Abstract
Although regulatory T (T reg) cells are thought to develop primarily in the thymus, the peripheral events that shape the protective T reg cell population are unclear. We analyzed the peripheral CD4+ T cell receptor (TCR) repertoire by cellular phenotype and location in mice with a fixed TCRβ chain. We found that T reg (Foxp3+) cells showed a marked skewing of TCR usage by anatomical location in a manner similar to antigen-experienced (CD44hiFoxp3−) but not naive (CD44loFoxp3−) cells, even though CD44hi and T reg cells used mostly dissimilar TCRs. This was likely unrelated to peripheral conversion, which we estimate generates only a small percentage of peripheral T reg cells in adults. Conversion was readily observed, however, during the immune response induced by Foxp3− cells in lymphopenic hosts. Interestingly, the converted Foxp3+ and expanded Foxp3− TCR repertoires were different, suggesting that generation of Foxp3+ cells is not an automatic process upon antigen activation of Foxp3− T cells. Retroviral expression of these TCRs in primary monoclonal T cells confirmed that conversion did not require prior cellular conditioning. Thus, these data demonstrate that TCR specificity plays a crucial role in the process of peripheral conversion and in shaping the peripheral T reg cell population to the local antigenic landscape.
The importance of natural Foxp3+ T reg cells for the maintenance of self-tolerance was recently reaffirmed by the observation that acute depletion of T reg cells creates a state of “immunologic anarchy” resulting in the rapid death of a previously healthy animal (1, 2). T reg cells are commonly thought to be generated in the thymus because of recognition of self-antigens (3, 4), which is supported by studies using mice with limited TCR diversity showing strong similarity between the thymic and peripheral T reg TCR repertoires (5–7). However, the peripheral and thymic T reg TCR repertoires were not identical, suggesting that certain TCRs are preferentially enriched or deleted in the periphery, akin to earlier observations in the total CD4+ T cell population (8).
The notion that postthymic T reg TCR repertoire shaping may play an important role in immune regulation was suggested by several studies demonstrating that the presence of an organ is accompanied by functionally enhanced suppression of autoimmunity to that organ (9–12). Based on these studies, it has been widely hypothesized that tissue-specific T reg cell number is increased because of the presence of the antigen. However, this has not been directly demonstrated, and alternative explanations for these classical observations include altered trafficking patterns (13–15) and activation status (12, 16) of T reg cells at particular locations. These other possibilities are consistent with reports suggesting that T reg cells may not require tissue specificity for suppression (17–19) nor recognize self-antigens (20). Thus, an important unresolved question is whether the peripheral T reg TCR repertoire is altered based on the presentation of tissue-specific antigens.
The development of Foxp3+ T cells from mature, naive Foxp3− T cells, a process often referred to as peripheral conversion, is another mechanism by which the peripheral repertoire may be altered. This process would allow for the generation of T reg cells specific for antigens not presented in the thymus, such as those from commensal microbiota or sequestered self-antigens (21, 22). Furthermore, it has been hypothesized that peripheral conversion may also generate T reg cells during the course of an immune response to limit immune pathology. Studies of TCRαβ transgenic T cells have shown that exposure to cognate antigen under noninflammatory or tolerogenic conditions in vivo results in the development of suppressive Foxp3+ T reg cells in a portion of the monoclonal T cell population (23–25). In polyclonal T cell populations, peripheral development of CD25+ cells characteristic of T reg cells has been observed after adoptive transfer of CD25− T cells into both lymphopenic and nonlymphopenic hosts (26, 27). However, a study using endogenous secondary TCRα chains on self-reactive BDC2.5 TCRαβ transgenic T cells to track T cell fates found no evidence that this TCR facilitates conversion (28). Thus, the role of peripheral conversion in generating the peripheral T reg cell population remains unresolved.
To address these questions, we analyzed the TCR repertoire based on anatomical location and cellular phenotype by sequencing TCRα chains in mice with a fixed TCRβ chain (29). We generated a database of nearly 18,000 TRAV14 (Vα2) TCRα chain sequences derived from T reg (Foxp3+), antigen-experienced (CD44hiFoxp3−), and naive (CD44loFoxp3−) CD4+ cells isolated from the spleen and from cervical, axillary, inguinal, and mesenteric LNs. We found considerable differences in TCR usage by location in the T reg and CD44hi but not CD44lo T cell populations, demonstrating that tissue-specific antigen recognition dramatically alters the peripheral T reg cell population. Shaping of the peripheral T reg TCR repertoire by location therefore suggests that immune regulation is a tissue-specific activity.
A potential explanation for parallel changes in both the T reg and CD44hi TCR repertoires by location is that peripheral conversion generates a large proportion of the peripheral T reg cell population. However, we found that few cells became Foxp3+ after adoptive transfer of either peripheral CD4+Foxp3− cells or mature CD4+CD8− thymocytes into normal adult hosts, resulting in an estimated 4–7% contribution to the peripheral T reg cell population. In contrast, we found a much higher percentage of conversion during the immune response induced by Foxp3− cells transferred into lymphopenic hosts. Interestingly, there was considerable disparity in TCR usage between the converted Foxp3+ and expanded Foxp3− T cell subsets within the same individual host. We excluded an absolute requirement for thymically derived T reg cell precursors by showing that retroviral expression of particular TCRs on monoclonal peripheral T cells was sufficient to permit peripheral conversion. Thus, these data favor a TCR-specific model of peripheral T reg cell development.
RESULTS
Mapping the peripheral CD4+ TCR repertoire
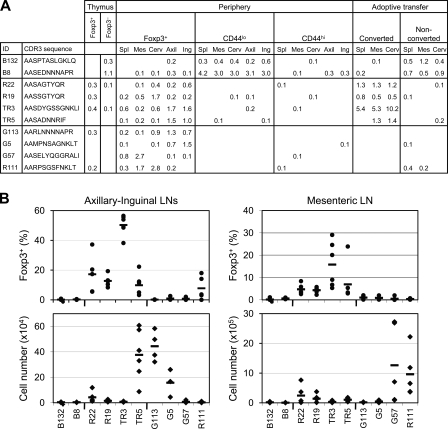
To determine the impact of anatomical location and cellular phenotype on the peripheral CD4+ TCR repertoire, we isolated T reg (Foxp3+), antigen-experienced (CD44hiFoxp3−), and naive (CD44loFoxp3−) CD4+ cells from the spleen and from cervical, axillary, inguinal, and mesenteric LNs of TCli TCRβ transgenic × Foxp3gfp × Tcra+/− mice (Table I; and Figs. S1 and S2, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). T cells from these mice therefore express a fixed TCRβ chain paired with a single endogenously rearranged TCRα chain. This facilitates experimental analysis because only one TCR needs to be sequenced per cell, and insures that only a single TCR specificity affects the cell fate decision of an individual T cell. We also analyze the TRAV14 (Vα2) subset, representing ∼10% of the population, to further restrict diversity to a manageable level. We considered using CD62L as an additional putative memory cell marker but found that only a minor subset of CD44hi cells are CD62Lhi (unpublished data). From these sorted T cell populations, we created a library of TRAV14 (Vα2) TCRα chain cDNA and obtained sequences from individual clones, as previously described (7), from four independent experiments, of which three experiments consisted of pooled cells from three to five mice and the fourth experiment consisted of three mice that were individually sequenced. As before, we used the CDR3 amino acid sequence as the primary identifier for a unique TCR sequence (7). Approximately 6,000 sequences were obtained from each of the T reg, naive, and memory cell populations, for a total of nearly 18,000 sequences.
Table I.
