Abstract
OBJECTIVE
To determine if the starting voltage in a step-wise ramping protocol for extracorporeal shock wave lithotripsy (SWL) alters the size of the renal lesion caused the SWs.
MATERIALS AND METHODS
To address this question, one kidney from 19 juvenile pigs (7–8 weeks old) was treated in an unmodified Dornier HM-3 lithotripter with either 2000 SWs at 24 kV (standard clinical treatment, 120 SWs/min), 100 SWs at 18 kV followed by 2000 SWs at 24 kV or 100 SWs at 24 kV followed by 2000 SWs at 24 kV. The latter protocols included a 3–4 minute interval, between the 100 SWs and the 2000 SWs, used to check the targeting of the focal zone. The kidneys were removed at the end of the experiment so that lesion size could be determined by sectioning the entire kidney and quantifying the amount of hemorrhage in each slice. The average parenchymal lesion for each pig was then determined and a group mean was calculated.
RESULTS
Kidneys that received the standard clinical treatment had a mean (SEM) lesion size of 3.93 (1.29)% functional renal volume (FRV). The mean lesion size for the 18 kV ramping group was 0.09 (0.01)% FRV, while lesion size for the 24 kV ramping group was 0.51 (0.14)% FRV. The lesion size for both of these groups was significantly smaller than the lesion size in the standard clinical treatment group.
CONCLUSIONS
The data suggest that initial voltage in a voltage-ramping protocol does not correlate with renal damage. While voltage ramping does reduce injury when compared with SWL with no voltage ramping, starting at low or high voltage produces lesions of the same approximate size. Our findings also suggest that the interval between the initial shocks and the clinical dose of SWs, in our one-step ramping protocol, is important for protecting the kidney against injury.
Keywords: Tissue injury, Animal models, Renal protection
INTRODUCTION
While extracorporeal shock wave lithotripsy (SWL) is considered a highly effective treatment for upper urinary tract stones, concerns about the safety and efficacy of SWL have dampened enthusiasm for the treatment [1–3]. These concerns have been heightened by the fact that second generation and more recent lithotripters appear less effective at breaking stones [4–7] and cause more tissue injury [8–9] than the original unmodified Dornier HM-3 lithotripter.
Our research has focused on the development of new treatment strategies to improve the safety and efficacy of SWL. One of these strategies involves “step-wise voltage ramping” where treatment commences at a low SW voltage and then is subsequently increased with time. Originally, voltage ramping appears to have been introduced in the clinic as a means to reduce patient discomfort during SWL by allowing patients to acclimate to the SWL treatment without anesthesia. Subsequent in-vitro [10–11] and in-vivo [12] studies applying this approach suggested that voltage ramping also improves stone fragmentation. More recently, a clinical comparison of voltage ramping against standard SWL treatment showed improved stone comminution with voltage ramping while using only a modest (11 to 13 kV) step-wise increase in SW voltage [13].
While voltage ramping appears promising for enhanced stone breakage, it is equally important to understand the consequences of step-wise voltage ramping on SWL-induced kidney injury. Willis et al. [14] provided the first data showing an effect of single-step voltage ramping on tissue injury. In that study porcine kidneys were treated with a limited number of low-energy (12 kV) SWs followed by a larger number of high-energy (24 kV) SWs, the latter being consistent with a standard dose of SWs used in the clinic. This strategy substantially reduced the acute hemorrhagic lesion normally observed in porcine kidneys after conventional SWL.
However, questions remain as to why a step-wise change in treatment voltage would “protect” kidneys from injury. One such question concerns the starting SW voltage. Some groups begin their voltage ramping protocol at 11 kV [13] while others report using 17 kV [15] or 18 kV [11–12], but no one has yet examined the relationship between starting voltage and renal injury. Because we have previously shown a positive correlation between voltage and lesion size [16], we hypothesized that as the starting voltage increases, the subsequent lesion sizes will increase. Accordingly, the present study was undertaken to determine if the starting voltage in a step-wise ramping protocol alters the size of the renal lesion caused by the SWs.
