Abstract
The risk determinants of type 1 diabetes, initiators of autoimmune response, mechanisms regulating progress toward β cell failure, and factors determining time of presentation of clinical diabetes are poorly understood. We investigated changes in the serum metabolome prospectively in children who later progressed to type 1 diabetes. Serum metabolite profiles were compared between sample series drawn from 56 children who progressed to type 1 diabetes and 73 controls who remained nondiabetic and permanently autoantibody negative. Individuals who developed diabetes had reduced serum levels of succinic acid and phosphatidylcholine (PC) at birth, reduced levels of triglycerides and antioxidant ether phospholipids throughout the follow up, and increased levels of proinflammatory lysoPCs several months before seroconversion to autoantibody positivity. The lipid changes were not attributable to HLA-associated genetic risk. The appearance of insulin and glutamic acid decarboxylase autoantibodies was preceded by diminished ketoleucine and elevated glutamic acid. The metabolic profile was partially normalized after the seroconversion. Autoimmunity may thus be a relatively late response to the early metabolic disturbances. Recognition of these preautoimmune alterations may aid in studies of disease pathogenesis and may open a time window for novel type 1 diabetes prevention strategies.
The incidence of type 1 diabetes among children and adolescents has increased markedly in the Western countries during the recent decades (1). The incidence has reached record levels in Finland, where, currently, 1/120 children develops type 1 diabetes before the age of 15 yr (2). The annual incidence, which showed a linear steady increase from 1965 to 1996 (3), was recently found to be increasing even faster than before, with the number of cases diagnosed at or before 14 yr of age expected to double in the next 15 yr (4). Although ∼70% of subjects with type 1 diabetes carry defined risk-associated genotypes at the HLA locus, only 3–7% of the carriers of such genetic risk markers develop the disease (5).
Seroconversion to islet autoantibody positivity has been the first detectable signal for the onset of autoimmunity and progression toward diabetes. Although seroconversion usually precedes the clinical disease by months to years, its occurrence may already be too late for therapeutic approaches aimed at preventing progression to overt diabetes. As long as the initiators of the autoimmune response remain unknown and the mechanisms supporting progression toward β cell failure are poorly understood, the estimation of the absolute disease risk and time of disease presentation in genetically susceptible individuals and the discovery of effective prevention remain a challenge.
In 1994, an ongoing birth cohort study (Type 1 Diabetes prediction and prevention study [DIPP]) was launched in Finland (6). After informed parental consent, HLA alleles associated with type 1 diabetes risk or protection were analyzed from cord blood, and subjects carrying risk-associated genotypes were followed over time to establish the time point of seroconversion and diabetes development. Over a period of 11.5 yr until 2006, >100,000 newborn infants have been screened for genetic risk and >8,000 at-risk children are being followed.
Metabolomics systematically studies the chemical fingerprints in cells, tissues, and biofluids in a given physiological and environmental context. New analytical methods combined with bioinformatics provide a way to measure the extended metabolome (7). The metabolic phenotype is sensitive to subtle but pathogenically relevant factors such as age, lifestyle, nutrition, and the microbe environment of the gut (8–10). Changes in the concentrations of metabolites during early development may thus reflect both genetic and environmental factors influencing later susceptibility to chronic diseases (11).
In this paper, we hypothesize that alterations in the metabolic phenotypes characterize the early pathogenesis of type 1 diabetes. We apply the metabolomics strategy to the unique preautoimmune and prediabetic sample series collected in DIPP and provide evidence that metabolic disturbances precede the autoimmunity that is characteristically observed before development of type 1 diabetes.
