoma | 97 | 53 |
| Desmoid/Fibromatosis† | 7 | 4 | |
| Dermatofibrosarcoma Protuberans (DFSP) | 1 | 0.5 | |
| Malignant Fibrous Histiocytoma | 21 | 12 | |
| Leiomyosarcoma | 12 | 7 | |
| Solitary Fibrous Tumor/Hemangiopericytoma | 6 | 3 | |
| Fibrosarcoma | 14 | 8 | |
| Chondrosarcoma¶¶ | 7 | 4 | |
| Other†† | 16 | 9 | |
| Primary Tumor Size | ≤ 5 cm | 10 | 6 |
| 5-10 cm | 50 | 28 | |
| > 10 cm | 105 | 58 | |
| Unknown | 16 | 9 | |
| Depth | Deep | 169 | 93 |
| Superficial | 11 | 6 | |
| Margin Status | R0 | 82 | 45 |
| R1 | 56 | 31 | |
| R2 | 29 | 16 | |
| Unknown | 14 | 8 | |
| Radiotherapy | Primary Tumor | 42 | 23 |
| Recurrent Disease | 52 | 29 | |
| Chemotherapy | Primary Tumor | 22 | 12 |
| Recurrent Disease | 82 | 45 | |
| Cause of Death | Local | 105 | 58 |
| Distant | 59 | 32 | |
| Unknown | 17 | 9 | |
| Median Time to Recurrence |
Local | 29 months (range 1 – 382) |
|
| Distant | 74 months (range 1 – 414) |
||
| Median Time to Sarcoma-Specific Death |
Local | 94 months (range 3 – 328) |
|
| Distant | 168 months (range 9 – 432) |
||
Because of rounding, not all percentages sum to 100.
Includes gynecologic 6/14 (43%), gastrointestinal 7/14 (50%), and genitourinary 1/14 (7%).
Analyses performed excluding patients with desmoids/fibromatosis obtained similar results.
Includes extraskeletal myxoid chondrosarcoma 7/7 (100%)
Comprises 9 histologic subtypes including endometrial stromal sarcoma, adenosarcoma, angiosarcoma, dendritic cell tumor, malignant mesenchymoma, and sarcoma NOS among others.
Among DOD patients, 105 patients (58%) experienced DOLR and 59 patients experienced DODR (32%). In 17 patients (9%), the mechanism of sarcoma-related death could not be verified. Retroperitoneal site (53%), liposarcoma histology (53%), and tumor size > 10 cm (58%) were all more prevalent among patients who died of disease. An R0 resection was less frequently achieved (45%) in this group of patients.
DOLR occurred earlier than DODR with median time to DOLR being 94 months (range 3 – 328) compared to 168 months (range 9 – 432) for DODR. Bowel obstruction, renal failure, and inanition were the dominant causes of DOLR. Rare causes of DOLR included airway invasion (4 patients) and direct central nervous system extension from head and neck sarcomas (3 patients). Respiratory failure was the predominant cause of DODR.
High Grade Recurrence
Although we could not identify the overall prevalence of high grade recurrence (HGR) in the entire dataset, there were 46 HGR among the 182 DOD patients. HGR was more frequent among retroperitoneal (54%), truncal (20%), and extremity (17%) sites. Although 52% of HGR occurred with liposarcoma, there was no significant difference in HGR between liposarcoma (52%) and non-liposarcoma (46%) histologies (P=0.42) among patients who DOD. Furthermore, HGR was comparable among DOLR (29 of 106, 27%) and DODR (15 of 59, 25%).
Predictors of Disease-Specific Survival
As depicted in Table 3, multivariate analysis revealed DOD to be statistically associated with primary tumor site, increasing primary tumor size, and margin status. There was a trend toward worse disease-specific survival with increasing age, but this association was not statistically significant (hazard ratio (HR) 1.01 [95% CI 0.998 – 1.02]). Extremity sites experienced the most favorable prognosis, while thoracic, head and neck, and skin (grouped as other, HR 2.80, 95% CI 1.36 – 5.76), visceral (HR 4.90, 95% CI 2.39 – 10.05), and retroperitoneal (HR 5.46, 95% CI 3.44 – 8.65) all had statistically worse DSS. Although truncal locations experienced worse DSS than extremity ones (HR 2.04, 95% CI 0.94 – 4.44), there was only a trend toward statistical significance (P = 0.07, see figure 2a). When patients with desmoid/fibromatosis were excluded, there were negligible differences in the results of multivariate analysis.