TRAV14 TCRα sequences obtained from TCli TCRβ transgenic mice
| Number of sequences by location
|
||||||
|---|---|---|---|---|---|---|
| LNs
|
||||||
| Phenotype | Spl | Mes | Cerv | Axil | Ing | Total |
| Foxp3+ | 1,310 | 1,362 | 1,347 | 1,013 | 941 | 5,973 |
| Foxp3−CD44lo | 1,263 | 1,345 | 1,326 | 1,009 | 1,060 | 6,003 |
| Foxp3−CD44hi | 1,297 | 1,417 | 1,330 | 937 | 966 | 5,947 |
| Total | 3,870 | 4,124 | 4,003 | 2,959 | 2,967 | 17,923 |
The total number of TRAV14 (Vα2) TCRα sequences obtained from cells of the indicated phenotype and location are shown. Axil, axillary; Cerv, cervical; Ing, inguinal; Mes, mesenteric; Spl, spleen.
The T reg TCR repertoire varies by anatomical location
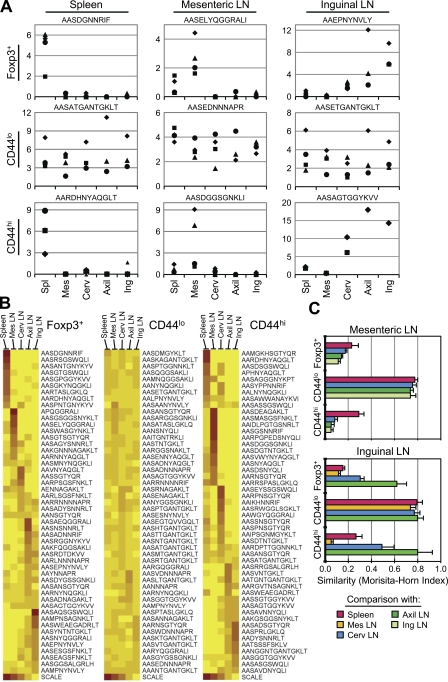
An important unresolved issue is the mechanism by which tissue-specific antigen presentation results in enhanced protection from autoimmunity, as it could derive from either an increased number or heightened potency of antigen-specific T reg cells. Analysis of TCRs from the various LNs and the spleen revealed that the T reg TCR repertoire varied considerably according to the anatomical location. This could be observed at the level of individual TCRs (Fig. 1 A, top; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1), which show a strong preference across multiple experiments for either the spleen, the mesenteric LNs, or the skin-draining axillary, inguinal, and cervical LNs. This is further illustrated by analyzing the relative frequency at each anatomical location for a large number of individual TCRs (Fig. 1 B). Finally, this pattern was confirmed at the population level by a statistical assessment of the similarity between two populations, the Morisita-Horn index (see Materials and methods; Fig. 1 C). In contrast, naive CD44lo T cells showed little skewing of their TCR repertoire by anatomical location, consistent with the notion that these cells circulate among the secondary lymphoid organs scanning for foreign antigens. Thus, these data provide direct evidence that the peripheral T reg cell population is shaped by local antigen presentation.
Figure 1.
The natural T reg and CD44hi but not naive CD44lo TCR repertoires vary by anatomical location. (A) Analysis of individual TCRs. Using the pooled TCR dataset described in Table I, we chose the most frequent TCR of a given phenotype (shown at left) at each location (shown on top). To decrease the effect of mouse-to-mouse variability, we only chose TCRs that were found in at least two independent experiments. Because the naive CD44lo TCRs were fairly uniform across locations, the three most frequent naive TCRs, irrespective of location, are shown from left to right. Symbols represent the frequency (as a percentage of total sequences) of the TCR, denoted above by its CDR3 amino acid sequence, within the dataset of that phenotype and the location in each of the four experiments. (B) Anatomical distribution of the most abundant TCR sequences within each phenotype. Using the pooled TCR datasets from all four experiments, the top 50 TCR sequences by frequency within a given phenotype were selected. For each TCR, the relative frequency with which it is found at each anatomical location was calculated by dividing its frequency at each location by the sum of the frequencies at all locations. The frequency is represented on the heat map by decile, with the scale indicating the color at 100 (red), 75, 50, 25, and 0% (yellow) for a TCR at a given location. The data are clustered such that those TCRs predominantly found in one location are shown together. (C) Statistical analysis of similarity. The mean Morisita-Horn similarity index (±SD; n = 3–4 independent experiments) is shown for the comparison between the mesenteric (Mes) LNs versus the spleen, cervical (Cerv), axillary (Axil), and inguinal (Ing) LNs (top), and the inguinal LNs versus the spleen, Mes, Cerv, and Axil LNs (bottom).
Interestingly, the CD44hiFoxp3− TCR repertoire behaves much like the Foxp3+ TCR repertoire with respect to repertoire changes based on location (Fig. 1). For example, we observed that in both subsets the TCR usage in the mesenteric LNs was very different from that in the other LNs, whereas the inguinal and axillary LNs were much more similar to each other (Fig. 1 C). This shared pattern of TCR localization by the T reg and CD44hi subsets may reflect common recirculation patterns and spectra of antigens in these LNs. For example, one might expect the axillary and inguinal LNs to share a similar antigenic repertoire because they both drain the skin and limbs. We also found, using generalized linear model testing, that out of the 15 most prevalent T reg and CD44hi TCRs, 10 T reg and 12 CD44hi TCRs showed a statistically significant level of skewing according to location (P < 0.05 with the Bonferroni correction; see Materials and methods), whereas only 1 out of the top 15 naive TCRs did so (Fig. S3). These data therefore suggest that the peripheral T reg population is shaped via a mechanism that is reminiscent of the generation and maintenance of memory cells.
The T reg and antigen-experienced CD44hi TCR repertoires are distinct
One possible explanation for the observed similarity in the pattern of localization between the T reg and CD44hi TCR repertoires is that these cells may codevelop from common precursors. This would be consistent with the hypothesis that peripheral T reg cells normally develop during the course of an immune response to limit pathology (22), and the observation in TCRαβ transgenic models that peripheral conversion only occurs in a portion of antigen-specific T cells after antigen administration (23–25). Thus, it is quite possible that TCRs that select for development into the antigen-experienced CD44hi phenotype may also be found within the T reg cell subset, because of peripheral conversion, and potentially account for the well-described overlap between the T reg and conventional non–T reg TCR repertoires (5, 6, 29).
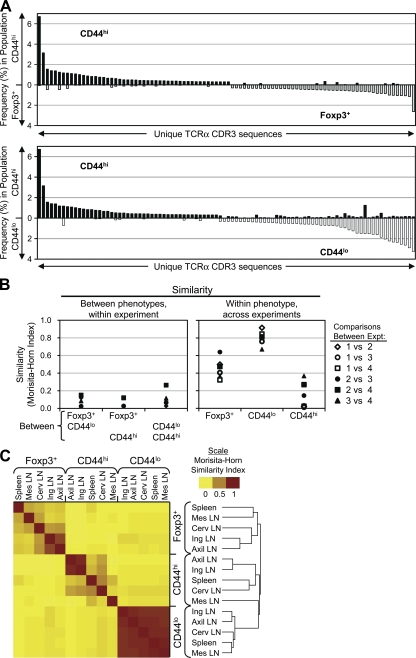
However, analysis of the sequences from the fractionated conventional T cell subsets revealed that the prominent TCRs in the CD44hi subset were not preferentially found in the Foxp3+ subset (Fig. 2 A; and Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). As expected, the naive (CD44lo) TCR repertoire showed little similarity with either the CD44hi or Foxp3+ TCR repertoires (Fig. 2 A, bottom; and Fig. S4 A), indicating differential requirements in TCR specificity for the development of these CD4+ T cell subsets. These differences between cellular phenotypes were also readily apparent within each individual experiment, even though the TCR repertoires varied between experiments depending on cellular phenotype (Fig. 2 B). The variability between experiments appeared to be the result of mouse-to-mouse variability (Fig. S4 B). In summary, we found little evidence from these studies of unimmunized adult animals to support the hypothesis that peripheral Foxp3+ T cell generation is an automatic occurrence after antigen encounter.
