Abstract
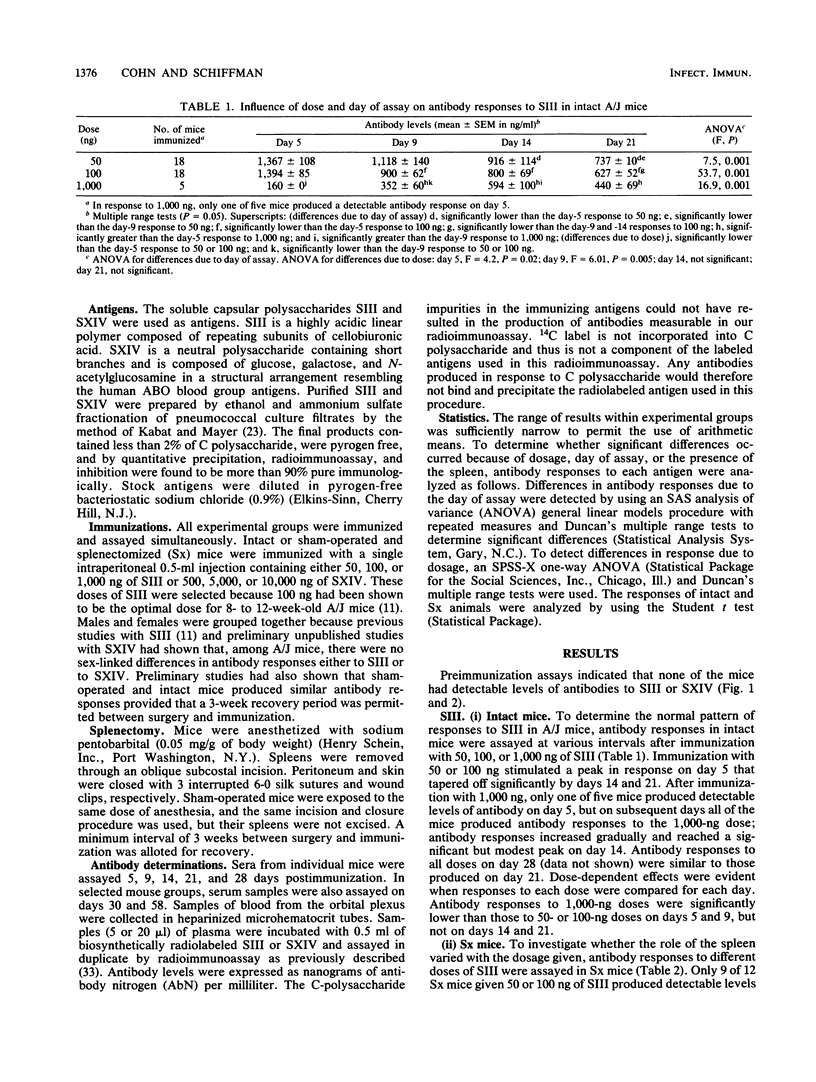
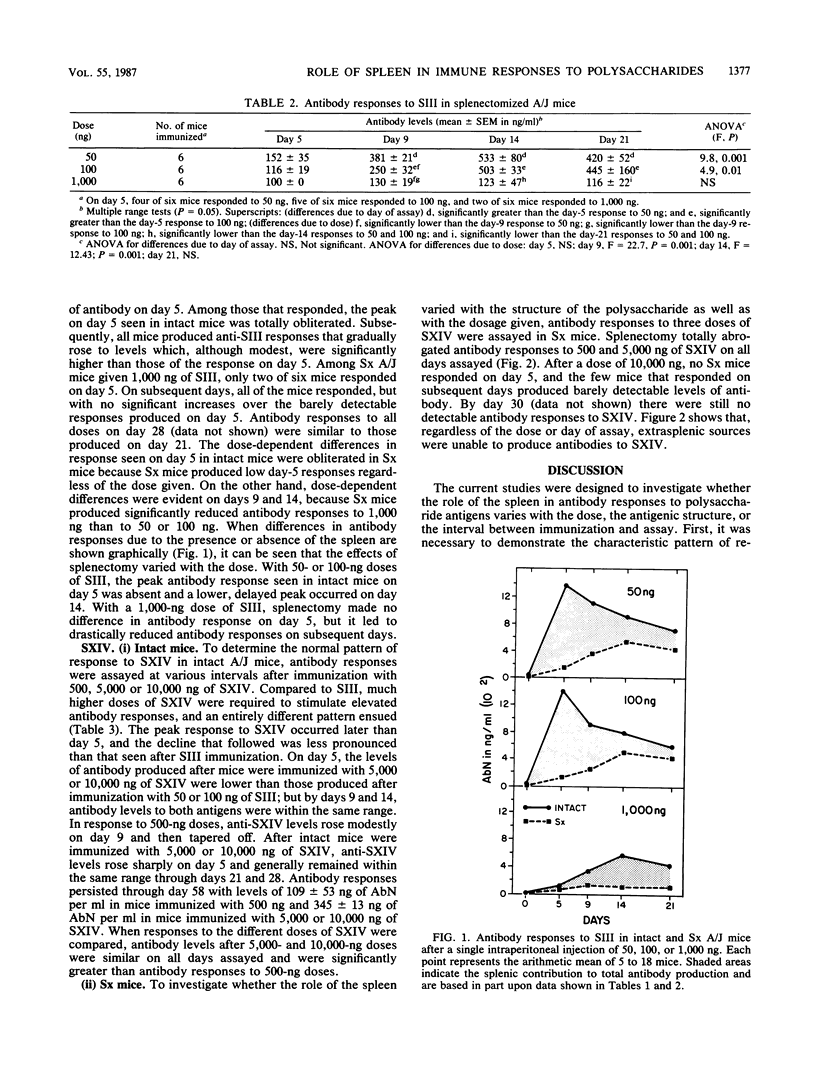
Antibody responses to two structurally different pneumococcal polysaccharides, type 3 (SIII) and type 14 (SXIV), were examined in intact and splenectomized (Sx) A/J mice to determine whether the role of the spleen in immune responses to these antigens varies with respect to the dosage, the antigenic structure, or the interval between immunization and assay. Antibody responses to SIII and SXIV, measured over a 4-week period by radioimmunoassay, differed in antigenic load requirements, kinetics, and extent of dependence upon the spleen. Intact mice given 50 or 100 ng of SIII produced peak antibody responses on day 5, which tapered off by days 14 and 21. Intact mice given SXIV required doses 100 times greater than those of SIII to stimulate high levels of antibody response; antibody responses increased on day 5 and remained elevated through day 28. In Sx mice given 50 or 100 ng of SIII, the peak antibody response on day 5 was obliterated, but extrasplenic sources produced low levels of antibody which peaked by day 14. In Sx mice given SXIV, all anti-SXIV responses were abrogated regardless of the dose or day of assay. Differences between the anti-SIII and anti-SXIV responses in dependence upon the spleen were probably due to structural differences between the two antigens and to the localization of each to different sites in the reticuloendothelial system. These results attest to the importance of the spleen in antibacterial resistance. They show that, even in the presence of extrasplenic antibody synthesis, the spleen is required for early antibody production, the timing of which is critical for the effective clearance of bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosino D. M., Schiffman G., Gotschlich E. C., Schur P. H., Rosenberg G. A., DeLange G. G., van Loghem E., Siber G. R. Correlation between G2m(n) immunoglobulin allotype and human antibody response and susceptibility to polysaccharide encapsulated bacteria. J Clin Invest. 1985 Jun;75(6):1935–1942. doi: 10.1172/JCI111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlot P. L., Grennan D., Humphrey J. H. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985 May;15(5):508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbaugh D. F., Prescott B., Baker P. J. Effect of splenectomy on the expression of regulatory T cell activity. J Immunol. 