RESULTS
Subjects
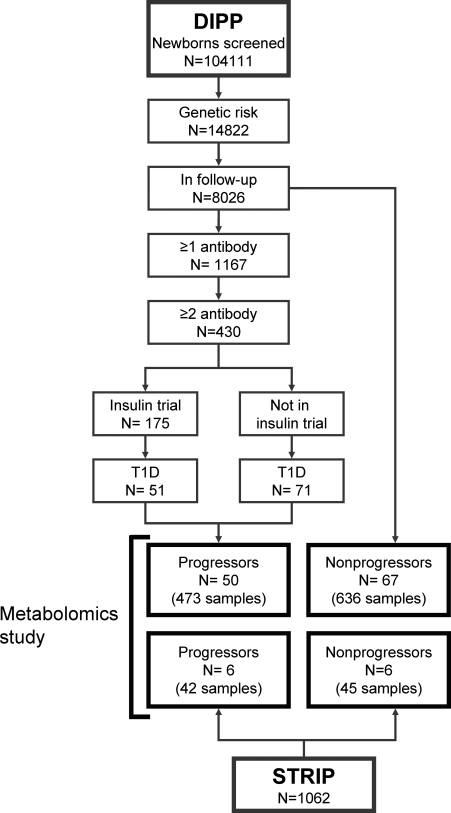
Serum metabolite profiles were compared between 50 DIPP children who progressed to type 1 diabetes (the progressors) and 67 nonprogressors, who remained healthy and autoantibody negative and were matched with the progressors for time and site of birth, gender, and HLA risk group (Fig. 1). The 117 DIPP children contributed 1,109 samples (9.5 samples per child on average) covering the time from 3 mo of age to clinical diabetes in the progressors. Additionally, the cord blood samples of 39 DIPP children from Turku, 15 of which progressed to diabetes before the age of 12 yr, were analyzed. Two progressors included in the cord blood series were also included in the longitudinal sample series.
Figure 1.
Subject selection flowchart for the metabolomics study. Nonprogressors were matched with progressors for time and site of birth, gender, and HLA risk group. Status of DIPP (6) and STRIP (12) studies as of June 6, 2006.
To compare the findings in the genetically defined DIPP cohort to those in another cohort of children that was not genetically defined, the metabolomes of another six children in the Special Turku Coronary Risk Factor Intervention Project for Children (STRIP) (12) who had developed type 1 diabetes were investigated before disease presentation and compared with their six age- and sex-matched nondiabetic controls from the same study. The STRIP series also comprised 87 samples, which had been collected annually between the ages of 7 mo and 5–15 yr. Three of the children who developed diabetes turned out to carry the defined HLA-DQB1 risk alleles, but all had had multiple diabetes-associated autoantibodies for 2–13 yr before the presentation of diabetes. Selected characteristics of the study subjects are shown in Table I.
Table I.
Selected characteristics of the subjects included in the study.
| Progressors | Nonprogressors | |
|---|---|---|
| Gender (girls, boys) | 31, 25 | 40, 33 |
| City of birth (Turku, Oulu) | 29, 27 | 45, 28 |
|
Season at time of birtha (winter, spring, summer, autumn) |
11, 19, 14, 12 | 15, 25, 19, 14 |
|
Season at time of diagnosis (winter, spring, summer, autumn) |
11, 21, 10, 13 | NA |
|
Age at time of diagnosis (median, range in months) |
44, 6–162 | NA |
|
Age at time of first seroconversion (median, range in months) |
12, 6–96 | NA |
| HLA risk haplotypes | ||
| Moderate riskb DR4-DQ8/x (x = any haplotype except DR2-DQ6, DR5-DQ7 or DR3-DQ2) |
33 | 46 |
| High risk (DR3-DQ2/DR4-DQ8) | 17 | 20 |
Seasons are defined as winter for December to February, spring for March to May, summer for June to August, and autumn for September to November.
HLA genotype information is shown for the DIPP children only. One nonprogressor, not included in the table, had the moderate risk DR3-DQ2/x haplotype.
Analytical platforms
Current lipidomics platforms constitute an efficient way to separate and quantify hundreds of lipid molecules (13, 14). For this study, ultra performance liquid chromatography coupled to mass spectrometry was applied as previously described (8, 15). The final lipidomics dataset, not including the cord blood data, comprised 53 identified lipids measured in each of the 1,196 samples. A total of 515 samples were from progressors to type 1 diabetes, among which 112 were collected before seroconversion to autoantibody positivity. In each of the 39 cord blood samples, 249 lipids were identified. Once the complete dataset was constructed, we first confirmed that the samples were not affected by the time of storage (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081800/DC1).
Serum samples were also analyzed by two-dimensional gas chromatography coupled to time of flight mass spectrometry (GCxGC-TOF/MS). This analytical technology provides a broad profile of small molecules (16). The analysis was performed on a random subset of DIPP study samples from Turku, comprising sample series from 13 progressors and 26 matched nonprogressors. The final metabolomics dataset obtained by GCxGC-TOF/MS consisted of 75 identified metabolites measured in each of the 419 samples.