Table 3.
Multivariate Model of Cancer-Specific Death for Patients Age ≥ 16 with Low Grade Soft Tissue Sarcoma Resected with Curative Intent
| Variable* | Hazard Ratio for Death from Soft Tissue Sarcoma (95% Confidence Interval) |
|---|---|
| Primary Site | |
| Extremity | 1.00 (referent) |
| Trunk | 2.04 (0.94 – 4.44) |
| Other¶ | 2.80 (1.36 – 5.76) |
| Visceral† | 4.90 (2.39 – 10.05) |
| Retroperitoneal | 5.46 (3.44 – 8.65) |
| Primary Tumor Size | |
| ≤ 5 cm | 1.00 (referent) |
| > 5 cm ≤ 10 cm | 4.62 (2.26 – 9.43) |
| > 10 cm | 5.56 (2.57 – 12.04) |
| Margin Status | |
| R0 | 1.00 (referent) |
| R1 | 1.38 (0.96 – 2.00) |
| R2 | 2.60 (1.72 – 3.95) |
Variables significant at the 0.10 level on univariate analysis were included in the multivariate model. Gender, depth, age, and histologic subtype were not statistically significant variables on multivariate analysis. Treatment-related variables were not included in the multivariate model since their association with the outcome of interest could not be assumed to be independent.
Includes thoracic, head and neck, and skin.
Includes gastrointestinal, gynecologic, and genitourinary.
Figure 2.
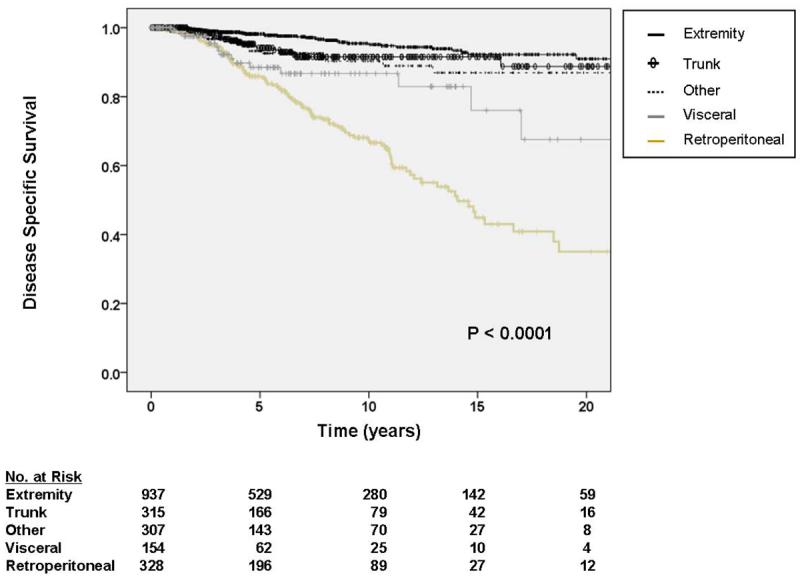
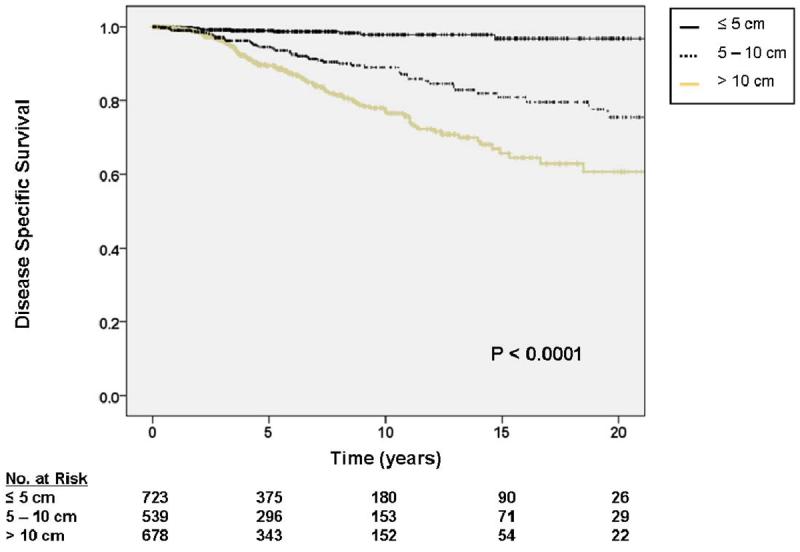
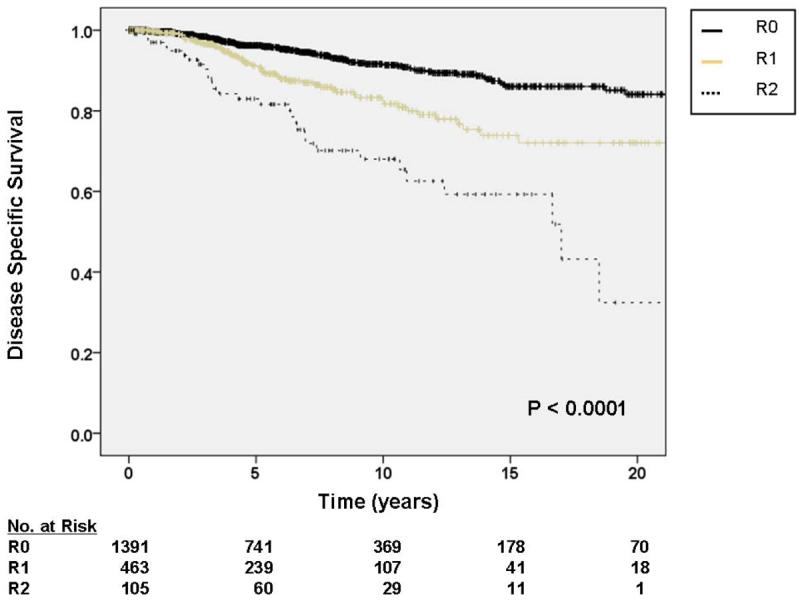
A. Kaplan-Meier curve depicting disease-specific survival grouped by site of primary tumor. Other sites include thoracic, head and neck, and skin locations. Visceral includes gastrointestinal, gynecologic, and genitourinary. Pooled univariate P value is shown. B. Kaplan-Meier curve depicting disease-specific survival grouped by primary tumor size. Pooled univariate P value is shown. C. Kaplan-Meier curve depicting disease-specific survival by completeness of resection/status of resection margins. Pooled univariate P value is shown.
