Abstract
Autoantibodies against double-stranded DNA (dsDNA) and nucleosomes represent a hallmark of systemic lupus erythematosus (SLE). However, the mechanisms involved in breaking the immunological tolerance against these poorly immunogenic nuclear components are not fully understood. Impaired phagocytosis of apoptotic cells with consecutive release of nuclear antigens may contribute to the immune pathogenesis. The architectural chromosomal protein and proinflammatory mediator high mobility group box protein 1 (HMGB1) is tightly attached to the chromatin of apoptotic cells. We demonstrate that HMGB1 remains bound to nucleosomes released from late apoptotic cells in vitro. HMGB1–nucleosome complexes were also detected in plasma from SLE patients. HMGB1-containing nucleosomes from apoptotic cells induced secretion of interleukin (IL) 1β, IL-6, IL-10, and tumor necrosis factor (TNF) α and expression of costimulatory molecules in macrophages and dendritic cells (DC), respectively. Neither HMGB1-free nucleosomes from viable cells nor nucleosomes from apoptotic cells lacking HMGB1 induced cytokine production or DC activation. HMGB1-containing nucleosomes from apoptotic cells induced anti-dsDNA and antihistone IgG responses in a Toll-like receptor (TLR) 2–dependent manner, whereas nucleosomes from living cells did not. In conclusion, HMGB1–nucleosome complexes activate antigen presenting cells and, thereby, may crucially contribute to the pathogenesis of SLE via breaking the immunological tolerance against nucleosomes/dsDNA.
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease of largely unknown etiology. Genetic and environmental factors such as smoking, UV exposure, and infections increase the risk of disease manifestation. Virtually every organ in the human body can be involved, especially skin, joints, hematopoietic system, kidneys, central nervous system, and heart, thereby causing an ample variety of symptoms.
Autoantibodies against double-stranded DNA (dsDNA) and nucleosomes represent a serological hallmark of SLE (1). These autoantibodies can form immune complexes and immune deposits within kidneys and blood vessels and, hence, crucially contribute to the pathogenesis of lupus nephritis and vasculitis (2–4). However, the mechanisms that cause breakage of the immunological tolerance against native chromosomal DNA and nucleosomes, which by themselves are poorly immunogenic, are not understood.
High mobility group box protein 1 (HMGB1) is an evolutionary conserved ubiquitously expressed chromosomal protein consisting of two positively charged DNA binding domains, called HMG box A and B, and a negatively charged C-terminal domain (5). HMGB1 binds to dsDNA, single-stranded DNA (ssDNA), distorted DNA, and nucleosomes. HMGB1 stabilizes the structure of nucleosomes and induces DNA bending, thereby participating in transcriptional regulation (6). Recently, it has been described that HMGB1 can also act as a proinflammatory mediator when released from cells. Binding of HMGB1 to the receptor for advanced glycation end products (RAGE) and Toll-like receptor (TLR) 2 and TLR4 leads to the recruitment of inflammatory cells and the release of proinflammatory cytokines including TNF-α, IL-1β, and IL-6 (7–11). In addition, HMGB1 induces up-regulation of activation markers (HLA-DR, CD83, CD80, and CD86) on DC (12, 13).
Extracellular HMGB1 may contribute to the pathogenesis of several diseases. Wang et al. (9) showed that HMGB1 is a crucial mediator of late lethality from septic shock. Extracellular HMGB1 has been reported in experimental arthritis models as well as in human rheumatoid arthritis (14–16). Importantly, systemic application of either the antagonistic box A or HMGB1-neutralizing antibodies ameliorated collagen-induced arthritis in rodents (14, 15).
During apoptosis, a substantial amount of nuclear HMGB1 gets tightly attached to hypoacetylated chromatin, thereby preventing HMGB1 release (17). We hypothesized that in case of clearance deficiency, as found in 30–50% of patients with SLE, uningested apoptotic cells may undergo secondary necrosis (18, 19). During secondary necrosis, the majority of HMGB1 remains bound to nucleosomes in the insoluble nuclear remnants (17); however, a fraction of the nucleosomes themselves are released in soluble form and may carry along the tightly bound proinflammatory HMGB1.
It was previously shown that extracellular HMGB1 is present within lesional skin in patients with chronic cutaneous lupus (20, 21). Moreover, we recently detected HMGB1 in serum and plasma of patients with SLE (22). In this paper, we demonstrate that HMGB1–nucleosome complexes are released from secondary necrotic cells and can be found in the blood of patients with SLE. HMGB1–nucleosome complexes purified from apoptotic cells induced cytokine expression in macrophages and maturation of DC. In contrast, neither nucleosomes from viable cells nor nucleosomes from apoptotic cells devoid of HMGB1 and with a low level of HMGB2 (HMGBlow) induced any marked cytokine release. Importantly, immunization with nucleosomes from apoptotic, but not with those from viable cells, resulted in significantly increased anti-dsDNA and antihistone antibody titers in nonautoimmune mice. Hence, nucleosomes with tightly bound HMGB1 may play a crucial role in breaking the immunological tolerance against dsDNA, which represents a key autoantigen in SLE.
RESULTS
Nucleosomes released from late apoptotic cells contain HMGB1
During apoptotic cell death, oligo- and mononucleosomes are generated by internucleosomal cleavage of chromatin by endonucleases (23, 24). In vivo, the appearance of nucleosomes in the circulation is normally prevented by the effective elimination of apoptotic cells by phagocytes (25–27). In vitro, nucleosomes are released into culture supernatants when the dying cells enter late stages of apoptosis, i.e., secondary necrosis (28). To investigate whether nucleosomes spontaneously released from secondary necrotic cells contain HMGB1, we induced apoptosis in Jurkat cells either by UV irradiation (unpublished data) or staurosporine treatment for 48 h, when virtually all cells had undergone secondary necrosis. Primary necrosis was induced by heat treatment. Using different antihistone and anti-dsDNA antibodies, HMGB1 was precipitated from supernatants of secondary necrotic cells but not from those of primary necrotic cells (Fig. 1). From supernatants of primary heat-induced necrotic cells, HMGB1 was precipitated only by anti-HMGB1 antibodies, indicating that HMGB1 released from primary necrotic cells is not associated with nucleosomes. Early apoptotic cells did not release detectable amounts of HMGB1 (unpublished data). Hence, nucleosomes released from cultured cells undergoing secondary necrosis are, at least partially, associated with HMGB1.
Figure 1.
HMGB1 is associated with nucleosomes spontaneously released during secondary necrosis. For induction of secondary necrosis (SN), Jurkat cells were treated with 2 μM staurosporine for 48 h. Primary necrosis was induced by heating cells at 56°C for 30 min followed by incubation for an additional hour at 37°C. Supernatants from apoptotic (top) and necrotic (bottom) Jurkat cells were collected and subjected to immune precipitations with anti-HMGB1, anti-dsDNA, antihistone H3, antihistone H2B, and antihistones H2A/H4 as well as with appropriate isotype control antibodies. Immune-precipitated material was then fractionated by SDS-PAGE on 12% polyacrylamide gel. Western blot analysis was performed using polyclonal anti-HMGB1 antibodies. WB, Western blotting; IP, immunoprecipitation. These experiments have been performed four times with virtually identical results.
Circulating nucleosomes in the blood of patients with SLE contain HMGB1
We and others have previously described that HMGB1 is frequently detectable in plasma and serum of patients with SLE (22, 29). Within the peripheral blood of some patients with SLE, high amounts of circulating immune complexes, which often contain nucleosomes, can be detected (30–32). To test if these circulating immune complexes contain HMGB1, immune complexes were precipitated from sera of patients with SLE and other diseases, including rheumatoid arthritis, allergies, and infections, as well as from healthy controls, and analyzed by Western blotting. In six of eight SLE sera, HMGB1 was readily detectable. In contrast, in precipitates of 4 healthy donors and 10 non-SLE patients, no or very little HMGB1 could be detected (Fig. 2 A and not depicted).
Figure 2.