Case report
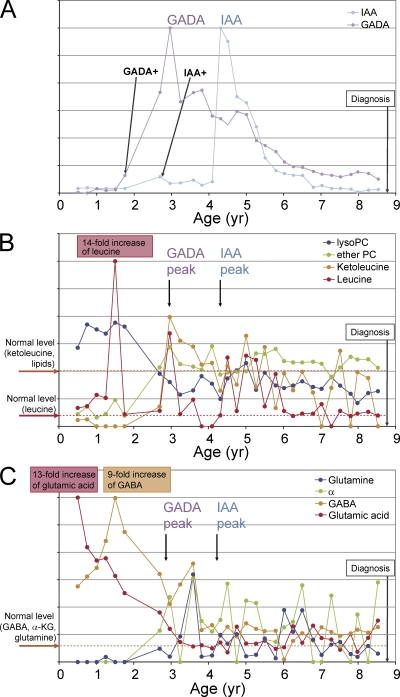
The interdependence of metabolic and immune system factors is first demonstrated in Fig. 2 for a girl with an extended sample series available. She developed overt type 1 diabetes close to 9 yr of age (Fig. 2 A). The time before the appearance of the first autoantibody was characterized by abnormally low succinic acid (not depicted), ketoleucine, and ether phosphatidylcholine (PC), as well as high lysoPC and leucine (Fig. 2 B). This period was also accompanied by abnormally high glutamic acid and γ-aminobutyric acid (GABA), as well as low glutamine and α-ketoglutarate (Fig. 2 C). During glutamic acid decarboxylase antibody (GADA) positivity, these metabolite concentrations almost normalized, whereas alanine diminished and 2-hydroxybutyric acid increased transiently (unpublished data).
Figure 2.
Selected autoantibody and metabolite changes during the prediabetic period in a girl who progressed to type 1 diabetes at close to 9 yr of age (HLA-haplotype DR7-DQ2/DR4-DQ8, moderate genetic risk). (A) GADA and IAA profiles. The relative autoantibody level values are calculated as the ratio of their measured levels and corresponding cutoff limits for autoantibody positivity. Scales are linear and adjusted separately for each autoantibody for clarity. Time of seroconversion to positivity for each autoantibody is marked, as is the time of diagnosis of type 1 diabetes. The child also seroconverted to ICA and IA-2A autoantibody positivity within 6 mo before the appearance of IAA. (B) Changes of ketoleucine, leucine, lysoPC (the profile shown is for the most abundant lysoPC species PC(18:0/0:0)), and ether PC (the profile shown is for the most abundant ether PC species PC(O-18:1/20:4)) with age. Fold changes are calculated as ratios of metabolite level at a given time point and the normal level, i.e., the mean value across all samples in the nonprogressors. Scales are linear and adjusted separately for each metabolite for clarity. (C) The changes of glutamic acid, GABA, α-ketoglutarate (α-KG), and glutamine with age.
Metabolome in prospective sample series and cord blood
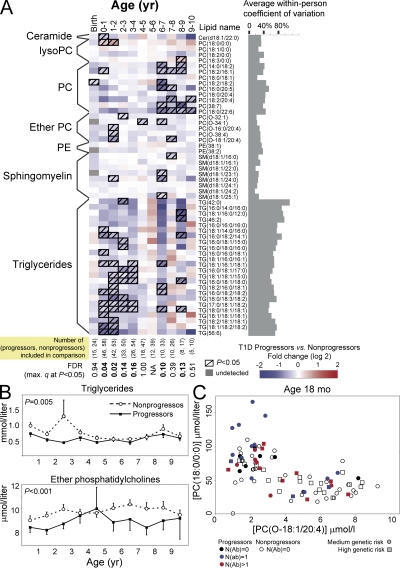
The analysis of longitudinal series of serum metabolomes during the asymptomatic period from children who later developed diabetes revealed characteristic metabolite patterns that preceded or accompanied the initial seroconversion to autoantibody positivity (Fig. 3). Compared with children who remained autoantibody negative, the serum lipidome of progressors showed a consistent decrease in triglycerides (P = 0.005) and multiple phospholipids, including ether PCs (P < 0.001), PCs (P = 0.04), and sphingomyelins (P = 0.09), in samples obtained from infancy to early school years (Fig. 3, A and B). The difference in lipidome was observed before the autoantibodies appeared and it persisted throughout the entire age range covered. Low phospholipid levels were also observed in cord blood, although only one of the lipids detected in the longitudinal sample series differed significantly between the progressors and nonprogressors. However, the total amount of cord serum PC was 1.22-fold decreased in the progressors (P = 0.004), as shown in Fig. S2 (available at http://www.jem.org/cgi/content/full/jem.20081800/DC1).