For tumors 5 – 10 cm in size, the HR for DOD was 4.62 (95% CI 1.55 – 4.63), and for tumors > 10 cm, the HR for DOD was 5.56 (95% CI 2.57 – 12.04) relative to tumors < 5 cm (figure 2b). Relative to an R0 resection, an R1 resection was associated with a HR for DOD of 1.38 (95% CI 0.96 – 2.00). This approached statistical significance (P = 0.08). R2 resections were statistically associated with worse DOD with an HR of 2.60 (95% CI 1.72 – 3.95). These associations are depicted graphically in figure 2c. On subgroup analysis, the impact of extent of resection on DOD was most significant for retroperitoneal tumors (P = 0.0001). For visceral tumors this variable was borderline significant (P = 0.05), and for extremity tumors it was not significant (P = 0.61).
Gender, tumor depth, and histologic subtype (using all 29 subtypes) were not statistically significant predictor variables for DOD on multivariate analysis. A subset analysis comparing myxoid liposarcoma, well-differentiated liposarcoma, and non-liposarcoma histologies revealed that myxoid liposarcoma histology was associated with statistically worse DSS (HR 1.89, 95% CI 1.12 – 3.12), while there was no difference in DSS among well-differentiated liposarcoma and non-liposarcoma histologies.
Administration of chemotherapy and radiotherapy were observed to correlate with worse DOD on multivariate analysis (data not shown), but these treatment-related variables were excluded from the final multivariate model because their association with the outcome variable of interest could not assumed to be independent.
Predictors of Local Cause-Specific Survival
As depicted in Table 4, multivariate analysis demonstrated primary tumor site, primary tumor size, liposarcoma histology, and margin status to be significantly associated with DOLR. Although there were significant differences in event-specific DOLR by primary tumor site (figure 3a), multivariate analysis demonstrated liposarcoma histology to be a more reliable predictor of DOLR than site, likely because site and histology are tightly linked covariables. Among histologic subtypes, liposarcoma histology was observed to be a significant predictor of DOLR with an HR of 1.87 (95% CI 1.08 – 3.25) while other histologies were not significant.
Table 4.