MATERIALS AND METHODS
The present study was carried out with an unmodified Dornier HM-3 lithotripter (Dornier Medical Systems, Kennesaw, GA) located at Methodist Hospital, Indianapolis, IN, USA. This lithotripter has an 80 nF capacitor and a focal zone (F2) of about 1.5 cm diameter × 2.5 cm length. Refurbished spark plugs (Healthtronics, Kennesaw, GA) were used for all experiments and were discarded after 1000 shots.
The experimental protocol used in this study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Nineteen female farm pigs, aged 7–8 weeks (Hardin Farms, Danville, IN), were assigned to receive either 2000 SWs at 24 kV (a standard clinical treatment protocol, n = 7), 100 SWs at 18 kV followed by 2000 SWs at 24 kV (n = 7) or 100 SWs at 24 kV followed by 2000 SWs at 24 kV (n = 5). Both ramping protocols included a 3–4 minute pause in SW delivery between the first 100 SWs and the remaining 2000 SWs to check targeting of F2. All SWs were delivered at a rate of 120 SWs/minute. This protocol builds on a previously published study using 100 SWs at 12 kV followed by 2000 SWs at 24 kV [14]. That study was carried out with the same lithotripter, pigs of the same size and the same protocol as the present experiment.
At the beginning of the experiment the pigs were rendered unconscious with an I. M. injection of ketamine (15–20 mg/kg) and xylazine (2 mg/kg). They were then intubated and anesthetized with isoflurane (1–3%) throughout the experiment. Sterile saline was infused through an ear vein at a rate of 1–3% of body weight per hour to maintain adequate hydration and urine flow. Surgical procedures for the placement of femoral artery and bilateral ureteric catheters have been described previously [17].
After a post-surgery acclimation period (2 to 2.5 hours), the pigs were disconnected from the anesthesia machine and transferred (unconscious) to the lithotripsy suite (a trip of ≅ 5 minutes) where administration of isoflurane anesthesia was resumed. The pigs were then placed supine in the gantry of the HM-3 lithotripter. The pigs were positioned in the water bath (39°C) so that one kidney could be exposed to the SWs. Positioning of each pig was accomplished by injecting a small amount of contrast medium (Renografin 60%, Bracco Diagnostics, Princeton, NJ) through the ureteric catheter into the urinary collection system of the kidney to be treated. Using the positioning fluoroscopes of the lithotripter, F2 was located on a lower pole calyx of that kidney. The pigs were then treated with one of the three protocols listed above.
After SWL, each pig was returned to the surgical suite (once again disconnected from the anesthesia machine for ≅ 5 minutes). At 4 h after the completion of the lithotripsy treatment, the kidneys were perfusion-fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH = 7.4) as previously described [18]. After perfusion, the kidneys were removed and submerged in fresh fixative for subsequent determination of lesion size.
Kidneys used for quantitation of lesion size were processed according to our previously published protocol [19]. Briefly, each kidney was cast, embedded in paraffin and serial sections were cut on a sliding microtome. A digital image of each section was captured and a computer-assisted segmentation technique was used to quantify the hemorrhagic lesion as a volume percent of the total functional volume (FRV) of each treated kidney. A mean (SEM) was calculated for lesion size in each of the treated pigs. The Kruskal-Wallis test, a non-parametric ANOVA for non-normally distributed data, was used for statistical analysis. Significant overall differences in the group medians were followed by post hoc comparisons adjusted by the Bonferroni method (comparing the standard clinical treatment protocol group, and the 18 kV and 24 kV voltage ramping groups). The criterion for statistical significance was set at P < 0.05.