Figure 2.
The CD44hi, CD44lo, and T reg TCR repertoires show little overlap. (A) Comparison of the frequencies of prevalent TCRs between the CD44hi and Foxp3+ or CD44lo datasets. In each graph, the 50 most abundant TCRs from each of the two indicated populations were selected and plotted on the x axis, with the frequency of the TCR in the indicated subset on the y axis. TCRs are arranged in order of decreasing frequency within the CD44hi subset, and increasing frequency within the other subset. (B) Statistical analysis of similarity. (left) The Morisita-Horn similarity values between the indicated phenotypes within each experiment are shown, with each experiment represented by a different symbol. (right) The interexperiment similarity within each phenotype is shown, with each comparison represented by a unique symbol. (C) Cluster analysis of the peripheral TCR dataset. Data shown are a heatmap representation of the Morisita-Horn similarity indices calculated pairwise for all cell phenotype and location combinations using the pooled TCR dataset. Each decile is represented by distinct shades, with the scale indicating the color at 0, 0.5, and 1. The mean linkage algorithm was used to obtain a hierarchical cluster (or dendrogram; right) by sequentially grouping the two most correlated observations on the basis of a distance metric provided by the square root of 1 (Morisita-Horn index).
The strong difference between the TCR repertoires of these three phenotypes is further demonstrated by the cluster analysis of a matrix of Morisita-Horn similarity indices, which includes comparisons based on both location and cell phenotype (Fig. 2 C). This plot reveals the dominant effect of the cell type over the location when all data are considered, as the calculated similarities cluster in 5 × 5 blocks according to cell phenotype first. This analysis also reaffirms the similarity in the naive T cell repertoires between locations, as shown by the uniformly red 5 × 5 block representing the naive subset repertoires, whereas the other blocks show relatively high similarity only between the axillary and inguinal LNs. Thus, these data demonstrate that even though T reg and CD44hi cells share a common behavioral feature at the population level, it is unlikely that they are generated via a common developmental pathway.
A minor role for peripheral conversion in generating the adult T reg cell population
One explanation for the unexpected lack of similarity between the T reg and antigen-experienced subsets is that peripheral conversion is an infrequent process in unimmunized adult animals. Current estimates of the magnitude of peripheral conversion in adult animals are quite divergent. One study suggested that many peripheral T reg cells could arise via conversion, as ∼8% of CD25− T cells became CD25+ after transfer into a congenic, nonlymphopenic host (27). If one extrapolates the behavior of the adoptively transferred population in this study to represent that of the normal non–T reg cell subset at large, conversion would account for ∼72% of peripheral T reg cells (see Materials and methods). On the other hand, it has also been suggested that conversion occurs infrequently based on a study of monoclonal T cells reactive to an islet self-antigen (28).
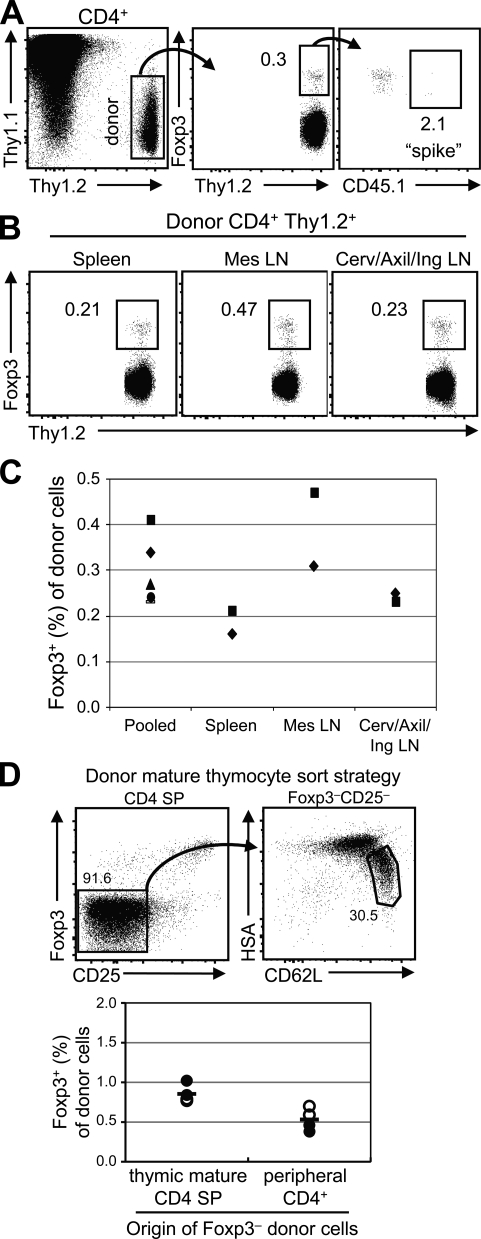
To measure the potential for peripheral conversion in a polyclonal T cell population, we used FACS to obtain a highly pure population of Foxp3−CD4+ donor T cells from Foxp3gfp mice. We achieved a purity of >99.998%, or <1 contaminating Foxp3+ cell per 50,000 Foxp3−CD4+ cells. Because T reg cells have been reported to divide at a faster rate than non–T reg cells (30), we also measured the projected expansion of these few contaminating natural T reg cells in this environment by adding ∼0.001% (100–200 cells per 107) FACS-purified CD45.1+Foxp3+ cells to the donor cell population. To detect sufficient numbers of donor cells by flow cytometry 3–4 wk after intravenous transfer, we analyzed nearly the entire T cell population from the pooled LNs and spleen of each recipient.
We found that the percentage of donor-derived cells that were Foxp3+ by 3 wk after transfer was quite low, ∼0.3% of the donor cells analyzed (Fig. 3 A). Consistent with previous studies (23, 26, 27), these cells appeared phenotypically similar to natural T reg cells (CD25hiGITRhi; not depicted). As the contribution from contaminating Foxp3+ donor cells to the final Foxp3+ population was estimated to be only ∼2% via analysis of the coinjected CD45.1+ cells, we believe that this frequency (0.3%) approximates the extent of conversion from peripheral Foxp3−CD4+ T cells.
Figure 3.
Peripheral conversion in nonlymphopenic hosts is infrequent. (A) Adoptive transfer of peripheral Foxp3− cells into congenic hosts. FACS-purified Foxp3−CD45.2+ (107) and Foxp3+CD45.1+ (1–2 × 102) T cells were intravenously transferred into normal Thy1.1 hosts. After 3–4 wk, acquisition of Foxp3 was analyzed by flow cytometry. Pooled spleen and LN cells were gated on CD4+ and Thy1.2+ (donor cells; left) to determine the frequency of Foxp3+ cells (middle). Within the Foxp3+ cells, the outgrowth of the contaminating cells in the original CD45.2+Foxp3− population was estimated by the spike of Foxp3+CD45.1+ cells (right). Data shown are representative of three independent experiments (n = 5 mice), and percentages are shown. (B) The frequency of conversion varies by anatomical location. The experiment was performed as in A, and after 4 wk the LNs or spleens from three animals were pooled for analysis. Data shown are representative of two independent experiments, and percentages are shown. (C) Summary of the flow cytometric data described in A and B. The frequency of Foxp3+ cells is shown. Each symbol represents data from an individual mouse (“pooled”; as in A) or an experiment in which cells from the organs of three mice were pooled (as in B) from the spleen, Mes LNs, and a pool of Cerv/Axil/Ing LNs. (D) Adoptive transfer of mature Foxp3− thymocytes. (top) Mature HSAloCD62LhiFoxp3−CD25− CD4SP thymocytes or Foxp3−CD4+ peripheral T cells were adoptively transferred into congenic hosts. Percentages are shown. (bottom) The frequency of Foxp3+ cells in pooled spleen and LN preparations was assessed at 3 wk by flow cytometry. Each symbol represents data from an individual recipient from two experiments in which 3.4 × 106 (closed circles) or 9 × 106 (open circles) cells were injected per mouse. The horizontal bars represent mean values.