1978 Oct;121(4):1483–1485. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Rudbach J. A., Prescott B., Caldes G., Evans C., Stashak P. W. Influence of multiple genes on the magnitude of the antibody response to bacterial polysaccharide antigens. Infect Immun. 1984 Jul;45(1):56–61. doi: 10.1128/iai.45.1.56-61.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Gray D., Platteau B., MacLennan I. C. Distinct delta + and delta - B-lymphocyte lineages in the rat. Ann N Y Acad Sci. 1982;399:157–174. doi: 10.1111/j.1749-6632.1982.tb25671.x. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Brown E. J. The role of the spleen in resistance to infection. Annu Rev Med. 1986;37:49–59. doi: 10.1146/annurev.me.37.020186.000405. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Requirements for activation of contrasuppressor T cells by type III pneumococcal polysaccharide. J Immunol. 1986 Jan;136(2):396–401. [PubMed] [Google Scholar]
- Church J. A., Mahour G. H., Lipsey A. I. Antibody responses after splenectomy and splenic autoimplantation in rats. J Surg Res. 1981 Oct;31(4):343–346. doi: 10.1016/0022-4804(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Cohn D. A. Sensitivity to androgen. A possible factor in sex differences in the immune response. Clin Exp Immunol. 1979 Nov;38(2):218–227. [PMC free article] [PubMed] [Google Scholar]
- Corazza G. R., Tarozzi C., Vaira D., Frisoni M., Gasbarrini G. Return of splenic function after splenectomy: how much tissue is needed? Br Med J (Clin Res Ed) 1984 Oct 6;289(6449):861–864. doi: 10.1136/bmj.289.6449.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes L. G., Malangoni M. A., Spiegel C. A., Schiffman G. Response to immunization after partial and total splenectomy. J Surg Res. 1985 Jul;39(1):53–58. doi: 10.1016/0022-4804(85)90161-1. [DOI] [PubMed] [Google Scholar]
- Di Padova F., Dürig M., Wadström J., Harder F. Role of spleen in immune response to polyvalent pneumococcal vaccine. Br Med J (Clin Res Ed) 1983 Dec 17;287(6408):1829–1832. doi: 10.1136/bmj.287.6408.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M. Phenotypic expression of B lymphocytes. III. Marginal zone B cells in the spleen are characterized by the expression of Tac and alkaline phosphatase. J Immunol. 1985 Jul;135(1):123–130. [PubMed] [Google Scholar]