Multivariate Model of Death from Locally Recurrent Disease for Patients Age ≥ 16 with Low Grade Soft Tissue Sarcoma Resected with Curative Intent
| Variable* | Hazard Ratio for Death from Soft Tissue Sarcoma (95% Confidence Interval) |
|---|---|
| Primary Site** | |
| Extremity | 1.00 (referent) |
| Trunk | 5.98 (1.01 – 35.48) |
| Other¶ | 19.80 (4.26 – 92.10) |
| Visceral† | 16.09 (2.86 – 90.62) |
| Retroperitoneal | 59.10 (13.22 – 264.32) |
| Primary Tumor Size | |
| ≤ 5 cm | 1.00 (referent) |
| > 5 cm ≤ 10 cm | 6.78 (1.98 – 23.14) |
| > 10 cm | 10.23 (3.06 – 34.27) |
| Histology | |
| Other¶¶ | 1.00 (referent) |
| Malignant Fibrous | 1.05 (0.32 – 3.48) |
| Histiocytoma (MFH) | |
| Leiomyosarcoma | 2.14 (0.73 – 6.27) |
| Liposarcoma | 1.87 (1.08 – 3.25) |
| Margin Status | |
| R0 | 1.00 (referent) |
| R1 | 2.26 (1.40 – 3.68) |
| R2 | 5.86 (3.42 – 10.04) |
Variables significant at the 0.10 level on univariate analysis were included in the multivariate model. Gender, depth, and age were not statistically significant variables on multivariate analysis. Treatment-related variables were not included in the multivariate model since their association with the outcome of interest could not be assumed to be independent.
Although there were significant differences in DOLR by primary tumor site, multivariate analysis demonstrated liposarcoma histology to be a more reliable predictor of DOLR than site, likely because site and histology are tightly linked covariables.
Includes thoracic, head and neck, and skin.
Includes gastrointestinal, gynecologic, and genitourinary.
Includes all histologies except MFH, leiomyosarcoma, and liposarcoma.
Figure 3.
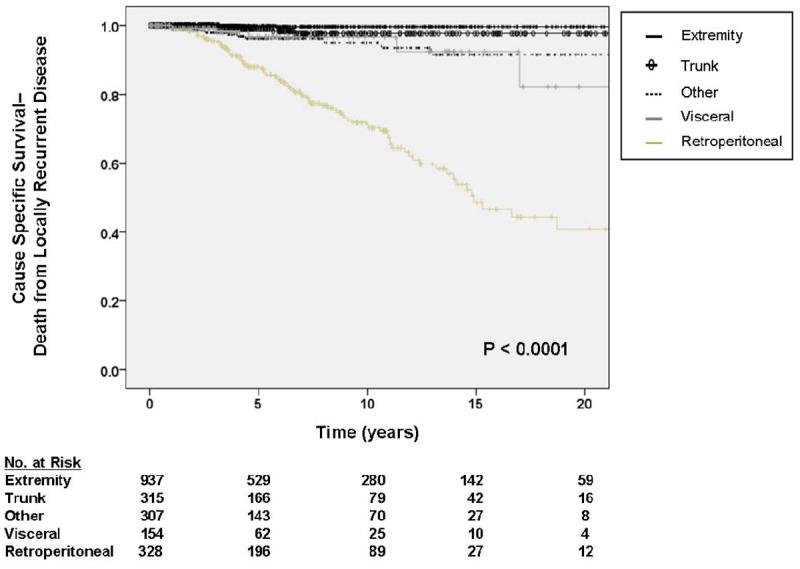
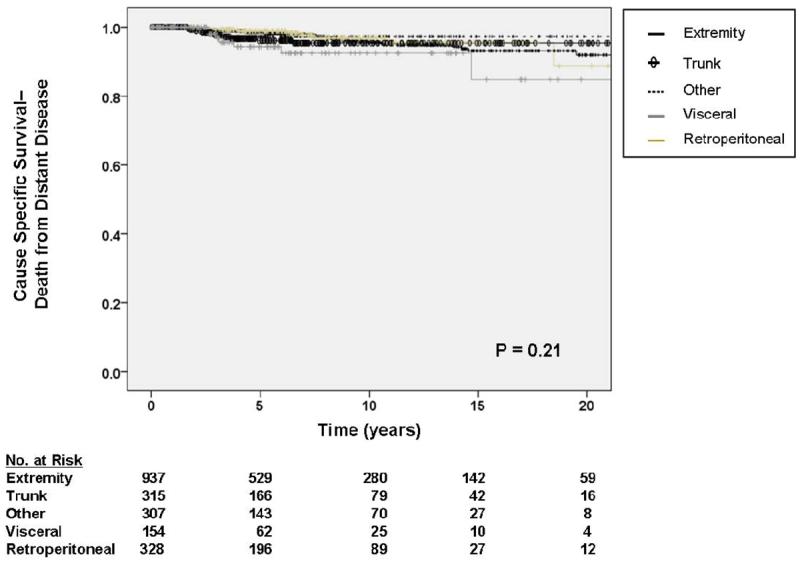
A. Kaplan-Meier curve depicting cause-specific survival (death from locally recurrent disease) grouped by site of primary tumor. Other sites include thoracic, head and neck, and skin locations. Visceral includes gastrointestinal, gynecologic, and genitourinary. Pooled univariate P value is shown. B. Kaplan-Meier curve depicting cause-specific survival (death from distant disease) grouped by site of primary tumor. Other sites include thoracic, head and neck, and skin locations. Visceral includes gastrointestinal, gynecologic, and genitourinary. Pooled univariate P value is shown.