Recent studies have suggested that the gut environment may preferentially facilitate peripheral conversion (14, 31, 32). We repeated the experiment described earlier in this section to compare the frequency of converted Foxp3+ cells in the mesenteric LNs, spleen, and the pooled cervical, axillary, and inguinal LNs. To observe sufficient conversion events by flow cytometry, each experiment consisted of tissues pooled from three individual mice that had received the same preparation of purified donor cells 4 wk earlier. Consistent with a previous paper (31), we found that the mesenteric LNs, in comparison with the peripheral LNs and spleen, contained an approximately twofold higher percentage of converted Foxp3+ cells (Fig. 3, B and C). However, converted Foxp3+ cells were found in all locations, at frequencies ranging from 0.2–0.5%.
Because conversion appears to occur naturally in lymphoreplete animals, it was possible that this process would reduce the number of cells that can undergo conversion in the peripheral Foxp3−CD4+ T cell population used. We therefore assessed the frequency of conversion in immediate precursors to the peripheral T cell population, mature CD4+CD8− (CD4 single-positive [SP]) thymocytes. We purified CD62LhiHSAloFoxp3−CD25− CD4SP thymocytes as well as Foxp3−CD4+ peripheral T cells to enable direct comparison between these populations. 3 wk after adoptive transfer into congenic hosts, we found that, on average, 0.5% of peripheral CD4+ donor cells and 0.8% of thymocytes became Foxp3+. If one extrapolates this behavior as representative of their respective T cell populations, then peripheral conversion would account for the development of 4–7% of peripheral CD4+Foxp3+ T cells in a normal mouse (see Materials and methods). Although this is a rough estimate, it suggests that peripheral conversion plays a relatively small role in generating the protective T reg cell population in adult individuals.
Peripheral conversion in lymphopenic hosts is TCR specific
The low rate of peripheral conversion in an adult lymphoreplete animal may be caused, in part, by inhibition by the existing T reg cell population. We hypothesized that peripheral conversion may play a greater role in a lymphopenic environment, such as that seen in the early ontogeny of the peripheral immune system, which is both lymphopenic (33, 34) and T reg cell–deficient because of delayed thymic T reg cell export (35, 36). Therefore, we used the adoptive transfer of CD4+Foxp3− T cells from mice containing the fixed TCRβ chain into lymphopenic hosts as a model of peripheral conversion, which has previously been reported to occur upon the transfer of non–T reg cells into lymphopenic hosts (26, 27).
Consistent with previously published results, we observed peripheral conversion of the highly purified CD4+Foxp3− T cells from TCli TCRβ chain transgenic mice 2.5–3 wk after intravenous transfer into Tcrb−/− αβT cell–deficient mice (see Fig. S6 A). The converted cells were phenotypically similar to T reg cells, expressing high levels of CD103, GITR, and CD25 (not depicted). Because the incidence of Foxp3 induction versus subsequent expansion cannot be discriminated, the extent of “peripheral conversion” in this system represents the aggregate outcome. Of note, the percentage of converted Foxp3+ cells was considerably higher after adoptive transfer into lymphopenic (see Fig. S6 A) as compared with nonlymphopenic (Fig. 3) hosts, potentially because of the empty T reg cell niche available in Tcrb−/− mice. A second difference between the results from these two hosts was the relative frequencies of Foxp3+ cells recovered from the various LNs. Lymphopenic hosts showed a decreased frequency of Foxp3+ cells in the mesenteric relative to other LNs, which may reflect the preferential accumulation of effector cells, as the transfer of CD4+ T cells depleted of T reg cells is known to result in colitis (37). In summary, the frequency of peripheral converted Foxp3+ cells arising from Foxp3− cells is much greater in a lymphopenic compared with a lymphoreplete environment.
Initial experiments analyzing TRAV14 TCRα sequences obtained from FACS-purified Foxp3+ and Foxp3−CD4+ T cells pooled from several recipient Tcrb−/− mice hinted at a fair degree of mouse-to-mouse variability (unpublished data), similar to that seen in the CD44hi T cell subset in normal mice (Fig. S4 B). To address whether random sampling was responsible for this variability, we obtained a duplicate sequence dataset from four samples (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). We found that all TCRs greater than ∼2% at this sample size (n = 150–200) are present in both datasets at similar frequencies. We therefore went on to generate a database of >7,000 Foxp3+ and Foxp3− TCR sequences from the cervical and mesenteric LNs and spleens of six individual recipient mice from two independent experiments (Fig. S6, B and C; and Fig. S7).
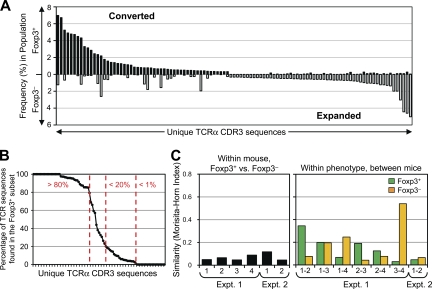
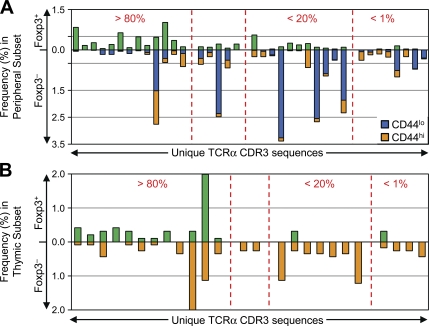
Based on studies of monoclonal TCR populations showing that the majority of the antigen-specific T cells remain Foxp3− during peripheral conversion (23–25), we were surprised to find relatively little overlap in TCR usage between the converted and nonconverted T cell subsets (Fig. 4 A). Most of the prevalent TCRs identified in these experiments showed a marked skewing toward either the Foxp3+ (converted) or Foxp3− (nonconverted) populations, with only a minority (9 out of 85) of the TCRs being found at nearly equal frequencies in both populations (Fig. 4 B). We observed considerable differences in the TCR repertoires between the individual mice even though the donor cell population was identical within each experiment (Fig. 4 C, right), which was suggestive of the preferential expansion of cells with a low precursor frequency. However, the level of dissimilarity between the Foxp3+ and Foxp3− cells from each animal was equivalent (Fig. 4 C, left). Although most TCRs were not found in all six individual mice, TCRs recovered from multiple animals were reproducibly found in either the converted or nonconverted subsets, with few exceptions (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). Thus, even though both peripheral conversion and lymphopenia-induced proliferation of non–T reg cells occurred within the same animal, these data demonstrate that TCR specificity played an important role in T reg cell fate determination during this immune response.
Figure 4.