RESULTS
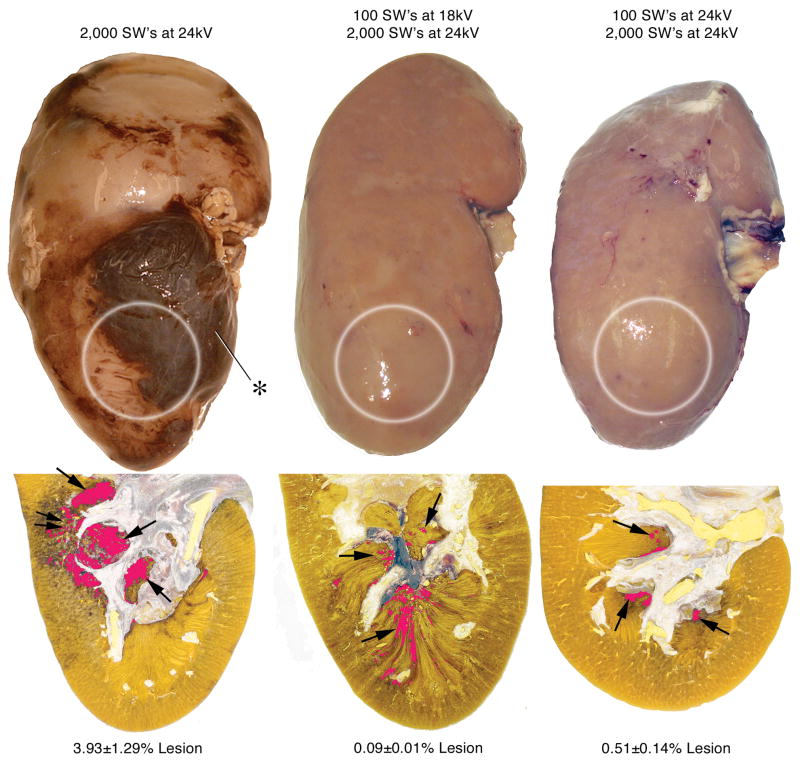
Figure 1 shows a digitized and pseudo-colored cross-section of a kidney from each of the three treatment groups. Pigs from the standard clinical treatment group had a mean (SEM, range) lesion size of 3.93 (1.29, 1.15–9.37)% FRV. These kidneys had many areas of intraparenchymal bleeding. These sites were localized at the focus of the SW and involved both the cortex and medulla. In some cases, the hemorrhage extended all the way from the papilla tip to the capsule resulting in a subcapsular hematoma (Figure 1). Kidneys from pigs in the 18 kV and 24 kV ramping groups lacked surface hematomas and contained very few areas of intraparenchymal hemorrhage. These damage sites were small, and were found almost exclusively in the medulla. The mean (SEM, range) lesion size for the 18 kV ramping group was 0.09 (0.01, 0.0–0.1)% FRV while lesion size for the 24 kV ramping group was 0.51 (0.14, 0.15–0.87)% FRV. The mean lesion size for both of these groups was significantly smaller than the lesion size of pigs in the standard clinical treatment group (P = 0.003 for 18 kV, P = 0.014 for 24 kV).
FIG. 1.
Gross appearance of kidneys treated with 2000 SWs at 24 kV (standard treatment), 100 SWs at 18 kV followed by 2000 SWs at 24 kV, or 100 SWs at 24 kV followed by 2000 SWs at 24 kV with an unmodified Dornier HM-3 lithotripter. The white circles show the approximate location of the SW focus (F2) on the lower pole of each kidney. Note that no sites of hemorrhage are evident on the kidneys using the one-step ramping protocol of 18 kV or 24 kV, while a large subcapsular hematoma (asterisk) is located on the kidney following standard treatment. Beneath the gross view of each kidney is a lower pole section showing the typical lesion found using each protocol and the average lesion size calculated in each group (expressed as mean (SEM) of percent of FRV). The lesion has been segmented and pseudo-colored (red), so that the size of the SWL-induced injury can be appreciated. Single arrows point to papillae showing evidence of hemorrhagic injury. Double arrows indicate an area where the injury extended up into the cortex, a common finding in the kidneys from pigs after the standard treatment.
DISCUSSION
These findings suggest that the beginning voltage is not the key determinant responsible for reduced lesion size in our ramping protocol. Starting voltages of 12 kV [14], 18 kV or 24 kV all produced the same degree of protection when compared to conventional nonramped SWL.
Studies over the last 20 years in our laboratory have shown that the application of 2000 SWs (24 kV, with a Dornier HM-3) to a juvenile pig kidney consistently produces a morphological lesion that averages 4–6% of the FRV [14,16, 20]. Recently, Willis et al. [14] reported that one can “protect” a kidney, i.e. reduce tissue injury, by treating that kidney with a series of low voltage shocks before delivering a clinical dose of SWs. While the cause of the protection is unknown, several factors could potentially trigger the response; e.g. the number of SWs given at the beginning of treatment, the starting voltage of the SWs, and the time interval between the SW applications.