Nucleosomes circulating in the blood of patients with SLE are complexed with HMGB1. (A) Circulating immune complexes in the blood of patients with SLE contain HMGB1. Immune complexes from serum samples of patients with SLE and rheumatoid arthritis (RA) as well as from healthy persons (NHD, normal healthy donors) were precipitated by 1.5 or 2% of polyethylene glycol (PEG). The precipitates were dissolved in SDS sample buffer and analyzed by Western blotting using polyclonal anti-HMGB1 antibodies. (B) Antihistone and anti-dsDNA antibodies coimmunoprecipitate HMGB1 from sera of patients with SLE. Serum samples obtained from patients with SLE and normal healthy donors were subjected to coimmunoprecipitation using antihistone and anti-dsDNA antibodies covalently linked to protein G Sepharose. Sepharose beads alone or linked to isotype control antibodies served as negative controls. The precipitates were separated by reducing SDS-PAGE on a 12.5% polyacrylamide gel followed by Western blot analysis using polyclonal anti-HMGB1 antibodies.
To explore whether HMGB1 is associated with circulating DNA/nucleosomes in the blood of SLE patients, we performed coimmunoprecipitations using antibodies covalently coupled to Sepharose beads. With antihistone and anti-dsDNA/nucleosome antibodies, we could coprecipitate HMGB1 from HMGB1-containing sera of SLE patients but not from sera of healthy donors (Fig. 2 B). Results similar to those shown in Fig. 2 B were obtained with additional sera from SLE patients (n = 6) and healthy controls (n = 5). These data show that nucleosomes circulating in the blood of SLE patients are associated with HMGB1.
Apoptotic cell-derived nucleosomes induce a proinflammatory response
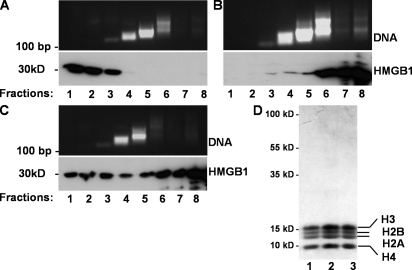
To investigate whether HMGB1-containing nucleosomes derived from apoptotic cells exhibit proinflammatory activity, we isolated nucleosomes by sucrose gradient ultracentrifugation from viable, from staurosporine-treated apoptotic, and from heat-induced necrotic Jurkat cells. Mononucleosomes were predominantly found in fraction 4 of all preparations as demonstrated by detection of mononucleosomal DNA and histones (Fig. 3). In the case of viable cells, HMGB1 was found almost exclusively at the top of the gradient in the fractions that did not contain nucleosomes/DNA (Fig. 3 A). In preparations from necrotic cells, only minute amounts of HMGB1 were detected in the mononucleosomes containing fractions 4 and 5 (Fig. 3 B). In cells necrotized by other means, such as freeze thaw, most HMGB1 was found at the top of the gradient, and again only minute amounts of HMGB1 were detected in association with mononucleosomes or in the high molecular mass fractions 6–8 (unpublished data). Only preparations from apoptotic cells contained substantial amounts of HMGB1 in nucleosomal fractions 4 and 5 (Fig. 3 C). In addition, relatively large quantities of HMGB1 were found at the top of the density gradient from apoptotic cells, indicating free HMGB1. Fraction 4, mainly containing mononucleosomes, was analyzed by SDS-PAGE and Coomassie blue staining of gels to proof the predominant composition of histones and the purity of the preparations (Fig. 3 D). Collectively, these data demonstrate that HMGB1 is primarily attached to nucleosomes from apoptotic cells.
Figure 3.
HMGB1 cofractionates with nucleosomes from apoptotic cells. For induction of apoptosis, Jurkat cells were treated with 1 μM staurosporine for 8 h. Necrosis was induced by heat treatment at 56°C for 30 min. Nuclei isolated from viable (A), necrotic (B), and apoptotic (C) Jurkat cells were digested with micrococcal nuclease, lysed, and fractionated by sucrose gradient ultracentrifugation. Eight fractions were collected in each gradient from top (1) to bottom (8), and individual fractions were analyzed by 1.5% agarose gel electrophoresis in the presence of 1% SDS. HMGB1 was detected by Western blotting. (D) Characterization of mononucleosomes. 10 μg of total protein of mononucleosomal fraction 4 purified from necrotic (lane 1), viable (lane 2), and apoptotic (lane 3) Jurkat cells was subjected to a 15% SDS-PAGE. The proteins were visualized by Coomassie blue staining.
To compare the capacity for macrophage activation, we exposed human monocyte-derived macrophages to 20 μg/ml of nucleosomes from viable, necrotic, and apoptotic cells. After 24 h, concentrations of TNF-α, IFN-α, IFN-γ, IL-1β, IL-6, IL-8, IL-10, and IL-18 were determined in culture supernatants (Fig. 4). Apoptotic nucleosomes, which contained substantial amounts of HMGB1, potently stimulated the secretion of TNF-α, IL-1β, IL-6, and IL-10, whereas nucleosomes from viable cells, which did not contain HMGB1, failed to induce cytokine release. Nucleosomes from necrotic cells containing less HMGB1 than those from apoptotic cells induced measurable cytokine secretion but less than apoptotic nucleosomes (Fig. 4). We could not detect IFN-α, IFN-γ, IL-8, and IL-18 secretion by human macrophages after exposure to nucleosomes (unpublished data). Similar results were obtained using mouse peritoneal macrophages (unpublished data). Nucleosomes isolated from cells treated with other inducers of apoptosis, such as anti-Fas antibodies or UV irradiation, elicited comparable cytokine release to nucleosomes from staurosporine-treated cells (unpublished data). Furthermore, there was no significant difference in cytokine induction by nucleosomes prepared from early or late apoptotic cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081165/DC1). Importantly, nucleosomes spontaneously released from late apoptotic cells also induced cytokine release from human monocyte-derived macrophages (Fig. S2). These data indicate that the cytokine-inducing capacity of mononucleosomes correlates with their HMGB1 content.
Figure 4.
HMGB1-containing nucleosomes from apoptotic cells induce secretion of cytokines by human monocyte-derived macrophages. Human macrophages were cultured in the absence or presence of 20 μg/ml of nucleosomes purified from viable (NC V), apoptotic (NC A), and necrotic cells (NC N) or diluent (PBS). Untreated human macrophages served as controls. Cell culture supernatants were harvested after 24 h and cytokine concentrations were determined. One representative of three independent experiments is shown. Mean values and SD were calculated from triplicates. Student's t test was used for statistical analysis. **, P ≤ 0.01; *, P ≤ 0.05.
HMGB proteins are required for the proinflammatory activity of apoptotic nucleosomes
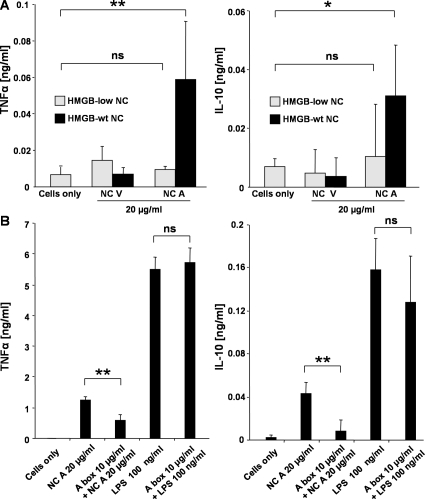
To further test whether HMGB1 contributes to the proinflammatory activity of apoptotic nucleosomes, we isolated nucleosomes from Hmgb1−/− (containing HMGB2) mouse embryonic fibroblasts (MEF) (33) and HMGBlow (deficient for HMGB1 and expressing only trace amounts of HMGB2) MEF from their WT counterparts and compared their cytokine-inducing capacity. Primary human monocyte-derived macrophages were incubated with nucleosomes purified from viable and apoptotic MEF, and the secretion of TNF-α and IL-10 was assessed. Nucleosomes purified from apoptotic Hmgb1−/− MEF induced only low amounts of TNF-α and IL-10 (unpublished data) and those prepared from HMGBlow fibroblasts induced virtually no cytokines, which is comparable to nucleosomes from viable cells. In contrast, nucleosomes purified from dying WT fibroblasts stimulated TNF-α and IL-10 secretion (Fig. 5 A). Therefore, HMGB1 and, presumably, HMGB2 are required for cytokine secretion induced by nucleosomes derived from apoptotic cells.