TCR specificity is important for peripheral conversion in lymphopenic hosts. (A) The converted and nonconverted TCR repertoires differ. TCRα chain sequences were obtained as described in Materials and methods. The 50 most abundant TCRs from each of the converted Foxp3+ and nonconverted Foxp3− pooled datasets are shown, with the frequency in the indicated subset on the y axis. The TCRs are ordered from the most abundant in the Foxp3+ subset (left) to the most abundant in the Foxp3− subset (right). (B) TCRs are preferentially skewed toward either the Foxp3+ or Foxp3− subset. For prevalent TCRs in the pooled dataset (summed frequencies >0.5%), the percentage of each TCR in the Foxp3+ subset (%Foxp3+/%Foxp3+ + %Foxp3−) is plotted in decreasing order. Arbitrary cutoffs (vertical dashed lines) are shown for TCRs highly (>80% Foxp3+), poorly (<20%), or not (<1%) associated with conversion. (C) Assessment of mouse-to-mouse variability. (left) Morisita-Horn index values comparing the Foxp3+ and Foxp3− TCR datasets within each individual recipient are shown. (right) The Foxp3+ TCR datasets from individual animals within the same experiment are compared in green, and the Foxp3− datasets are compared in orange. There were four and two recipients for the first and second independent experiments, respectively.
TCRs that facilitate peripheral conversion are also found within the normal peripheral and thymic T reg TCR repertoire
Although we were unable to determine which TCRs undergo peripheral conversion in normal hosts because of the low rate of conversion, we did identify TCRs that facilitate peripheral conversion during the immune response in lymphopenic hosts. As expected, these converted TCRs were found in the normal non–T reg cell subsets, because the donor cells for these studies were normal Foxp3− cells (Fig. 5 A). Interestingly, these TCRs were also often found in the normal T reg cell subset and constitute ∼5% of the sequences in the normal peripheral T reg cell dataset (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). Although the extent to which these TCRs affect the T reg TCR repertoire in lymphoreplete individuals by peripheral conversion is unknown, these data support our observation that peripheral conversion makes only a small contribution to the normal T reg cell population.
Figure 5.
TCRs that facilitate peripheral conversion are found in the normal peripheral and thymic T reg cell TCR repertoires. (A) Comparison with the normal peripheral TCR repertoire. Prevalent TCRs recovered after the transfer of Foxp3− cells into lymphopenic hosts that are also found in the normal peripheral dataset (Table I) at a summed frequency of >0.15% were identified (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). The frequencies of these overlapping TCRs in the normal dataset are plotted. The TCRs are sorted by their ability to facilitate conversion, as designated by the vertical dashed lines (Fig. 4B). (B) Comparison with the normal thymic TCR repertoire. The frequency of the TCR in the normal thymic dataset (reference 38) is shown as in A.
It has been proposed that peripheral conversion may play an important role in generating T reg cells to antigens that are not present in the thymus, such as those found in the gut (14, 31, 32). Therefore, this would predict that converted TCRs should generally be found within the peripheral but not thymic T reg cell subset. We found that of the highly converting TCRs that were also present in our thymic sequence dataset (38), most (11 out of 12) could be found within the Foxp3+ subset (Fig. 5 B). By comparison, only 2 out of 11 TCRs from the poorly converting category were found within the thymic Foxp3+ dataset. One potential explanation for this observation is the presence of recirculating mature peripheral CD4+Foxp3+ T cells in the thymus. Although the frequency of recirculating T cells appears low (39, 40), a direct assessment of Foxp3+ T reg cell recirculation in normal hosts was not performed. To test this possibility, we injected 20 × 106 enriched peripheral CD4+ T cells into a congenic host and assessed the percentage of donor cells in the CD4SP thymocyte population after 1 wk (Fig. S10, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). If one assumes that the behavior of the donor cells is representative of all peripheral CD4+ T cells, then ∼4% of thymic T reg cells arise from recirculation. Because these shared TCRs are not grossly overrepresented in the periphery compared with the thymus, recirculation alone is unlikely to account for the thymic observation of TCRs that facilitate peripheral conversion. Rather, we favor the hypothesis that some TCRs are able to facilitate both thymic and peripheral T reg cell development. Because we expect primarily self-antigens to be presented in a normal thymus, these data suggest that some self-reactive cells, upon escaping T reg cell selection in the thymus, may get a “second chance” to become Foxp3+ regulatory cells via peripheral conversion.
TCR specificity plays a crucial role in peripheral conversion
The observation that many TCRs found preferentially on converted T reg cells are also found on normal thymic T reg cells raised the possibility that we were merely observing Foxp3 induction in Foxp3− T reg cell precursors that arise in the thymus and are then exported before Foxp3 is expressed (41). We therefore asked whether TCR specificity alone, in the absence of thymic conditioning, is sufficient to direct peripheral conversion by introducing selected TCRα chains into peripheral monoclonal T cells from TCli αβTCR transgenic × Foxp3gfp mice on a Rag1−/− background and then transferring them into Tcrb−/− hosts (7). As the cells were isolated and treated identically, they should differ only by the additional retroviral TCRα chain at the time of transfer.
We first tested two TCRα chains (B2 and B132) that were previously identified in naive T cells and were also found primarily in the nonconverted T cell subset in our lymphopenic expansion experiments (Fig. 6 A). These TCRs were previously shown to be unable to facilitate in vivo expansion of T cells in Rag1−/− hosts (29). Consistent with their low frequencies in the converted TCR datasets, we found that these TCRs did not facilitate either Foxp3 induction or expansion (Fig. 6 B). Therefore, TCR activation and retroviral introduction of an additional TCRα chain alone are not sufficient to induce Foxp3 expression in these cells.
Figure 6.
TCR specificity is sufficient to direct peripheral conversion in lymphopenic hosts. (A) Frequency distribution of TCRs tested. Data shown are the frequencies (percentages) of the TCRs in the datasets from thymocytes (reference 38), normal peripheral T cells (Table I), and T cells recovered after adoptive transfer into lymphopenic hosts (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20081359/DC1). (B) Retroviral manipulation of TCR specificity in peripheral T cells. Monoclonal TCli αβTCR transgenic T cells were transduced with TCRα chains, as described in Materials and methods. (top) The frequency of Foxp3+ cells in the transduced (Vα2+Vβ6+CD4+) T cell population was assessed by flow cytometry 2.5–3 wk after adoptive transfer into Tcrb−/− mice. (bottom) The absolute number of transduced (Vα2+) cells recovered was calculated using the cell count obtained via either a Coulter counter (Beckman Coulter) or hemacytometer (Hausser), and the frequency of Vα2+Vβ6+CD4+ T cells within the live gate, as determined by flow cytometry. Data shown are from individual mice and were obtained from five independent experiments constituting at least two independent retroviral transductions per TCR. Each symbol represents data from an individual recipient (n = 4–6), and the horizontal bars represent mean values.
We then tested four TCRα chains that were identified in the highly converting Foxp3+ TCR dataset (R19, R22, TR3, and TR5; Fig. 6 A and Fig. S9) and found that they also facilitated peripheral conversion in retrovirally transduced cells (Fig. 6 B, top). TCR-dependent conversion occurred even though the T cells underwent in vitro activation and retroviral transduction, in contrast with the previous study in which freshly isolated polyclonal T cells were transferred (Fig. 4). Thus, these data directly demonstrate that TCR specificity alone, in the absence of a unique thymic signal, is sufficient to facilitate the process of peripheral conversion.