For tumors 5 – 10 cm in size, the HR for DOLR was 6.78 (95% CI 1.98 – 23.14), and for tumors > 10 cm, the HR for DOD was 10.23 (95% CI 3.06 – 34.27) relative to tumors < 5 cm. Relative to an R0 resection, an R1 resection was associated with an HR for DOLR of 2.26 (95% CI 1.40 – 3.68), and an R2 resection was associated with an HR of 5.86 (95% CI 3.42 – 10.04). Kaplan-Meier analysis of DOLR by margin status is shown in figure 3b.
Similar to DOD, gender and tumor depth were not statistically significant predictors of DOLR on multivariate analysis. There was a trend toward increased DOLR with increasing age, but this association did not reach statistical significance. For each one year increment of age, the HR for DOLR was 1.01 (95% CI 0.999 – 1.02) with a P value of 0.08. Also similar to the results for DOD, negligible differences were observed in the results of multivariate analysis when patients with desmoids tumors/fibromatosis were excluded.
Predictors of Distant Cause-Specific Survival
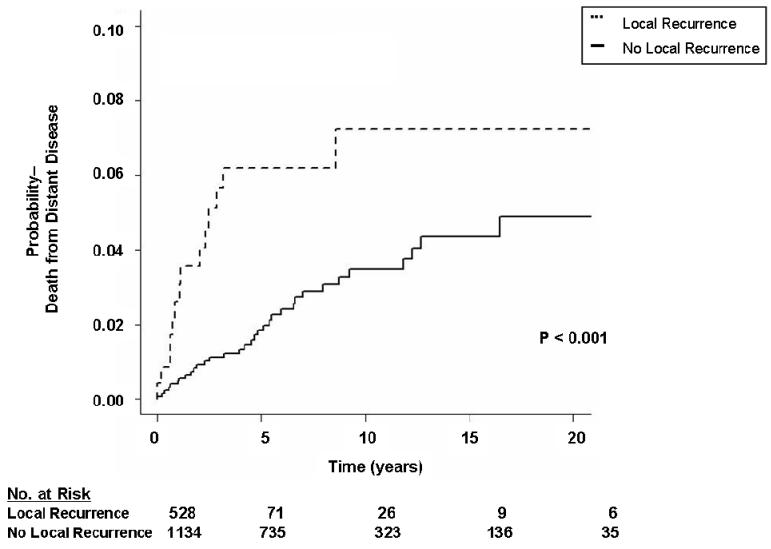
As depicted in Table 5, multivariate analysis demonstrated primary tumor size (HR 3.54, 95% CI 1.50 – 8.36 for tumors 5 – 10 cm, HR 3.24, 95% CI 1.34 – 7.85 for tumors > 10 cm), and local recurrence (HR 1.90, 95% CI 1.26 – 2.87) to be significantly associated with DODR. In contrast to DOLR, primary tumor site (figure 3b) and margin status (figure 4b) were not statistically significant predictors of DODR on multivariate analysis. As demonstrated in figure 5, patients who developed a local recurrence experienced a worse event-specific DODR. Myxoid liposarcoma histology was independently associated with worse DODR on subgroup analysis (data not shown), while negligible differences were observed when patients with desmoids/fibromatosis were excluded.