Figure 5.
HMGB1 contributes to the proinflammatory activity of apoptotic cell–derived nucleosomes. (A) Nucleosomes isolated from apoptotic WT, but not from HMGBlow MEF, stimulated human monocyte-derived macrophages to release TNF-α and IL-10. Macrophages were incubated with 20 μg/ml of nucleosomes purified from viable (NC V) and apoptotic (NC A) WT or HMGBlow MEF. Untreated macrophages served as control. After 24 h, concentrations of TNF-α and IL-10 were measured by ELISA. (B) Apoptotic nucleosome-induced cytokine release was suppressed by the antagonistic A box domain of HMGB1. Macrophages were stimulated either with 20 μg/ml of nucleosomes purified from apoptotic cells or 100 ng/ml LPS in the absence or presence of 10 μg/ml of the recombinant A box fragment. After incubation for 24 h, cell culture supernatants were harvested and concentrations of the cytokines TNF-α and IL-10 were measured by ELISA. Untreated macrophages served as a control. One representative example of three experiments is shown. Mean values and SD were calculated from triplicates. Student's t test was used for statistical analysis. **, P ≤ 0.01; *, P ≤ 0.05.
To specifically antagonize the effects of HMGB1, we used recombinant box A (the truncated N-terminal domain of HMGB1), which acts as a competitive inhibitor of the full-length HMGB1 protein (34, 35). Human monocyte-derived macrophages were incubated with nucleosomes purified from apoptotic Jurkat cells in the presence or in the absence of 10 μg/ml of recombinant A box. Treatment with the recombinant A box strongly inhibited the cytokine stimulating activity of apoptotic nucleosomes, whereas LPS-induced TNF-α and IL-10 release were not altered (Fig. 5 B).
Apoptotic nucleosome-induced cytokine release is dependent on TLR2 but not on TLR4, TLR9, and RAGE
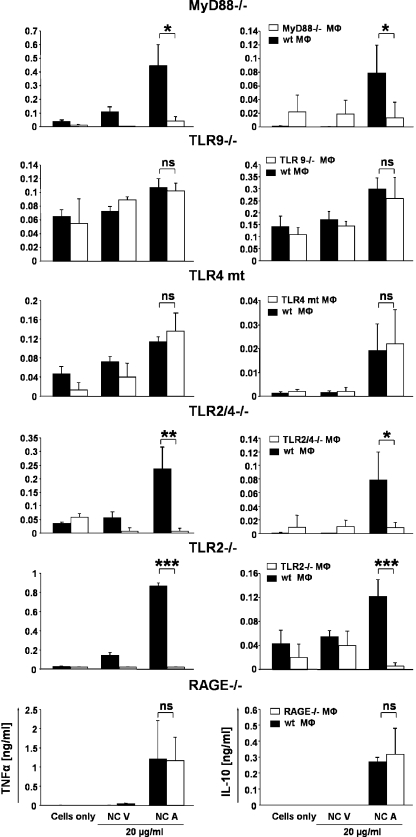
Previous studies indicated that HMGB1 is an endogenous ligand of RAGE, TLR2, and TLR4, whereas CpG oligonucleotide signaling depends on TLR9. The adaptor protein MyD88 (myeloid differentiation primary response protein 88) is critical for signal transduction leading to proinflammatory cytokine gene induction upon activation of all these TLRs (36, 37). To evaluate the relevance of RAGE and TLR signaling in mediating the proinflammatory effect of apoptotic nucleosomes, we incubated nucleosomes purified from viable and apoptotic cells with thioglycollate-elicited peritoneal macrophages obtained from WT and mice defective for RAGE (38), MyD88 (39), TLR9 (40), TLR2, TLR4 (41), or TLR2/4 signaling. The release of TNF-α and IL-10 upon exposure to apoptotic nucleosomes was completely abolished in MyD88-deficient macrophages as well as in TLR2/4- and TLR2-deficient macrophages. In contrast, cytokine secretion from RAGE-deficient, TLR4 mutant, and TLR9-deficient macrophages was not impaired (Fig. 6). Therefore, TLR2 is critical for proinflammatory signaling induced by HMGB1 containing nucleosomes, whereas RAGE, TLR4, and TLR9 are dispensable.
Figure 6.
Apoptotic nucleosome–induced cytokine release by macrophages is dependent on MyD88 and TLR2 but not on TLR4, TLR9, and RAGE. Thioglycollate-elicited peritoneal macrophages were obtained from WT, MyD88-, TLR9-, TLR2/4-, TLR2-, and RAGE-deficient or TLR4 mutant mice and incubated with 20 μg/ml of nucleosomes purified from viable (NC V) and apoptotic (NC A) Jurkat cells for 24 h. Untreated macrophages served as a control. Concentrations of TNF-α and IL-10 were measured by ELISA. Mean values and SD were calculated from triplicates. Student's t test was used for statistical analysis. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
Nucleosomes induce maturation of DC
DC, as the most potent APC, may be critically involved in the pathogenesis of SLE by activating naive autoreactive T helper lymphocytes (42, 43). Efficient T cell priming requires mature DC expressing MHC class II together with costimulatory molecules such as members of the B7 family, namely B7-1/CD80 and B7-2/CD86 (44). To determine whether nucleosomes, particularly those derived from apoptotic cells, can induce DC maturation, monocyte-derived immature DC were cultured in the absence or presence of 20 μg/ml of nucleosomes purified from viable and apoptotic Jurkat cells. After 48 h, the expression of MHC class II, CD86, and CD83 molecules on the surface of DC was assessed by flow cytometry. Only the HMGB1-containing nucleosomes from apoptotic cells significantly induced surface expression of MHC class II, the costimulatory molecule CD86, and the DC maturation marker CD83 (Fig. 7 A). In addition to maturation, apoptotic nucleosomes also induced TNF-α release from monocyte-derived DC (unpublished data).
Figure 7.
Nucleosomes induce maturation of DC. (A) Monocyte-derived DC were cultured in the absence or presence of 20 μg/ml of nucleosomes purified from viable (NC V) or apoptotic (NC A) Jurkat cells. After incubation for 48 h, expression of MHC class II, CD86, and CD83 molecules was assessed by flow cytometry. The results are expressed as mean fluorescence intensity (MFI). On the left, bar graphs show means and SD of triplicates. On the right, corresponding representative histograms are shown. Gray line, unstimulated DC; black line, DC exposed to nucleosomes from viable cells; filled gray, DC exposed to nucleosomes from apoptotic cells. (B) DC exposed to nucleosomes from apoptotic cells are more potent stimulators of allogeneic T cells than DC exposed to nucleosomes from viable cells. Immature DC were incubated for 48 h with nucleosomes purified from viable or apoptotic cells or LPS as positive control. DC were then cocultured with allogeneic T cells at a DC/T cell ratio of 1:100. T cell proliferation was assessed by measuring the amount of 3H thymidine incorporation. Mean values and SD were calculated from triplicates. Student's t test was used for statistical analysis. **, P ≤ 0.01. (C) HMGB1 contributes to the apoptotic nucleosome–induced DC activation. Human monocyte-derived DC were left untreated or incubated with 20 μg/ml of nucleosomes purified from viable or apoptotic WT or HMGBlow MEF. After 48 h, expression of CD83 and CD86 on the surface of DC was determined by flow cytometry. The results are expressed as mean fluorescence intensity. Student's t test was used for statistical analysis. **, P ≤ 0.01; *, P ≤ 0.05. Experiments were repeated three times using DC generated from different donors.
To investigate if apoptotic nucleosome-induced maturation of DC is also relevant for DC function, we cocultured monocyte-derived DC in the absence or in the presence of nucleosomes isolated from either viable or apoptotic cells. After 48 h, allogeneic T cells were added and their proliferative response was assessed. The mixed leukocyte reaction was significantly enhanced by DC preincubated with nucleosomes from apoptotic cells but not by DC preincubated with nucleosomes from viable cells (Fig. 7 B). These data indicate that the capacity of DC to activate T cells is increased by apoptotic nucleosomes.