Because these highly converting TCRs were also found in the normal T reg TCR dataset, we tested the ability of other natural T reg TCRs that were not found in the conversion experiments to facilitate conversion. Interestingly, only one out of the four T reg TCRs tested (R111) was able to induce Foxp3 in a sizable proportion of cells, whereas the others did not (G113, G5, and G57). The inability to augment conversion was presumably not caused by a lack of TCR engagement, as these other TCRs did facilitate in vivo expansion (Fig. 6 B, bottom), suggesting that their cognate antigens were available in the periphery. It also appeared that certain TCRs favored expansion in different anatomical LNs, which was consistent with our analysis of the normal T reg TCR repertoire (Fig. 2). For example, TR5 and G113 showed expansion mostly in the axillary and inguinal LNs, whereas G57 and R111 expanded mostly in the mesenteric LNs, which is consistent with the relative frequencies at which these TCRs are normally found in the Foxp3+ subsets in these LNs (Fig. 6 A) and would suggest that their cognate antigens reside preferentially in these locations. In summary, these data suggest that TCR specificity determines whether a cell is capable of peripheral conversion but that not all TCR specificities associated with peripheral T reg cells have this capability.
DISCUSSION
Our analysis of the peripheral TRAV14 TCR repertoire strongly suggests that immune regulation is a tissue-specific activity, as the antigen specificity of the protective T reg cell population changes considerably by anatomical location (Fig. 1). These data are consistent with our previous observations of individual T reg TCR–driven proliferation in normal hosts, in which CFSE dilution was more pronounced in certain LNs (7). Thus, the observation that tissue-specific antigens greatly modify the local T reg TCR repertoire provides direct evidence for a mechanism by which the presence of an organ facilitates T reg cell–mediated tolerance to that organ (9–11).
Another intriguing observation is that the T reg and CD44hi TCR repertoires showed similar patterns of variability by anatomical location. This suggests that T reg and CD44hi populations share similar fundamental behaviors governing their interaction with tissue-specific antigens, even though the conditions for their development may be different. These data therefore support previous studies using monoclonal TCR transgenic mice (42), demonstrating that both T reg and non–T reg cells undergo clonal expansion upon antigen encounter. The expansion and maintenance of T reg cells specific for antigens normally presented in the peripheral self would favor tolerogenic T reg cell responses to those antigens, rather than novel foreign antigens. Thus, we hypothesize that shaping of the T reg cell population to the local antigenic landscape provides another mechanism for self/nonself discrimination by the immune system.
Contrary to previous studies (27), we did not find that peripheral conversion played a prominent role in the generation of the peripheral T reg cell population (Fig. 3). This difference could be explained in part by our use of Foxp3 as a marker for T reg cells, rather than CD25 (43), to isolate non–T reg cells for adoptive transfer and analysis of conversion. Nonetheless, the use of mature Foxp3−CD4+ thymocytes may still overestimate the role of peripheral conversion because we cannot exclude the presence of rare CD25−Foxp3− T reg cell precursors in this subset. We can also not be certain that these mature thymocytes are phenotypically identical to recent thymic emigrants in terms of their potential to undergo peripheral conversion. On the other hand, the use of peripheral Foxp3−CD4+ T cells may underestimate the role of peripheral conversion because we can only assess the fraction of Foxp3− cells capable of converting at steady state, and those T cells with the greatest propensity to undergo peripheral conversion are therefore likely to be underrepresented. Thus, we believe our estimate that peripheral conversion contributes ∼4–7% of the normal adult peripheral T reg cell population represents the best currently available figure.
We also studied the process of peripheral conversion during the immune response of Foxp3− cells transferred into αβT cell–deficient hosts, which may mimic the lymphopenic and T reg cell–deficient conditions during the early neonatal period. We found that peripheral conversion occurred much more readily in this environment than in a lymphoreplete host, and this made it technically feasible to perform TCR usage analysis. Interestingly, we found that peripheral conversion in polyclonal populations did not mirror previous experiments using monoclonal T cell populations, where a portion (∼20%) of cells became Foxp3+ after noninflammatory antigen encounter in both nonlymphopenic and lymphopenic models (23, 41, 44). Rather, we observed no consistent patterns governing peripheral conversion during T cell expansion, finding that individual TCRs ranged from being highly skewed toward or against peripheral T reg cell development (Fig. 4 B and Fig. S8). Thus, the analysis of polyclonal TCR repertoires from both lymphopenic and nonlymphopenic environments does not support a model in which peripheral conversion is an automatic consequence of T cell activation, but rather one in which peripheral conversion is highly dependent on TCR specificity.
Although our data do not directly allow us to determine whether peripheral conversion occurs upon recognition of nonself-antigens, we hypothesize that many TCRs that facilitate conversion are reactive to self-antigens. This is strictly based on the observation that several TCRs from converted T reg cells in lymphopenic hosts can also be found in the normal thymic T reg cell subset (Fig. 5 and Fig. S9). As previously argued, we believe that thymic T reg cell development occurs via recognition of self- (38) rather than nonself-antigens (20). Although we cannot exclude the possibility of peripheral nonself-antigen presentation in the thymus, nor the possibility that different peptides are recognized in the thymus and periphery, the most straightforward explanation is that the same self-reactivity that induces T reg cell development in the thymus also does so in the periphery. Although future experiments will be required to prove that the observed bias of converted TCRs toward the thymic T reg TCR repertoire is caused by the recognition of the same self-antigens, these data suggest that some cells that escape thymic T reg cell development express self-reactive TCRs that facilitate a second chance for T reg cell development in the periphery.
Peripherally converted T reg cells may develop directly from naive T cells or instead differentiate from memory/effector T cells (41). In vivo data suggests that an appreciable frequency of Foxp3+ cells requires 1 wk or more to develop (23, 25, 44). This time frame favors a multistep process but may also reflect a need for postconversion expansion. In contrast, in vitro studies have suggested that TGF-β–mediated induction of Foxp3 is considerably more efficient in naive than in memory T cells (45). In fact, it has been reported that TGF-β is unable to induce Foxp3 in previously in vitro–differentiated T cells (46). Because in vitro TCR stimulation is used in the retroviral transduction protocol, our data directly demonstrate that in vivo, peripheral conversion from previously activated T cells can still occur in the context of an appropriate TCR specificity (Fig. S7), hinting at possible differences between in vitro and in vivo conversion.
It is clear, however, that conversion alone is insufficient to maintain immune homeostasis, as shown by the development of colitis and other autoimmune manifestations upon transfer of T reg cell–depleted CD4+ T cells (3, 9–11, 37). However, conversion may diminish the severity of disease (25, 47). The inability to prevent disease may be caused by the limited T reg TCR repertoire generated by peripheral T reg cell development (Fig. S7), which has been suggested to permit autoimmunity in lymphopenic mice (48). Alternatively, it may be that peripheral conversion occurs too late to fully prevent autoimmune pathology. One interesting hypothesis is that autoimmune disease may also be exacerbated by self-reactive Foxp3− T cells expressing T reg TCRs that escape into the periphery (7) but are unable to undergo peripheral conversion because of their antigen specificity (Fig. 6).
These data demonstrate a critical role for TCR specificity in the decision to undergo peripheral conversion. One potential mechanism by which this occurs is that the “nature” of the TCR–ligand interaction itself could generate a unique signal that results in Foxp3 expression. This has been suggested by an αβTCR × cognate antigen double transgenic model of thymic T reg cell development (4). In this model, the high affinity ligand induced both deletion and T reg cell development, whereas a slightly different, lower affinity ligand could only induce deletion. However, a mechanism dependent on unique TCR signals would be unable to distinguish the context of T cell activation in the periphery, making it teleologically less appealing. An alternative, nonmutually exclusive hypothesis is that the TCR specificity results in activation within an environment that facilitates peripheral T reg cell development. We favor this latter model, in which antigen presentation can occur on APCs and/or within microenvironments that determine whether a given T cell will become a regulatory or effector cell. Based on current data, we suspect that a microenvironment promoting peripheral conversion would contain TGF-β and potentially retinoic acid (14, 31, 32, 44). Therefore, the balance of antigen presentation between two different microenvironments would determine the pathological or tolerogenic outcome of the immune response to an antigen.