Table 5.
Multivariate Model of Death from Distant Metastatic Disease for Patients Age ≥ 16 with Low Grade Soft Tissue Sarcoma Resected with Curative Intent
| Variable* | Hazard Ratio for Death from Soft Tissue Sarcoma (95% Confidence Interval) |
|---|---|
| Primary Tumor Size | |
| ≤ 5 cm | 1.00 (referent) |
| > 5 cm ≤ 10 cm | 3.54 (1.50 – 8.36) |
| > 10 cm | 3.24 (1.34 – 7.85) |
| Local Recurrence¶ | |
| No | 1.00 (referent) |
| Yes | 1.90 (1.26 – 2.87) |
Variables significant at the 0.10 level on univariate analysis were included in the multivariate model. Gender, depth, age, histologic subtype, primary tumor site, and margin status at initial operation were not statistically significant variables on multivariate analysis. Treatment-related variables were not included in the multivariate model since their association with the outcome of interest could not be assumed to be independent.
The association between local recurrence and DODR was tested using landmark analysis since a local recurrence cannot be considered a baseline variable.
Figure 4.
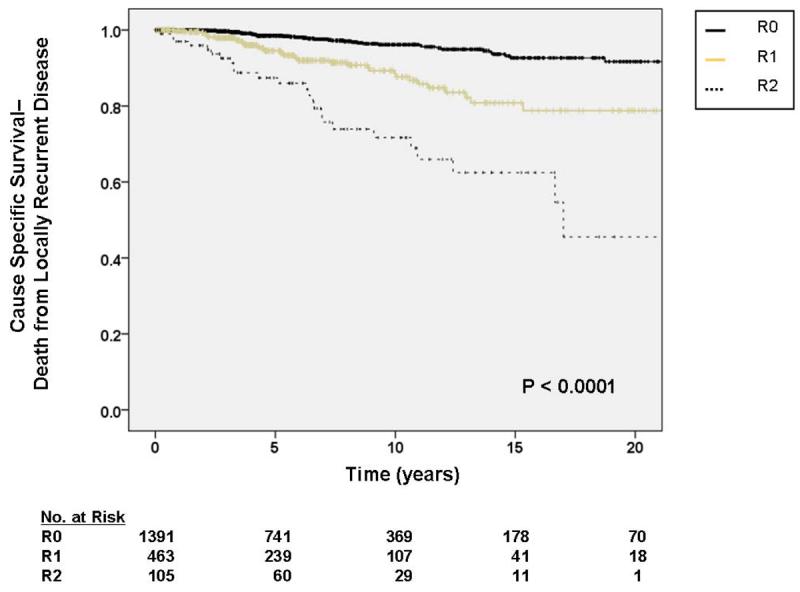
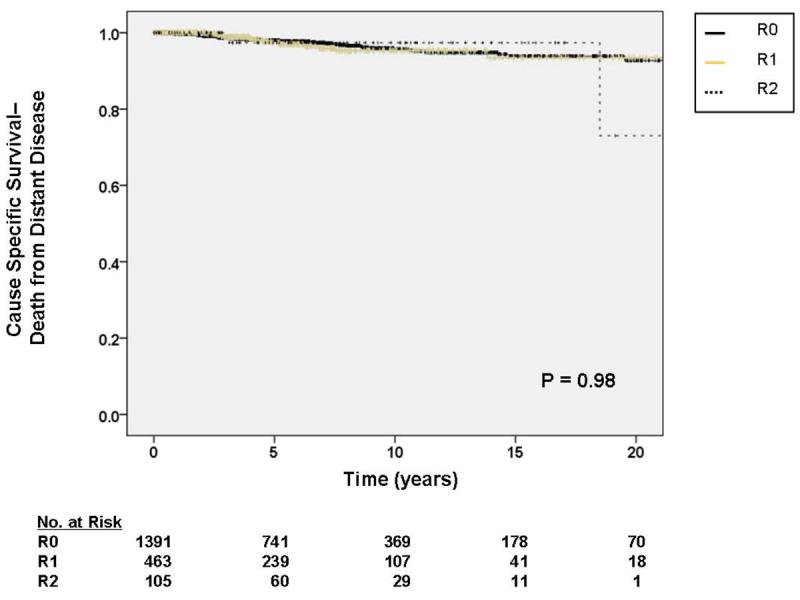
A. Kaplan-Meier curve depicting cause-specific survival (death from locally recurrent disease) grouped by completeness of resection/status of resection margins. Pooled univariate P value is shown. B. Kaplan-Meier curve depicting cause-specific survival (death from distant disease) grouped by completeness of resection/status of resection margins. Pooled univariate P value is shown.