To further test if HMGB1 is involved in DC maturation induced by nucleosomes from apoptotic cells, immature DC were exposed to nucleosomes isolated from either WT or HMGBlow MEF for 48 h. Expression of CD83 and CD86 on the surface of DC was assessed by flow cytometry. Nucleosomes isolated from apoptotic WT MEF significantly up-regulated expression of CD83 and CD86 on DC, whereas nucleosomes from HMGBlow MEF displayed only a subtle effect on the surface expression of CD83 and CD86 (Fig. 7 C). These data demonstrate that HMGB1-containing nucleosomes from apoptotic cells can induce maturation of DC, which is essential for efficient antigen presentation.
IFN-α released from plasmacytoid DC (pDC) may critically contribute to the immunopathogenesis of SLE and can be induced by CpG oligonucleotides and, even more efficiently, by HMGB1 CpG oligonucleotide complexes in a TLR9- and RAGE-dependent manner (10, 11). In our experiments, neither nucleosomes from viable nor from apoptotic cells induced expression of IFN-α or maturation markers in pDC (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081165/DC1; and not depicted). However, if pDC were incubated with nucleosomes in addition to anti-dsDNA containing SLE serum, there was a strong induction of IFN-α release (Fig. S3). These date indicate that IFN-α secretion from pDC does not depend on HMGB1 attached to nucleosomes but on immune complex formation.
Increased immunogenicity of nucleosomes from apoptotic cells
To test whether HMGB1-containing nucleosomes from apoptotic cells display increased immunogenicity in vivo, we immunized BALB/c mice with nucleosomes isolated from viable and apoptotic Jurkat cells. Adult female mice were injected three times i.v. with nucleosomes without any adjuvant. Controls were injected with PBS. The concentrations of anti-dsDNA, anti-ssDNA, and antihistone antibodies were measured by ELISA.
Mice injected with apoptotic cell–derived nucleosomes displayed a significant increase in serum IgG antibodies directed to dsDNA and histones compared with mice immunized with nucleosomes from viable cells, which showed only slight or no anti-dsDNA and antihistone responses. None of the groups displayed a relevant increase in anti-ssDNA IgG titers (Fig. 8 A). In addition, sera were tested for extractable nuclear antigens. Mice immunized with nucleosomes from viable cells showed no relevant increase in these autoantibodies, whereas two of the mice immunized with nucleosomes from apoptotic cells displayed high titers of anti-Scl70 antibodies. One of these mice produced also antibodies to Jo-1, Sm, SSA/Ro, and SSB/La. Furthermore, most mice injected with apoptotic nucleosomes showed a trend toward increased concentrations of antibodies to U1-RNP, SSA/Ro, and SSB/La. Antibodies to the lupus-specific Sm antigen were significantly increased in mice immunized with nucleosomes from apoptotic cells compared with those immunized with nucleosomes from viable cells (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081165/DC1; and not depicted). Nucleosomes from apoptotic MEF also induced anti-dsDNA antibodies in BALB/c mice (unpublished data).
Figure 8.
Increased immunogenicity of nucleosomes derived from apoptotic cells and involvement of TLR2 in the autoimmune response. (A) Groups of five BALB/c mice each were i.v. immunized three times with 50 μg of purified nucleosomes from viable and apoptotic Jurkat cells in intervals of 3 wk. A control group received PBS. Sera were collected before the first immunization and 3 wk after each immunization. Concentrations of IgG antibodies against nucleosomes, ssDNA, dsDNA, and histones were determined by ELISA. All sera were tested in the same assay. Mean values of the OD and SD are shown. Statistical analysis was performed using the nonparametric Mann-Whitney U test for unpaired samples. **, P ≤ 0.01; *, P ≤ 0.05. (B) To evaluate the requirement of TLR2 for the production of autoantibodies in vivo, groups of TLR2−/−, TLR2/4−/−, and C57BL/6 mice were i.v. injected with 75 μg of purified nucleosomes from apoptotic cells in intervals of 2 wk. 2 wk after the third immunization, anti-dsDNA and antihistone IgG antibodies were quantified in serum samples by ELISA. All sera were tested in the same assay. Bars indicate the mean values. Statistical analyses were performed using the Student's t test for unpaired samples. *, P ≤ 0.05.
To evaluate the requirement of TLR2 for the immunogenicity of apoptotic cell–derived nucleosomes in vivo, TLR2- and TLR2/4-deficient mice, as well as C57BL/6 WT mice, were injected i.v. three times with mononucleosomes prepared from apoptotic cells. TLR2- and TLR2/4-deficient mice developed very low concentrations of anti-dsDNA and antihistone antibodies, which were markedly lower than in C57BL/6 WT controls (Fig. 8 B).
These in vivo experiments demonstrate that nucleosomes derived from apoptotic cells more efficiently induce antibodies to dsDNA, histones, and various extractable nuclear antigens compared with those from viable cells. Moreover, the engagement of TLR2 is critical for the induction of anti-dsDNA and histone IgG autoantibodies in mice.
DISCUSSION
Nucleosomes appear to be a crucial primary autoantigen in SLE, and autoantibodies against dsDNA and nucleosomes are a characteristic feature of SLE (30, 45, 46). Usually, purified dsDNA and nucleosomes are poorly immunogenic (47, 48). It is still unknown why nucleosomes, which are ubiquitous and abundant self components, become immunogenic in SLE. Mono- and oligonucleosomes are formed during apoptosis by cleavage of chromatin and might appear on the cell surface and in apoptotic blebs at later stages of the apoptotic death process (23, 24, 49). Normally, apoptotic cells are removed swiftly by phagocytes before nucleosomes are released. However, if dying cells enter late stages of apoptosis, nucleosomes are released (28). We detected nucleosomes complexed with HMGB1 in the supernatants of secondary necrotic but not primary necrotic cells. Hence, in conditions with impaired clearance of apoptotic cells, as is observed in a subset of patients with SLE (18, 19), nucleosomes complexed with the endogenous adjuvant HMGB1 may get released and foster an autoimmune response.
In fact, nucleosomes are often detectable in the blood of patients with SLE and are thought to result from in vivo cell death (30, 50). Nucleosomes and DNA appeared in a dose-dependent manner in the plasma of mice treated with apoptosis-inducing agents (51). In SLE, nucleosomes predominantly exist in the form of immune complexes because of the presence of respective autoantibodies (52). We detected HMGB1 in circulating immune complexes from SLE patients but not from controls. Because of the presence of autoantibodies to HMGB1 in a substantial number of patients with SLE (22, 53), there could also be immune complexes containing only HMGB1. However, coimmunoprecipitation experiments revealed that HMGB1 that was detectable in the serum and plasma of SLE patients was, at least partially, associated with dsDNA and nucleosomes. No HMGB1-containing immune complexes were detectable in healthy individuals, although anti-HMGB1 antibodies were also present (22, 53). Most likely, in healthy subjects HMGB1 is usually not released because of a rapid clearance of dying cells; hence, no immune complexes are formed. Previous studies demonstrated that, in apoptotic cells, HMGB1 is firmly bound to hypoacetylated chromatin, even after undergoing secondary necrosis. In contrast, HMGB1 is rapidly lost from chromatin of primary necrotic cells (17). Therefore, HMGB1–nucleosome complexes in the blood of patients with SLE might be predominantly derived from secondary necrotic rather than from primary necrotic cells.
Recent findings of Bell et al. (54) demonstrated that HMGB1 release may not be an exclusive feature of primary necrosis. At least in certain cells, HMGB1 release occurs also during late apoptotic cell death, even before release of DNA (54). In this paper, we show that at least some of the HMGB1, which is released from secondary necrotic cells, is bound to nucleosomes. Nucleosomes from viable cells contained virtually no HMGB1. Only a fraction of apoptotic cell–derived nucleosomes contained HMGB1, suggesting that the formation of high-affinity complexes between nucleosomes and HMGB1 is restricted to certain subsets of nucleosomes and/or HMGB1. The amount of these complexes may depend on cell types and different apoptotic pathways.