MATERIALS AND METHODS
Mice and reagents.
Foxp3gfp reporter knockin, and TCli TCRβ and TCRαβ transgenic mice were provided by A. Rudensky (University of Washington, Seattle, WA). B6.SJL (CD45.1), B6.PL (Thy1.1), Tcrb−/−, and Tcra−/− mice were obtained from the Jackson Laboratory. Animals were housed in a specific pathogen-free animal facility at Washington University and were used according to protocols approved by the Animal Studies Committee of Washington University. Monoclonal antibodies were purchased from eBioscience and BD. Human IL-2 was obtained from the National Institutes of Health Biological Resources Branch.
Cell purification.
CD4+ T cells were initially purified from the tissues indicated in the figures using the CD4 Untouched kit, which uses AutoMACS magnetic separation to remove non-CD4+ cells (Miltenyi Biotec). The remaining cells were stained with fluorescently labeled antibodies and were sorted by flow cytometry using a MoFlo (Dako) or a FACSAria (BD) instrument.
Isolation of T cells for TCR sequencing.
CD4+Vβ6+ T cells were purified by FACS into the Foxp3+, Foxp3−CD44hi, and Foxp3−CD44lo subsets from normal mice, and Foxp3+ and Foxp3− subsets after proliferation and conversion in lymphopenic hosts. CD44 was not used to purify T cells from lymphopenic hosts, as the Foxp3− population was CD44hi after lymphopenia-induced proliferation. Postsort purity was >95%. Cell numbers for all sorted Foxp3− samples were between 1.7 × 105 and 1.5 × 106, and between 2 and 5 × 104 for Foxp3+ samples from all mice except one, for which they were between 7 × 103 and 1.6 × 104. The percentage of Vα2+ cells was found to range from 6–14%, suggesting that the proportion of these cells was relatively stable between mice and experiments. Cloning of TRAV14 TCRα sequences was performed as previously described (7). As before, we used the CDR3 amino acid sequence provided by IMGT/V-QUEST as a unique identifier for individual TCRs (49). Although differences in J-region usage between T reg and non–T reg TCRs have been noted, much greater differences are seen when comparing CDR3 amino acid sequences (29). Thymic Foxp3+ and Foxp3−CD4+CD8− datasets have been previously described (38).
Statistical analysis.
The estimated contribution of peripheral conversion to the T reg cell population is a simple extrapolation of the percentage of Foxp3+ cells in the adoptively transferred Foxp3− population to the entire Foxp3− population multiplied by the proportion of Foxp3− cells of CD4+ T cells, divided by the proportion of T reg cells of CD4+ T cells. Thus, an 8% conversion in normal mice would represent 7.2% of total CD4+ T cells (8 × 90%), which would be 72% the size of the T reg cell subset (7.2 of 10% T reg cells in the total CD4+ T cell population). Our observed conversion frequency of 0.8% from mature thymocytes and 0.4% (average of all experiments) from peripheral cells would therefore translate into an estimated contribution of conversion to the peripheral T reg cell population of 7.2 and 3.6%, respectively.
We used the Morisita-Horn index as a statistical measure of similarity between two datasets (50). This unitless index ranges from 0 to 1, representing complete dissimilarity to similarity. The Morisita-Horn index takes into account the frequency of each TCR and is relatively resistant to the sample size, as compared with other similarity indices such as the Jaccard. A sequence found in both datasets at greatly different frequencies would therefore contribute to dissimilarity by this index. For reference, comparison of the first 200 sequences with the last 200 sequences of an ∼400-sequence dataset results in a Morisita-Horn index of ∼0.9, which represents an experimentally derived value for the maximum expected similarity that incorporates the inherent variability because of random sampling.
The abundance coverage estimator was used to estimate the total number of unique species in the population based on the observed number of species in the dataset, as previously described (29).
To determine whether anatomical location affected TCR distribution, we performed generalized linear model testing using the raw counts of all experiments for each of the phenotypes. We used the Bonferroni correction (0.05 divided by the total number of sequences analyzed) to provide the most stringent p-value for rejection of the null hypothesis that TCR distribution is unaffected by location.
Assessment of peripheral conversion in vivo.
For nonlymphopenic hosts, 107 FACS-purified Foxp3−CD4+CD45.2+ T cells from the pooled spleen and LNs of Foxp3gfp mice were intravenously injected into congenic Thy1.1 hosts. 100–200 Foxp3+CD4+CD45.1+ T cells were also cotransferred to assess the contribution of Foxp3+ contaminants in the Foxp3− population. Alternatively, mature HSAloCD62LhiFoxp3−CD25− CD4SP thymocytes (3.4 or 9 × 106) were FACS purified from thymocytes depleted of CD8+ cells by AutoMACS from 13 or 17 mice. Acquisition of Foxp3 and other markers by flow cytometry was assessed after 3–4 wk.
For lymphopenic hosts, 2 × 106 FACS-purified Foxp3−CD4+ T cells from Foxp3gfp or TCli TCRβ × Foxp3gfp × TCRα+/− mice were intravenously injected into Tcrb−/− hosts, and were analyzed for acquisition of Foxp3 and other markers by flow cytometry after 19–21 d. We also verified that the converted Foxp3+ cells were unlikely to originate from the contaminating Foxp3+ cells in the sorted population using “spike” experiments similar to those in Fig. 3 A (not depicted).
Facilitation of peripheral conversion by retrovirally expressed TCRα chains.
Peripheral T cells from TCli (Vα18 and Vβ6) TCRαβ transgenic × Foxp3gfp × Rag1−/− mice were depleted by magnetic bead selection of CD25+ cells (Miltenyi Biotec), and were retrovirally transduced on days 1 and 2 after activation, as previously described (7). After 7 d, a T cell population containing 4 × 105 transduced Vα2+CD4+ cells was injected intravenously into a Tcrb−/− host. Acquisition of Foxp3 on Vα2+ cells was assessed at 2.5–3 wk by flow cytometry.
Online supplemental material.
Figs. S1–S5 provide additional detail regarding the TRAV14 sequences obtained from TCli TCRβ chain transgenic T cells, as summarized in Table I. The number of sequences in each dataset, as well as the sorting strategy, is shown in Fig. S1. The diversity of the TCR datasets is illustrated in Fig. S2. Detailed information regarding the anatomical preference of the 15 most prevalent TCRs in the CD44hi, CD44lo, and Foxp3+ subsets is presented in Fig. S3. Additional comparisons between TCR datasets from different T cell subsets are shown in Fig. S4. Finally, an assessment of random sampling is provided in Fig. S5.
Figs. S6–S10 offer additional information regarding the studies of T reg cell conversion in lymphopenic hosts. The frequency of Foxp3+ cells, numbers of TRAV14 TCRα sequences obtained, and detailed information regarding the top 14 Foxp3− and Foxp3+ TCRs are shown in Fig. S6. The diversity of the TCR datasets is illustrated in Fig. S7. The frequency of the TCR in the converted and nonconverted datasets is shown at the individual mouse level in Fig. S8. A comparison of the normal TCR datasets with those obtained after transfer into lymphopenic hosts is shown in Fig. S9. Finally, the frequency of T reg cell recirculation into the thymus is estimated in Fig. S10. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081359/DC1.
Supplementary Material
Acknowledgments
We would like to thank P. Simmons and J. Hunn for expert technical assistance; J. Scott-Brown, J. Kim, W. Yokoyama, and C.-W. Lio for helpful discussions and critical review of the manuscript; A. Rudensky for providing the Foxp3gfp reporter and TCli TCRβ and TCRαβ transgenic mice; L. Fulton, W. Courtney, and B. Theising for their assistance at the Genome Sequencing Center; B. Eades and J. Hughes at the Siteman Cancer Center High Speed Cell Sorter Core; and W. Harrington of BD Immunocytometry Systems.