Figure 5.
Kaplan-Meier curve depicting landmark analysis of probability of death from distant disease grouped by presence or absence of local recurrence within two years of diagnosis of the primary tumor.
Discussion
Although disagreement exists among pathologists regarding what constitutes the most accurate and reproducible histologic grading system for soft tissue sarcoma,23, 24 there is little question regarding the value of pathologic grade in determining a patient's prognosis. Multiple reports, using either of the three dominant methods (three-tier NCI,4 three-tier FNCLCC,5 or two-tier MSKCC1), have all established pathologic grade as a critical prognostic variable, and survival of patients with low grade (MSKCC) or grade I (NCI, FNCLCC) STS is consistently favorable. Five-year metastasis-free survival rates range from 90 to 98% for these patients,10, 13, 14 compared to 40 to 60% for patients with high grade sarcoma.10, 25 Similarly, Marcus et al.13 reported a 94% 10-year disease-specific survival (DSS) among 87 low grade sarcoma patients compared to 44% among 572 patients with FNCLCC grade 3 sarcoma.6 In the current series of 2041 patients with low grade sarcoma, the five-year rates of metastasis-free survival and DSS were 93% and 94%, respectively.
Although DSS is characteristically favorable among patients with low grade sarcoma, few reports have examined risk factors and patterns of disease-specific death for those patients who experience DOD. We observed non-extremity site, increasing tumor size, and less than R0 resection as statistically significant independent predictors of worse DSS in this large cohort of exclusively low grade sarcoma patients. In general, with the exception of depth, the risk factors for DOD among low grade sarcoma patients parallel those found in studies analyzing risk factors for DOD among all sarcoma patients.10 Age and histologic subtype remain inconsistently reported as risk factors for DSS in studies including all grades of STS patients.26
After adjusting for other prognostic factors, we did not observe depth to be a statistically significant factor for DOD, perhaps because we included all anatomic sites in this study. This may have confounded the results since retroperitoneal and visceral sites are by definition deep. Nevertheless, the overall percentage of deep tumors (77%) in this series is comparable to other series analyzing exclusively extremity tumors. Although depth may be a significant independent predictor of DOD for the subset of low grade STS patients with extremity only tumors, our results among greater than 2000 patients with a median follow-up of 66 months suggest that depth is not a clinically or statistically significant independent predictor of outcome among all low grade STS patients.
Similarly, although certain trends emerged with respect to the prevalence of specific histologic types among DOD patients (e.g., liposarcoma 36% of index cases but 53% of deaths, desmoids tumors 18% of cases but 4% of deaths, extraskeletal myxoid chondrosarcoma 2% of cases but 4% of deaths), our data did not demonstrate histologic subtype to reliably predict DOD after controlling for other factors. With 17 histologic subtypes represented among 181 events, it is possible that our analysis is underpowered to detect an association between DOD and histology. However, given the sample size of this study, it is unlikely that another study with as rigorous pathologic review and mature follow-up will be sufficiently powered to discern such a relationship.
Local recurrences (33%) occurred with an approximate four-fold greater frequency than distant recurrences (8%) in this series. Local recurrences, in absolute numbers, were also responsible for more patient deaths than distant recurrences (106 versus 59, respectively). However, as a percentage of total recurrences, distant recurrences (34%) were more lethal than local recurrences (16%), likely reflecting a greater ability by the treating physicians to successfully salvage a patient with a local recurrence.
Multivariate analysis of predictors of DOLR demonstrated similar findings to those of DOD with increasing non-extremity sites, increasing tumor size, and less than R0 resection being strongly predictive of worse event-specific survival. Furthermore, liposarcoma histology was significantly associated with DOLR. Non-extremity site, increasing tumor size, and less than R0 resection all likely correlate with an increased risk of a local recurrence, which is a necessary component of DOLR. These results are concordant with the finding that retroperitoneal tumors had the highest HR for DOLR with a 59-fold increase in DOLR relative to extremity tumors.