Most importantly, apoptotic cell–derived nucleosomes displayed a potent proinflammatory effect. Nucleosomes purified from HMGB1-deficient apoptotic MEF induced very little TNF-α and IL-10 secretion. Those from HMGBlow cells, which are deficient for HMGB1 and very low in HMGB2, did not stimulate any TNF-α or IL-10 release. These data indicate a direct and critical role of HMGB1 in the proinflammatory properties of nucleosomes derived from apoptotic cells and suggest that HMGB2, which displays a similar amino acid sequence and shares many if not all of the biochemical properties with HMGB1 (5, 55), also exhibits a proinflammatory potential. Notably, TNF-α, IL-6, and IL-10, which are induced by apoptotic nucleosomes, are elevated in the serum of patients with SLE and may contribute to the pathogenesis of SLE (56, 57). However, the role of TNF-α appears to be ambivalent in SLE. On one side, IFN-α/β production, which may contribute to the immune pathogenesis of SLE, is normally down-regulated by TNF-α (58). On the other side, TNF-α is found in the serum and inflamed kidneys of SLE patients and might contribute to the inflammatory process and organ damage (59). In SLE, TNF-α may fail to regulate IFN-α production adequately (56), and a combined increase of both cytokines could promote the pathogenesis.
Most of the biological effects of HMGB1 are proposed to be mediated by the multiligand receptor RAGE as well as TLR2 and 4 (8, 10, 11, 15, 60). A very recent study demonstrated that HMGB1, in complex with CpG oligonucleotides, binds more efficiently to RAGE than HMGB1 alone. The CpG oligonucleotide-mediated TLR9-dependent cytokine release from B cells and pDC was strongly enhanced by HMGB1 and was dependent on RAGE and TLR9. However, the exact mechanism remained unclear (10).
In contrast to these data on HMGB1–CpG oligonucleotide complexes, HMGB1-containing nucleosomes from apoptotic cells induced cytokine release from macrophages independently of RAGE, TLR4, and TLR9, whereas only TLR2 signaling was essential. The immunization of TLR2-deficient mice with apoptotic nucleosomes did not result in a marked induction of anti-dsDNA or antihistone antibodies, a fact which confirmed the crucial role of TLR2 for the immune stimulatory effect of HMGB1–nucleosome complexes in vivo. Interestingly, the presence of a single loss-of-function allele of the Mal/TIRAP protein, which is essential for TLR2- and TLR4-mediated signal transduction, was found to be protective against SLE, whereas it had no influence on the occurrence of rheumatoid arthritis (61). Based on our results, HMGB1 might represent the link between TLR2 signaling and the pathogenesis of SLE. As it has been shown in MRL/lpr mice (62), the formation of autoantibodies to dsDNA and ribonucleoprotein particles may additionally require TLR9 and TLR7, respectively.
In contrast to CpG oligonucleotides and CpG–HMGB1 complexes, HMGB1–nucleosome complexes by themselves did not cause IFN-α release from pDC. However, in the presence of sera containing high concentrations of anti-dsDNA antibodies together with nucleosomes, there was a strong induction of IFN-α, which may play a crucial role in the immunopathogenesis of SLE (Fig. S3) (63). Moreover, preliminary data indicate that in mice immunized with nucleosomes from apoptotic cells, but not from viable cells, subsequent injections of nucleosomes induced IFN-α release in vivo. These data suggest that apoptotic nucleosomes may initiate autoantibody formation to dsDNA. Subsequent release of nucleosomes leads to the formation of immune complexes and IFN-α secretion, which then acts as an amplification loop of autoimmunity and inflammation (Fig. S5).
DC are important regulators of immune responses. Mature DC are especially required for the priming of T helper cells. DC mature upon recognition of pathogens (64). DC-mediated autoimmune responses can occur also in the absence of pathogens. Under such conditions, endogenous molecules acting as alarmins may activate APC and enhance the expression of costimulatory and MHC class II molecules (65). It has been previously demonstrated that recombinant HMGB1 and the HMGB1 B box (12) as well as HMGB1-containing supernatants from WT MEF, but not from their Hmgb1−/− counterparts, induced maturation of DC (13). In addition, other groups demonstrated that chromatin/nucleosome-containing immune complexes (66) and free nucleosomes purified from calf thymus directly interact with DC and can activate them via TLR9-dependent and -independent pathways (46). In this paper, we demonstrated that apoptotic cell–derived nucleosomes induced markedly stronger DC maturation as compared with those from viable cells. In addition, nucleosomes derived from apoptotic HMGBlow MEF did not induce DC maturation, again demonstrating a critical role of HMGB proteins acting as endogenous adjuvants enhancing the antigen-presenting function of DC.
Although it is believed that nucleosomes constitute one of the primary autoantigens in SLE, previous attempts to immunize nonautoimmune mice with nucleosomes purified from viable cells failed to trigger relevant autoantibody production (30). In agreement with these previous studies, we also did not observe a significant induction of anti-dsDNA antibodies after immunization with nucleosomes from viable cells. Importantly, immunization with HMGB1–nucleosome complexes from apoptotic cells, which activate macrophages and induce DC maturation in vitro, translated into significantly increased anti-dsDNA antibody generation in nonautoimmune mice. Mevorach et al. (67) immunized nonautoimmune mice with whole apoptotic cells but could not detect antibodies against dsDNA, only antibodies against ssDNA. The failure of whole apoptotic cells to induce disease relevant anti-dsDNA antibodies in nonautoimmune mice may be caused by the rapid clearance of apoptotic cells and their antiinflammatory and immunosuppressive properties (68).
In summary, HMGB1-containing apoptotic cell–derived nucleosomes may critically contribute to break peripheral tolerance against nuclear antigens in SLE via their proinflammatory actions on various cell types, especially macrophages and DC. Moreover, our findings implicate that antagonizing of HMGB1 might represent a novel treatment strategy for SLE.
MATERIALS AND METHODS
Serum samples.
For serum preparation, venous blood was collected from healthy donors and patients with SLE or other diseases into tubes without anticoagulants (Monovette) and centrifuged at 2,000 g for 30 min at 4°C. Serum was collected and stored at −20°C. The study was approved by the ethics committee of the Medical Faculty at the University of Erlangen-Nuremberg (Ethikkommission der Medizinischen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg) and informed consent of blood donors was obtained.
Cell culture.
Jurkat (TIB-152) cells were cultured as previously described (22). Hmgb1+/+ and Hmgb1−/− MEF were obtained from sib embryos from crosses of Hmgb1 heterozygotes on a pure BALB/c genetic background (>10 backcrosses) and grown according to the 3T3 protocol, which prescribes splitting the growing cells 1:3 every 3 d for 3 mo. Fibroblasts obtained at the end of this procedure can be maintained indefinitely as monolayer cultures in complete DMEM. Fibroblast lines derived in this way express a low level of HMGB2 protein and no detectable HMGB3 mRNA in addition to being KO for HMGB1. We then refer to them as HMGBlow, and the WT counterparts are referred to as HMGB+. Human monocyte-derived macrophages were differentiated as described previously (69). Human monocyte-derived DC were generated as previously described (13). Thioglycollate-elicited mouse peritoneal macrophages were obtained according Michl et al. (70). T lymphocytes were isolated by negative immunomagnetic selection from PBMC of healthy donors using the Pan T Cell Isolation kit II (Miltenyi Biotec).
Animals.