- Humphrey J. H., Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981 Mar;11(3):221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981 Mar;11(3):212–220. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide (SSS-III). I. Use of 125I-labeled SSS-III to study serum antibody levels, as well as the distribution and excretion of antigen after immunization. J Immunol. 1976 Jan;116(1):41–51. [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide. II. Factors influencing the serum antibody levels after immunization with an optimally immunogenic dose of antigen. J Immunol. 1976 Jan;116(1):52–64. [PubMed] [Google Scholar]
- Markham R. B., Nicholson-Weller A., Schiffman G., Kasper D. L. The presence of sialic acid on two related bacterial polysaccharides determines the site of the primary immune response and the effect of complement depletion on the response in mice. J Immunol. 1982 Jun;128(6):2731–2733. [PubMed] [Google Scholar]
- Mäkelä O., Pasanen V. J., Sarvas H., Lehtonen M. A gene of the immunoglobulin H-chain cluster controls the murine antibody response to pneumococcal polysaccharide type 14. Scand J Immunol. 1980;12(2):155–158. doi: 10.1111/j.1365-3083.1980.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J. L., Ellegaard J., Marqversen J., Hansen H. H. Detection of splenosis and ectopic spleens with 99mTc-labelled heat damaged autologous erythrocytes in 90 splenectomized patients. Scand J Haematol. 1981 Jul;27(1):51–56. doi: 10.1111/j.1600-0609.1981.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Patel J., Williams J. S., Naim J. O., Hinshaw J. R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Surgery. 1982 Jun;91(6):638–641. [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Ochs H. D., Schiffman G., Hammerschlag M. R., Miser J., Vichinsky E., Wedgwood R. J. Immune response after splenectomy. Lancet. 1978 Jan 28;1(8057):178–181. doi: 10.1016/s0140-6736(78)90612-8. [DOI] [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Chiang J., Leiserson W. M., Caldes G., Prescott B., Baker P. J. Characteristics of amplifier T cells involved in the antibody response to the capsular polysaccharide of type III Streptococcus pneumoniae. J Immunol. 1984 Jun;132(6):3103–3108. [PubMed] [Google Scholar]
- Velcek F. T., Kugaczewski J. T., Jongco B., Shaftan G. W., Rao P. S., Schiffman G., Kottmeier P. K. Function of the replanted spleen in dogs. J Trauma. 1982 Jun;22(6):502–506. doi: 10.1097/00005373-198206000-00011. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]