The SW number was tested when Willis et al. [14] reduced the initial treatments of low voltage (12 kV) SWs from 2000 to 500 in one series of experiments, and then to 100 in another series. Similar protective responses occurred in each instance, indicating that if a threshold exists for the number of SWs needed to trigger the protection, it must ≤ 100. Certainly, further study will be needed to determine if <100 SWs will still invoke tissue protection.
The second potential factor, starting voltage, was examined in the present study. Previous experience has shown us that tissue injury increases as treatment voltage increases [16]. In fact, we have shown that lesion size can increased 20-fold with only a doubling of SW voltage (12 kV to 24 kV) [16], and this led us to hypothesize that as the initial ramping voltage was increased the size of the renal lesion would also increase. However, the data showed that protection was comparable whether the treatment started at 12 kV [14], 18 kV or 24 kV. This suggests that, as a starting voltage of 24 kV was as effective as 12 kV at preventing renal injury, voltage ramping per se is not solely responsible for limiting lesion size. What mechanisms initiate the protective effect and how these mechanisms work to reduce lesion size are unknown. Recent work by Handa et al. [21] suggests that an increase in renal vascular resistive index, presumably from constriction of renal blood vessels during SWL, is involved in mediating the protective response, but these findings tell us nothing about what initiates the response.
The present findings support the rationale for using a voltage ramping protocol in clinical SWL, as step-wise voltage ramping (from low to high voltage) improves stone fragmentation [10–13], and also limits renal injury. The present results indicate that a range of starting voltages (12–24 kV) can work to initiate the protective effect in the treated kidney. And, at least as conducted in the present experiment, voltage ramping causes less injury to the kidney than conventional nonramping protocols. Accordingly, clinical voltage-ramping protocols could be designed where the treatment regimens are optimized for stone fragmentation with the expectation that the ramping protocol will also initiate the protective response and limit injury. Clinical studies are needed to confirm this expectation.
The most intriguing and new implication arising from the present findings concerns, oddly enough, the 3–4 minute interval of inactivity between the two applications of SWs. If starting voltage is not the factor that initiates the protective response, as appears to be the case in the present study, then the 3–4 minute interval between the initial and clinical doses of SWs emerges as the principle factor that could be responsible for the protection. Otherwise, the 100 SWs at 24 kV ramping protocol, which includes the 3–4 minute interval, should have produced a lesion at least as large as that observed without voltage ramping [14,16,20]. Although the present studies have not tested that the interval between SWs initiates the protection response, our data clearly suggest such a possibility. This, in turn, raises concerns for ramping protocols currently in use that do not include a resting interval between SWs applied at different energies. If a resting interval is critical for reducing SWL-related tissue damage, ramping protocols lacking this interval may predispose patients to unnecessary injury. Clearly, further study is needed to determine exactly if and how a period of inactivity between groups of SWs protects renal tissue from SWL-induced injury, but prudence suggests that brief resting intervals be added to clinical ramping protocols.
In conclusion, the present findings suggest that the initial voltage of a one-step voltage ramping protocol for SWL does not correlate with renal damage. That is, voltage ramping reduced the amount of renal injury when compared with nonramped SWL regardless of whether low or high voltage SW were applied to start the ramping protocol. Our findings also suggest that the time interval between the first and second sets of SWs, as used in our experiments, may initiate the response that limits the renal injury caused by SWL.
Acknowledgments
This project was supported in part by PHS Grants# P01-DK43881 and R01-DK67133. The authors are indebted to Kelli Wind and Cynthia Johnson for their expert assistance. The definitive version of this article is available at www.blackwell-synergy.com.