Animal experiments were approved by the government of Mittelfranken (Regierung von Mittelfranken). Mice were housed in the animal facility of the University of Erlangen-Nuremberg. BALB/c and C57BL/6 mice were purchased from Charles River Laboratories. RAGE−/− mice were provided by B. Arnold (German Cancer Research Center, Heidelberg, Germany) (38), TLR9−/− mice by T. Winkler (Department of Biology, University of Erlangen-Nürnberg, Erlangen, Germany) (40), TLR2−/−, TLR4−/− (41), and TLR2/4−/− mice by M. Schnare (Institute of Medical Microbiology and Immunology, University of Erlangen-Nürnberg, Erlangen, Germany), and MyD88−/− (39), TLR4 mutant (C3H/HeJ), and corresponding WT control (C3H/HeN) mice by A. Gessner and J. Glaesner (Institute of Medical Microbiology and Immunology, University of Erlangen-Nürnberg, Erlangen, Germany).
Induction of apoptosis and necrosis.
For induction of apoptosis, Jurkat cells (2.5 × 106 cells/ml) were treated with 1 μM staurosporine (Enzo Biochem, Inc.) for 4–8 h for preparation of nucleosomes and for 48 h for coimmunoprecipitations. For preparation of nucleosomes, HMGBlow and WT MEF were treated with 0.5 μM staurosporine for 4–8 h. Primary necrosis was induced by heating cells at 56°C for 30 min followed by incubation for an additional hour at 37°C. Apoptotic and necrotic cell death were confirmed by staining with propidium iodide/annexin V-FITC.
PEG precipitation of immune complexes.
Circulating immune complexes from serum samples were precipitated with either 1.5 or 2% PEG 6000 (Carl Roth). The precipitates were separated in a 12% SDS polyacrylamide gel, and HMGB1 was detected by Western blotting using polyclonal anti-HMGB1 antibodies (BD).
Preparation of nucleosomes.
Nucleosomes were prepared from Jurkat cells (5 × 108 cells per preparation) and HMGB WT and HMGBlow MEF (2 × 108 cells per preparation) after staurosporine treatment for 4–8 h. Nuclei were isolated according to Vaux et al. (71). Chromatin fractions containing mononucleosomes were obtained based on the method described by Suer et al. (72). In brief, isolated nuclei were digested with 500 U of micrococcal nuclease (S7; Roche) for 30 min at 37°C, lysed with 10 mM Tris and 2 mM EDTA, and the suspension was layered onto 12 ml of a 10–50% sucrose gradient. The gradients were centrifuged at 34,000 rpm for 22 h at 4°C in a SW41 Beckman rotor. Fractions of 1.5 ml were collected and analyzed for DNA content by 1.5% agarose gel electrophoresis in the presence of 0.1% SDS followed by visualization with ethidium bromide. Histones and HMGB1 were detected by 15% SDS PAGE and Coomassie staining as well as Western blotting. Before use, the nucleosome fractions were extensively dialysed against PBS. To exclude contaminations with LPS, we used sterile endotoxin-free plastic ware and reagents for nucleosome preparations. All nucleosome preparations were tested for LPS contaminations using the E-TOXATE kit (Sigma-Aldrich) and the QCL-1000 chromogenic endotoxin detection assay (Cambrex). Nucleosome preparations were used only if the Limulus (E-TOXATE) assay was negative and the endotoxin concentration was <0.2 U/ml as detected by QCL-1000.
Western blot analyses.
Equal amounts of each fraction obtained by sucrose gradient ultracentrifugation were subjected to 12% SDS-PAGE and transferred onto a PVDF membrane (Millipore). Proteins were visualized as described previously (22).
Coimmunoprecipitation.
Coimmunoprecipitations were performed using the Seize X Mammalian Immunoprecipitation kit (Thermo Fisher Scientific) according to the manufacturer's instructions with minor modifications. In brief, 7.5 μg of anti-HMGB1 (R&D Systems), anti-dsDNA (33H11; provided by T. Winkler, University of Erlangen-Nürnberg, Erlangen, Germany), antihistone pan (Roche), antihistone KM1 and KM2 (provided by J. Berden, Nijmegen Medical Center, Nijmegen, Netherlands), and antihistone H3 (Abcam) antibodies and 7.5 μg/ml of appropriate isotype control antibodies were covalently coupled to protein G–Sepharose beads and incubated either with serum samples or cell culture supernatants of apoptotic or necrotic Jurkat cells at 4°C for 4 h. Precipitated proteins were analyzed by Western blotting with polyclonal anti-HMGB1 antibodies (Millipore).
Measurement of cytokines.
Concentrations of the cytokines TNF-α, IL-10, and IL-1β in cell culture supernatants of human monocyte-derived macrophages were determined using pairs of matched monoclonal antibodies (BD). IL-6, IL-8, IL-18, IFN-γ, and IFN-α were measured by FlowCytomix bead-based assay (Bender MedSystems) according to the manufacturer's instructions.
Flow cytometric analyses.
Monocyte-derived DC (5 × 105) were incubated at 4°C for 30 min with fluorochrome-conjugated monoclonal antibodies against CD83-PE (Beckman Coulter), CD86-biotin (BD), HLA-DR (Beckman Coulter), or appropriate isotype controls in PBS containing 2% FBS. Labeled cells were washed twice and analyzed using a FACSCalibur (BD). Data were analyzed using the Cell Quest software (BD).
Mixed leukocyte reaction.
Day-7 immature monocyte-derived DC were either left untreated or incubated with 20 μg/ml of nucleosomes from viable or apoptotic Jurkat cells in RPMI 1640 medium supplemented with 10% autologous human serum. After incubation for 48 h, 1 × 103 irradiated (30 Gy) DC were plated together with 1 × 105 allogeneic T cells in a final volume of 200 μl in round-bottomed 96-well plates. Cells were cocultured for 5 d and pulsed with 1 μCi 3H thymidine per well (Amersham Biosciences) during the final 16 h of culture. Incorporation of 3H thymidine was measured on a 1205 Wallac Beta counter (GE Healthcare). Thymidine incorporations are reported as mean cpm of triplicates ± SD.
Immunization.
6-wk-old female BALB/c mice were i.v. injected three times at an interval of 3 wk with 50 μg of nucleosomes purified either from apoptotic or viable Jurkat cells. The control group received PBS. Serum samples were taken before immunization and 3 wk after each immunization.
8-wk-old TLR2−/− or TLR2/4−/− mice (41) were i.v. injected with 75 μg of nucleosomes purified from apoptotic Jurkat cells. C57BL/6 mice served as controls. All groups of mice received three injections of apoptotic nucleosomes at an interval of 2 wk.
Determination of autoantibodies.
The concentrations of IgG antibodies to dsDNA, ssDNA, and histones in the sera were determined by ELISA as previously described (73, 74).
Statistical analyses.
Statistical analyses were performed using either the two-tailed Student's t test for heteroscedastic samples or Mann-Whitney U test for unpaired samples as appropriate and indicated in the figure legends.
Online supplemental material.
Fig. S1 shows similar cytokine secretion by human macrophages stimulated with nucleosomes biochemically purified from early versus late apoptotic Jurkat cells using sucrose gradient ultracentrifugation as described in Materials and methods. Fig. S2 shows that nucleosomes, which were spontaneously released from late apoptotic/secondary necrotic cells, contain HMGB1 and induce TNF-α and IL-10 release from human macrophages. Fig. S3 demonstrates that nucleosomes isolated from either viable or apoptotic cells do not stimulate IFN-α release from human pDC. However, nucleosomes together with anti-dsDNA antibodies containing serum induced a strong IFN-α release that was comparable to CpG oligonucleotides. Fig. S4 shows the induction of anti-Sm antibodies in BALB/c mice that had been immunized three times with apoptotic nucleosomes but not in those mice that had been immunized with viable nucleosomes. Fig. S5 displays a proposed model of the role of HMGB1–nucleosome complexes in immunopathogenesis of SLE. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081165/DC1.
Supplementary Material
Acknowledgments
We thank Benjamin Frey, Damian Maseda, Eva Gueckel and Kirsten Neubert for critical reading of the manuscript and their help during preparation of the manuscript. We are grateful to Bernd Arnold (German Cancer Research Center [DKFZ] Heidelberg) for kindly providing RAGE-deficient mice, to Andre Gessner and Joachim Glaesner for C3H/HeJ and C3H/HeN mice, to Markus Schnare for TLR2- and TLR2/4-deficient mice, and to Thomas Winkler for TLR9-deficient mice and the 33H11 monoclonal antibody (all University of Erlangen-Nürnberg).