There was a reproducible, but non-significant, trend for age to predict cause-specific survival in all multivariate analyses. The explanation of age as a potential risk factor for DOD, DOLR, and DODR remains somewhat elusive. Age has sometimes been viewed as a surrogate for good performance status and/or an ability to tolerate additional aggressive therapies, which would predispose patients to a more favorable outcome. However, with so few patients receiving adjuvant therapies, which are ineffective for low grade STS, this rationale appears less likely. It is also possible that older age is associated with different tumor biology or a decline in antitumor cell-mediated immunity among STS patients, but these hypotheses are not a priori obvious.27, 28
Unlike DOD and DOLR, primary tumor site and margin status were not predictive of DODR in multivariate analysis while increasing tumor size and positive local recurrence were. These findings are consistent with those of Stojadinovic et al.29 who observed that local recurrence is not inevitable for STS patients when positive margins are obtained at operation. However, once local recurrence does occur, it is associated with DOLR, DODR, and DOD. This relationship appears to be true for the subset of patients with low grade STS. It is also notable that intermediate-size tumors (5 to 10 cm) carried a slightly greater HR (3.54) for DODR than tumors greater than 10 cm (HR 3.24). Our data suggest that for tumors greater than 10 cm, the majority of DOD occur secondary to DOLR and that DOLR competes with DODR as a cause of death.
In summary, prognosis for low grade STS is overall excellent, with a median DSS of greater than 20 years. Nevertheless, approximately 9% of low grade STS patients die of sarcoma-related causes. Site, size, and margin status govern prognosis in low grade STS resected with curative intent, and distinct patterns of recurrence and death are predicted by primary tumor site.
Acknowledgement
This work was supported by Soft Tissue Sarcoma Program Project grant P01 CA 047179 (LXQ, RGM, SS, MFB)
Footnotes
Presented in part at the Society of Surgical Oncology 61st Annual Cancer Symposium, Chicago, IL, March 13 – 16, 2008.
REFERENCES
- 1.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12(3):326–31. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 2.Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006;48(1):42–50. doi: 10.1111/j.1365-2559.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MF, Singer S, Maki RG, O'Sullivan B. Sarcomas of the Soft Tissues and Bone. In: DeVita Vincent, Hellman Samuel, Rosenberg Steven., editors. Cancer: Principles and Practice of Oncology. 7th Edition Lippincott, Williams, and Wilkins; 2005. pp. 1581–1637. [Google Scholar]
- 4.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53(3):530–41. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 6.Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–77. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor JJ, Tan CC, Casper ES, et al. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol. 1992;10(8):1317–29. doi: 10.1200/JCO.1992.10.8.1317. [DOI] [PubMed] [Google Scholar]
- 9.Le Doussal V, Coindre JM, Leroux A, et al. Prognostic factors for patients with localized primary malignant fibrous histiocytoma: a multicenter study of 216 patients with multivariate analysis. Cancer. 1996;77(9):1823–30. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1823::AID-CNCR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 11.Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 225 patients. Cancer. 2003;97(10):2530–43. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 12.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–6. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 13.Marcus SG, Merino MJ, Glatstein E, et al. Long-term outcome in 87 patients with low-grade soft-tissue sarcoma. Arch Surg. 1993;128(12):1336–43. doi: 10.1001/archsurg.1993.01420240044008. [DOI] [PubMed] [Google Scholar]
- 14.Donohue JH, Collin C, Friedrich C, et al. Low-grade soft tissue sarcomas of the extremities. Analysis of risk factors for metastasis. Cancer. 1988;62(1):184–93. doi: 10.1002/1097-0142(19880701)62:1<184::aid-cncr2820620128>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103(2):402–8. doi: 10.1002/cncr.20778. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 17.Kooby DA, Antonescu CR, Brennan MF, Singer S. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2004;11(1):78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54. [PubMed] [Google Scholar]
- 19.Prentice RL, Kalbfleisch JD. Hazard rate models with covariates. Biometrics. 1979;35(1):25–39. [PubMed] [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 21.Fine JP, Gray JP. A proportional hazards model for the subdistribution of a competing risk. Jour Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 23.Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol. 1999;72(2):77–82. doi: 10.1002/(sici)1096-9098(199910)72:2<77::aid-jso7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–62. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 25.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219(2):165–73. doi: 10.1097/00000658-199402000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koea JB, Leung D, Lewis JJ, Brennan MF. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10(4):432–40. doi: 10.1245/aso.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Ikeda H, Bruce AT, et al. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32(13):231–45. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 28.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 29.Stojadinovic A, Leung DH, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235(3):424–34. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]