References
- 1.Kerbl K, Rehman J, Landman J, Lee D, Sundaram C, Clayman RV. Current management of urolithiasis: progress of regress? J Endourol. 2002;16:281–8. doi: 10.1089/089277902760102758. [DOI] [PubMed] [Google Scholar]
- 2.Lingeman JE, Kim SC, Kuo RL, McAteer JA, Evan AP. Shockwave lithotripsy: anecdotes and insights. J Endourol. 2003;17:687–93. doi: 10.1089/089277903770802191. [DOI] [PubMed] [Google Scholar]
- 3.Clayman RV. Untipped: an endourologist’s lament. J Urol. 2008;179:402. doi: 10.1016/j.juro.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 4.Eichel L, Batzold P, Erturk E. Operator experience and adequate anesthesia improve treatment outcome with third-generation lithotripters. J Endourol. 2001;15:671–3. doi: 10.1089/08927790152596217. [DOI] [PubMed] [Google Scholar]
- 5.Portis AJ, Yan Y, Pattaras JG, Andreoni C, Moore R, Clayman RV. Matched pair analysis of shock wave lithotripsy effectiveness for comparison of lithotriptors. J Urol. 2003;169:58–62. doi: 10.1016/S0022-5347(05)64034-7. [DOI] [PubMed] [Google Scholar]
- 6.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005–9. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]
- 7.Gerber R, Studer UE, Danuser H. Is newer always better? A comparative study of 3 lithotriptor generations. J Urol. 2005;173:2013–6. doi: 10.1097/01.ju.0000158042.41319.c4. [DOI] [PubMed] [Google Scholar]
- 8.Piper NY, Dalrymple N, Bishoff JT. Incidence of renal hematoma formation after ESWL using the new Dornier Doli-S lithotriptor. J Urol. 2001;165(5):377. [Google Scholar]
- 9.Dhar NB, Thornton J, Karafa MT, Streem SB. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol. 2004;172:2271–4. doi: 10.1097/01.ju.0000143459.03836.2d. [DOI] [PubMed] [Google Scholar]
- 10.McAteer JA, Baird T, Williams JC, Hatt EK, Evan AP, Cleveland RO. Voltage-stepping during SWL influences stone breakage independent of total energy delivered: in vitro studies with model stones. J Urol. 2003;169(4):487–8. [Google Scholar]
- 11.Zhou Y, Cocks FH, Preminger GM, Zhong P. The effect of treatment strategy on stone comminution efficiency in shock wave lithotripsy. J Urol. 2004;172:349–54. doi: 10.1097/01.ju.0000132356.97888.8b. [DOI] [PubMed] [Google Scholar]
- 12.Maloney ME, Marguet CG, Zhou Y, et al. Progressive increase of lithotripter output produces better in-vivo stone comminution. J Endourol. 2006;20:603–6. doi: 10.1089/end.2006.20.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demirci D, Mustafa S, Yalcin E, Ekmekcioglu O, Gulmez I, Karacagil M. Comparison of conventional and step-wise shockwave lithotripsy in management of urinary calculi. J Endourol. 2007;21:1407–10. doi: 10.1089/end.2006.0399. [DOI] [PubMed] [Google Scholar]
- 14.Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 15.Pace KT, Ghiculete D, Harju M, Honey RJ University of Toronto Lithotripsy Associates. Shock wave lithotripsy at 60 or 120 shocks per minute: a randomized, double-blind trial. J Urol. 2005;174:595–9. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- 16.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 17.Willis LR, Evan AP, Connors BA, Blomgren PM, Fineberg NS, Lingeman JE. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol. 1999;10:1753–62. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 18.Shao Y, Connors BA, Evan AP, Willis LR, Lifshitz DA, Lingeman JE. Morphological changes induced in the pig kidney by extracorporeal shock wave lithotripsy: nephron injury. Anat Rec. 2003;275A:979–89. doi: 10.1002/ar.a.10115. [DOI] [PubMed] [Google Scholar]
- 19.Blomgren PM, Connors BA, Lingeman JE, Willis LR, Evan AP. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. Anat Rec. 1997;249:341–8. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Evan AP, McAteer JA, Connors BA, Blomgren PM, Lingeman JE. Renal injury during shock wave lithotripsy is significantly reduced by slowing the rate of shock wave delivery. BJU Int. 2007;100:624–8. doi: 10.1111/j.1464-410X.2007.07007.x. [DOI] [PubMed] [Google Scholar]
- 21.Handa RK, Bailey MR, Paun M, et al. Resistive index measurements of renal perfusion during SWL. J Urol. 2008;179(4):507. [Google Scholar]