This work is supported by the Interdisciplinary Center for Clinical Research (Interdisciplinary Centers for Clinical Research [IZKF], project number N2) and the German Research Society (Collaborative Research Centers SFB 643, project B3) and the MIUR (Prin 2004 and 2005).
Potential conflict of interests: M.E. Bianchi is founder and part owner of HMGBiotech, a biotech company that provides materials and services for HMGB1 research; and M.E. Bianchi and A.A. Manfredi are named as coinventors on several patents on the use of HMGB1. The other authors have no other conflicting financial interests.
Abbreviations used: dsDNA, double-stranded DNA; HMGB1, high mobility group box protein 1; MEF, mouse embryonic fibroblast; pDC, plasmacytoid DC; PEG, polyethylene glycol; RAGE, receptor for advanced glycation end products; SLE, systemic lupus erythematosus; ssDNA, single-stranded DNA; TLR, Toll-like receptor.
References
- 1.Tan, E.M., P.H. Schur, R.I. Carr, and H.G. Kunkel. 1966. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J. Clin. Invest. 45:1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn, B.H. 1998. Antibodies to DNA. N. Engl. J. Med. 338:1359–1368. [DOI] [PubMed] [Google Scholar]
- 3.Winfield, J.B., I. Faiferman, and D. Koffler. 1977. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J. Clin. Invest. 59:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenstein, M.R., D.R. Katz, M.H. Griffiths, L. Papadaki, T.H. Winkler, J.R. Kalden, and D.A. Isenberg. 1995. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 48:705–711. [DOI] [PubMed] [Google Scholar]
- 5.Thomas, J.O., and A.A. Travers. 2001. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem. Sci. 26:167–174. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi, M.E., and A. Agresti. 2005. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15:496–506. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, U., H. Wang, K. Palmblad, A.C. Aveberger, O. Bloom, H. Erlandsson-Harris, A. Janson, R. Kokkola, M. Zhang, H. Yang, and K.J. Tracey. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park, J.S., D. Svetkauskaite, Q. He, J.Y. Kim, D. Strassheim, A. Ishizaka, and E. Abraham. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279:7370–7377. [DOI] [PubMed] [Google Scholar]
- 9.Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, A. Frazier, H. Yang, S. Ivanova, L. Borovikova, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251. [DOI] [PubMed] [Google Scholar]
- 10.Tian, J., A.M. Avalos, S.Y. Mao, B. Chen, K. Senthil, H. Wu, P. Parroche, S. Drabic, D. Golenbock, C. Sirois, et al. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8:487–496. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov, S., A.M. Dragoi, X. Wang, C. Dallacosta, J. Louten, G. Musco, G. Sitia, G.S. Yap, Y. Wan, C.A. Biron, et al. 2007. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 110:1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messmer, D., H. Yang, G. Telusma, F. Knoll, J. Li, B. Messmer, K.J. Tracey, and N. Chiorazzi. 2004. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 173:307–313. [DOI] [PubMed] [Google Scholar]
- 13.Rovere-Querini, P., A. Capobianco, P. Scaffidi, B. Valentinis, F. Catalanotti, M. Giazzon, I.E. Dumitriu, S. Muller, M. Iannacone, C. Traversari, et al. 2004. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 5:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokkola, R., E. Sundberg, A.K. Ulfgren, K. Palmblad, J. Li, H. Wang, L. Ulloa, H. Yang, X.J. Yan, R. Furie, et al. 2002. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 46:2598–2603. [DOI] [PubMed] [Google Scholar]
- 15.Kokkola, R., A. Andersson, G. Mullins, T. Ostberg, C.J. Treutiger, B. Arnold, P. Nawroth, U. Andersson, R.A. Harris, and H.E. Harris. 2005. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 61:1–9. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi, N., K. Kawahara, K. Yone, T. Hashiguchi, M. Yamakuchi, M. Goto, K. Inoue, S. Yamada, K. Ijiri, S. Matsunaga, et al. 2003. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48:971–981. [DOI] [PubMed] [Google Scholar]
- 17.Scaffidi, P., T. Misteli, and M.E. Bianchi. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 418:191–195. [DOI] [PubMed] [Google Scholar]
- 18.Baumann, I., W. Kolowos, R.E. Voll, B. Manger, U. Gaipl, W.L. Neuhuber, T. Kirchner, J.R. Kalden, and M. Herrmann. 2002. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 46:191–201. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann, M., R.E. Voll, O.M. Zoller, M. Hagenhofer, B.B. Ponner, and J.R. Kalden. 1998. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41:1241–1250. [DOI] [PubMed] [Google Scholar]
- 20.Popovic, K., M. Ek, A. Espinosa, L. Padyukov, H.E. Harris, M. Wahren-Herlenius, and F. Nyberg. 2005. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 52:3639–3645. [DOI] [PubMed] [Google Scholar]
- 21.Barkauskaite, V., M. Ek, K. Popovic, H.E. Harris, M. Wahren-Herlenius, and F. Nyberg. 2007. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 16:794–802. [DOI] [PubMed] [Google Scholar]
- 22.Urbonaviciute, V., B.G. Furnrohr, C. Weber, M. Haslbeck, S. Wilhelm, M. Herrmann, and R.E. Voll. 2007. Factors masking HMGB1 in human serum and plasma. J. Leukoc. Biol. 81:67–74. [DOI] [PubMed] [Google Scholar]
- 23.Oliveri, M., A. Daga, C. Cantoni, C. Lunardi, R. Millo, and A. Puccetti. 2001. DNase I mediates internucleosomal DNA degradation in human cells undergoing drug-induced apoptosis. Eur. J. Immunol. 31:743–751. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida, H., Y. Pommier, and T. Ueda. 2006. Endonuclease activation and chromosomal DNA fragmentation during apoptosis in leukemia cells. Int. J. Hematol. 84:367–376. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi, A.A., P. Rovere, G. Galati, S. Heltai, E. Bozzolo, L. Soldini, J. Davoust, G. Balestrieri, A. Tincani, and M.G. Sabbadini. 1998. Apoptotic cell clearance in systemic lupus erythematosus. I. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 41:205–214. [DOI] [PubMed] [Google Scholar]
- 26.Manfredi, A.A., P. Rovere, S. Heltai, G. Galati, G. Nebbia, A. Tincani, G. Balestrieri, and M.G. Sabbadini. 1998. Apoptotic cell clearance in systemic lupus erythematosus. II. Role of beta2-glycoprotein I. Arthritis Rheum. 41:215–223. [DOI] [PubMed] [Google Scholar]
- 27.Platt, N., R.P. da Silva, and S. Gordon. 1998. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 8:365–372. [DOI] [PubMed] [Google Scholar]
- 28.van Nieuwenhuijze, A.E., T. van Lopik, R.J. Smeenk, and L.A. Aarden. 2003. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann. Rheum. Dis. 62:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, W., and D.S. Pisetsky. 2008. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann. Rheum. Dis. 67:727–728. [DOI] [PubMed] [Google Scholar]
- 30.Amoura, Z., J.C. Piette, J.F. Bach, and S. Koutouzov. 1999. The key role of nucleosomes in lupus. Arthritis Rheum. 42:833–843. [DOI] [PubMed] [Google Scholar]
- 31.Mok, C.C., and C.S. Lau. 2003. Pathogenesis of systemic lupus erythematosus. J. Clin. Pathol. 56:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nezlin, R., D. Alarcon-Segovia, and Y. Shoenfeld. 1998. Immunochemical determination of DNA in immune complexes present in the circulation of patients with systemic lupus erythematosus. J. Autoimmun. 11:489–493. [DOI] [PubMed] [Google Scholar]
- 33.Calogero, S., F. Grassi, A. Aguzzi, T. Voigtlander, P. Ferrier, S. Ferrari, and M.E. Bianchi. 1999. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 22:276–280. [DOI] [PubMed] [Google Scholar]