This work was supported by the Arthritis Foundation, the Burroughs Wellcome Fund, and the National Institutes of Health (C.-S. Hsieh).
The authors have no financial conflicts of interest to report.
Abbreviation used: SP, single positive.
References
- 1.Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. [DOI] [PubMed] [Google Scholar]
- 2.Lahl, K., C. Loddenkemper, C. Drouin, J. Freyer, J. Arnason, G. Eberl, A. Hamann, H. Wagner, J. Huehn, and T. Sparwasser. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: Production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 4.Jordan, M.S., A. Boesteanu, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 5.Wong, J., R. Obst, M. Correia-Neves, G. Losyev, D. Mathis, and C. Benoist. 2007. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J. Immunol. 178:7032–7041. [DOI] [PubMed] [Google Scholar]
- 6.Pacholczyk, R., H. Ignatowicz, P. Kraj, and L. Ignatowicz. 2006. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 25:249–259. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh, C.S., Y. Zheng, Y. Liang, J.D. Fontenot, and A.Y. Rudensky. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7:401–410. [DOI] [PubMed] [Google Scholar]
- 8.Correia-Neves, M., C. Waltzinger, D. Mathis, and C. Benoist. 2001. The shaping of the T cell repertoire. Immunity. 14:21–32. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi, O., K. Kontani, H. Ikeda, T. Kezuka, M. Takeuchi, and T. Takahashi. 1994. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 82:365–369. [PMC free article] [PubMed] [Google Scholar]
- 10.Samy, E.T., L.A. Parker, C.P. Sharp, and K.S. Tung. 2005. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 202:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon, B., and D. Mason. 1999. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 189:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, E.A., Y. Choi, and R.A. Flavell. 2002. Pancreatic lymph node-derived CD4+ CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 16:183–191. [DOI] [PubMed] [Google Scholar]
- 13.Benson, M.J., K. Pino-Lagos, M. Rosemblatt, and R.J. Noelle. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mucida, D., Y. Park, G. Kim, O. Turovskaya, I. Scott, M. Kronenberg, and H. Cheroutre. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260. [DOI] [PubMed] [Google Scholar]
- 15.Sather, B.D., P. Treuting, N. Perdue, M. Miazgowicz, J.D. Fontenot, A.Y. Rudensky, and D.J. Campbell. 2007. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 204:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavin, M.A., S.R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2002. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3:33–41. [DOI] [PubMed] [Google Scholar]
- 17.Grajewski, R.S., P.B. Silver, R.K. Agarwal, S.B. Su, C.C. Chan, G.I. Liou, and R.R. Caspi. 2006. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J. Exp. Med. 203:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori, S., M. Haury, A. Coutinho, and J. Demengeot. 2002. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 99:8213–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 20.Pacholczyk, R., J. Kern, N. Singh, M. Iwashima, P. Kraj, and L. Ignatowicz. 2007. Nonself-antigens are the cognate specificities of foxp3(+) regulatory T cells. Immunity. 27:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875–888. [DOI] [PubMed] [Google Scholar]
- 22.Akbar, A.N., M. Vukmanovic-Stejic, L.S. Taams, and D.C. Macallan. 2007. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 7:231–237. [DOI] [PubMed] [Google Scholar]
- 23.Apostolou, I., and H. von Boehmer. 2004. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobbold, S.P., R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, and H. Waldmann. 2004. Induction of Foxp3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010. [DOI] [PubMed] [Google Scholar]
- 25.Knoechel, B., J. Lohr, E. Kahn, J.A. Bluestone, and A.K. Abbas. 2005. Sequential development of interleukin 2–dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curotto de Lafaille, M.A., A.C. Lino, N. Kutchukhidze, and J.J. Lafaille. 2004. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173:7259–7268. [DOI] [PubMed] [Google Scholar]
- 27.Liang, S., P. Alard, Y. Zhao, S. Parnell, S.L. Clark, and M.M. Kosiewicz. 2005. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 201:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, J., D. Mathis, and C. Benoist. 2007. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J. Exp. Med. 204:2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh, C.-S., Y. Liang, A.J. Tyznik, S.G. Self, D. Liggitt, and A.Y. Rudensky. 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 21:267–277. [DOI] [PubMed] [Google Scholar]
- 30.Fisson, S., G. Darrasee-Jeze, E. Litvinova, F. Septier, D. Klatzmann, R. Liblau, and B.L. Salomon. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun, C.M., J.A. Hall, R.B. Blank, N. Bouladoux, M. Oukka, J.R. Mora, and Y. Belkaid. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coombes, J.L., K.R. Siddiqui, C.V. Arancibia-Carcamo, J. Hall, C.M. Sun, Y. Belkaid, and F. Powrie. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Campion, A., C. Bourgeois, F. Lambolez, B. Martin, S. Leaument, N. Dautigny, C. Tanchot, C. Penit, and B. Lucas. 2002. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc. Natl. Acad. Sci. USA. 99:4538–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min, B., R. McHugh, G.D. Sempowski, C. Mackall, G. Foucras, and W.E. Paul. 2003. Neonates support lymphopenia-induced proliferation. Immunity. 18:131–140. [DOI] [PubMed] [Google Scholar]
- 35.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontenot, J.D., J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izcue, A., J.L. Coombes, and F. Powrie. 2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212:256–271. [DOI] [PubMed] [Google Scholar]
- 38.Lio, C.W., and C.S. Hsieh. 2008. A two-step process for thymic regulatory T cell development. Immunity. 28:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirberg, J., N. Bosco, J.C. Deloulme, R. Ceredig, and F. Agenes. 2008. Peripheral T lymphocytes recirculating back into the thymus can mediate thymocyte positive selection. J. Immunol. 181:1207–1214. [DOI] [PubMed] [Google Scholar]
- 40.Bosco, N., H.C. Hung, N. Pasqual, E. Jouvin-Marche, P.N. Marche, N.R. Gascoigne, and R. Ceredig. 2006. Role of the T cell receptor alpha chain in the development and phenotype of naturally arising CD4+CD25+ T cells. Mol. Immunol. 43:246–254. [DOI] [PubMed] [Google Scholar]
- 41.Lohr, J., B. Knoechel, and A.K. Abbas. 2006. Regulatory T cells in the periphery. Immunol. Rev. 212:149–162. [DOI] [PubMed] [Google Scholar]
- 42.Klein, L., K. Khazaie, and H. von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 44.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. [DOI] [PubMed] [Google Scholar]
- 45.Zheng, S.G., J. Wang, P. Wang, J.D. Gray, and D.A. Horwitz. 2007. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 178:2018–2027. [DOI] [PubMed] [Google Scholar]
- 46.Davidson, T.S., R.J. DiPaolo, J. Andersson, and E.M. Shevach. 2007. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 178:4022–4026. [DOI] [PubMed] [Google Scholar]
- 47.Izcue, A., S. Hue, S. Buonocore, C.V. Arancibia-Carcamo, P.P. Ahern, Y. Iwakura, K.J. Maloy, and F. Powrie. 2008. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 28:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milner, J.D., J.M. Ward, A. Keane-Myers, and W.E. Paul. 2007. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. USA. 104:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giudicelli, V., D. Chaume, and M.P. Lefranc. 2004. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 32:W435–W440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magurran, A.E. 1988. Ecological Diversity and Its Measurement. Princeton University Press, Princeton, NJ. 179 pp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.