- 34.Sitia, G., M. Iannacone, S. Muller, M.E. Bianchi, and L.G. Guidotti. 2007. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 81:100–107. [DOI] [PubMed] [Google Scholar]
- 35.Yang, H., M. Ochani, J. Li, X. Qiang, M. Tanovic, H.E. Harris, S.M. Susarla, L. Ulloa, H. Wang, R. DiRaimo, et al. 2004. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 101:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–787. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625–635. [DOI] [PubMed] [Google Scholar]
- 38.Liliensiek, B., M.A. Weigand, A. Bierhaus, W. Nicklas, M. Kasper, S. Hofer, J. Plachky, H.J. Grone, F.C. Kurschus, A.M. Schmidt, et al. 2004. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J. Clin. Invest. 113:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 40.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 41.Spiller, S., S. Dreher, G. Meng, A. Grabiec, W. Thomas, T. Hartung, K. Pfeffer, H. Hochrein, H. Brade, W. Bessler, et al. 2007. Cellular recognition of trimyristoylated peptide or enterobacterial lipopolysaccharide via both TLR2 and TLR4. J. Biol. Chem. 282:13190–13198. [DOI] [PubMed] [Google Scholar]
- 42.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 43.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 44.Jin, Y., L. Fuller, G. Ciancio, G.W. Burke III, A.G. Tzakis, C. Ricordi, J. Miller, and V. Esquenzai. 2004. Antigen presentation and immune regulatory capacity of immature and mature-enriched antigen presenting (dendritic) cells derived from human bone marrow. Hum. Immunol. 65:93–103. [DOI] [PubMed] [Google Scholar]
- 45.Decker, P. 2006. Nucleosome autoantibodies. Clin. Chim. Acta. 366:48–60. [DOI] [PubMed] [Google Scholar]
- 46.Decker, P., H. Singh-Jasuja, S. Haager, I. Kotter, and H.G. Rammensee. 2005. Nucleosome, the main autoantigen in systemic lupus erythematosus, induces direct dendritic cell activation via a MyD88-independent pathway: consequences on inflammation. J. Immunol. 174:3326–3334. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann, M., O.M. Zoller, M. Hagenhofer, R. Voll, and J.R. Kalden. 1996. What triggers anti-dsDNA antibodies? Mol. Biol. Rep. 23:265–267. [DOI] [PubMed] [Google Scholar]
- 48.Madaio, M.P., S. Hodder, R.S. Schwartz, and B.D. Stollar. 1984. Responsiveness of autoimmune and normal mice to nucleic acid antigens. J. Immunol. 132:872–876. [PubMed] [Google Scholar]
- 49.Casciola-Rosen, L.A., G. Anhalt, and A. Rosen. 1994. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 179:1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, R.C. Jr., C.C. Malone, C. Meyers, P. Decker, and S. Muller. 2001. Detection of nucleosome particles in serum and plasma from patients with systemic lupus erythematosus using monoclonal antibody 4H7. J. Rheumatol. 28:81–94. [PubMed] [Google Scholar]
- 51.Jiang, N., C.F. Reich III, M. Monestier, and D.S. Pisetsky. 2003. The expression of plasma nucleosomes in mice undergoing in vivo apoptosis. Clin. Immunol. 106:139–147. [DOI] [PubMed] [Google Scholar]
- 52.Sano, H., O. Takai, N. Harata, K. Yoshinaga, I. Kodama-Kamada, and T. Sasaki. 1989. Binding properties of human anti-DNA antibodies to cloned human DNA fragments. Scand. J. Immunol. 30:51–63. [DOI] [PubMed] [Google Scholar]
- 53.Uesugi, H., S. Ozaki, J. Sobajima, F. Osakada, H. Shirakawa, M. Yoshida, and K. Nakao. 1998. Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J. Rheumatol. 25:703–709. [PubMed] [Google Scholar]
- 54.Bell, C.W., W. Jiang, C.F. Reich III, and D.S. Pisetsky. 2006. The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 291:C1318–C1325. [DOI] [PubMed] [Google Scholar]
- 55.Bustin, M. 1999. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 19:5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grondal, G., I. Gunnarsson, J. Ronnelid, S. Rogberg, L. Klareskog, and I. Lundberg. 2000. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 18:565–570. [PubMed] [Google Scholar]
- 57.al-Janadi, M., A. al-Dalaan, S. al-Balla, M. al-Humaidi, and S. Raziuddin. 1996. Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J. Clin. Immunol. 16:198–207. [DOI] [PubMed] [Google Scholar]
- 58.Palucka, A.K., J.P. Blanck, L. Bennett, V. Pascual, and J. Banchereau. 2005. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc. Natl. Acad. Sci. USA. 102:3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aringer, M., and J.S. Smolen. 2008. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res. Ther. 10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merenmies, J., R. Pihlaskari, J. Laitinen, J. Wartiovaara, and H. Rauvala. 1991. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J. Biol. Chem. 266:16722–16729. [PubMed] [Google Scholar]
- 61.Castiblanco, J., D.C. Varela, N. Castano-Rodriguez, A. Rojas-Villarraga, M.E. Hincapie, and J.M. Anaya. 2008. TIRAP (MAL) S180L polymorphism is a common protective factor against developing tuberculosis and systemic lupus erythematosus. Infect. Genet. Evol. 8:541–544. [DOI] [PubMed] [Google Scholar]
- 62.Christensen, S.R., J. Shupe, K. Nickerson, M. Kashgarian, R.A. Flavell, and M.J. Shlomchik. 2006. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 25:417–428. [DOI] [PubMed] [Google Scholar]
- 63.Means, T.K., E. Latz, F. Hayashi, M.R. Murali, D.T. Golenbock, and A.D. Luster. 2005. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Granucci, F., M. Foti, and P. Ricciardi-Castagnoli. 2005. Dendritic cell biology. Adv. Immunol. 88:193–233. [DOI] [PubMed] [Google Scholar]
- 65.Tsan, M.F., and B. Gao. 2004. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 76:514–519. [DOI] [PubMed] [Google Scholar]
- 66.Boule, M.W., C. Broughton, F. Mackay, S. Akira, A. Marshak-Rothstein, and I.R. Rifkin. 2004. Toll-like receptor 9–dependent and –independent dendritic cell activation by chromatin–immunoglobulin G complexes. J. Exp. Med. 199:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mevorach, D., J.L. Zhou, X. Song, and K.B. Elkon. 1998. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voll, R.E., M. Herrmann, E.A. Roth, C. Stach, J.R. Kalden, and I. Girkontaite. 1997. Immunosuppressive effects of apoptotic cells. Nature. 390:350–351. [DOI] [PubMed] [Google Scholar]
- 69.Bottcher, A., U.S. Gaipl, B.G. Furnrohr, M. Herrmann, I. Girkontaite, J.R. Kalden, and R.E. Voll. 2006. Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 54:927–938. [DOI] [PubMed] [Google Scholar]
- 70.Michl, J., M.M. Pieczonka, J.C. Unkeless, and S.C. Silverstein. 1979. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J. Exp. Med. 150:607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaux, D.L., S. Wilhelm, and G. Hacker. 1997. Requirements for proteolysis during apoptosis. Mol. Cell. Biol. 17:6502–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suer, W., C. Dahnrich, W. Schlumberger, and W. Stocker. 2004. Autoantibodies in SLE but not in scleroderma react with protein-stripped nucleosomes. J. Autoimmun. 22:325–334. [DOI] [PubMed] [Google Scholar]
- 73.Wellmann, U., M. Letz, A. Schneider, K. Amann, and T.H. Winkler. 2001. An Ig mu-heavy chain transgene inhibits systemic lupus erythematosus immunopathology in autoimmune (NZB x NZW)F1 mice. Int. Immunol. 13:1461–1469. [DOI] [PubMed] [Google Scholar]
- 74.Comby, E., P. Tanaff, D. Mariotte, V. Costentin-Pignol, C. Marcelli, and J.J. Ballet. 2006. Evolution of antinuclear antibodies and clinical patterns in patients with active rheumatoid arthritis with longterm infliximab therapy. J. Rheumatol. 33:24–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.