Figure 3.
Serum lipidome in cord blood and prospective sample series. (A) Differences in serum lipidome between the progressors to type 1 diabetes and nonprogressors who remained autoantibody negative throughout the follow up. The age groups are divided into birth (cord blood) and then into groups covering 1-yr cohorts. Only one sample per subject, closest to the mean age within the time window, is used in each comparison. The number of subjects included in each age cohort is shown at the bottom. The last sample of each progressor was the sample drawn last before diagnosis of diabetes. (B) Total triglyceride and ether PC concentrations for the age cohorts shown in A calculated as the sum of lipid concentrations within each class. Both lipid classes were found consistently down-regulated in the progressors, as tested by the linear mixed effects model for the overall trend throughout the follow up. The error bars show SEM. (C) Levels of the lysoPC PC(18:0/0:0) and the ether PC PC(O-18:1/20:4) at the age of 18 mo for DIPP children. The two lipids were up-regulated (P = 0.0009) and down-regulated (P = 0.04) in progressors, respectively. Only one measurement, closest to the age of 18 mo within the 12–24-mo age interval is shown for each subject. Subjects who already seroconverted to autoantibody positivity against one or multiple islet autoantigens are specifically marked with different colors, whereas the subjects in moderate and high HLA-conferred risk groups, as defined in Table I, are marked with circles and squares, respectively.
We also compared the lipid concentration differences between the children at high and moderate HLA-associated genetic risk of developing type 1 diabetes. No significant differences between the genetic risk groups were identified among the progressors or the nonprogressors (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081800/DC1), thus confirming that the observed lipid changes are not attributable to HLA-associated genetic risk.
One subclass of phospholipids that was repeatedly low in progressors and showed low within-person variability were ether PCs (Fig. 3, A and B). Ethanolamine plasmalogen levels were also low in progressors (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081800/DC1). Concentration of the most abundant lysoPC, PC(18:0/0:0), was elevated in the progressors during the first 2 yr of life (Fig. 3 A). During this period, concentrations of PC(18:0/0:0) and PC(O-18:1/20:4), together with most other ether phospholipids, were inversely related (Fig. 3 C).
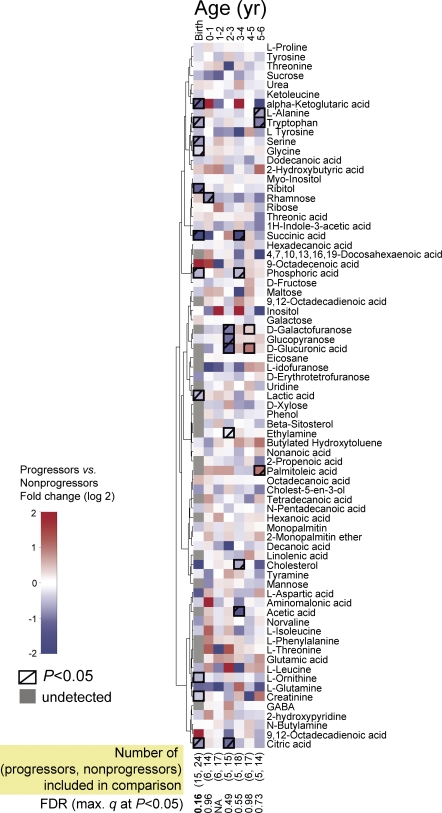
Although no clear persistent changes were observed in small molecule profiles using GCxGC-TOF/MS (Fig. 4), succinic acid, a key metabolite of citric acid cycle, was diminished fourfold in progressors at birth and during the first year of life (P = 0.04), whereas the citric acid was 1.7-fold decreased at birth (P = 0.02).
Figure 4.
Comparison of serum metabolomes between progressors to type 1 diabetes and nonprogressors who remained autoantibody negative throughout the follow up. The age groups are divided into birth (cord blood) and then into groups covering 1-yr periods of followup. Only one sample per subject, closest to the mean age within the time window (0.5, 1.5, 2.5 yr, etc.), is used in each comparison. Clustering was performed for the profiles across all available samples (n = 419) using Ward linkage and the nonparametric Spearman rank correlation–based distance metric. The number of subjects included in each age cohort is shown at the bottom.
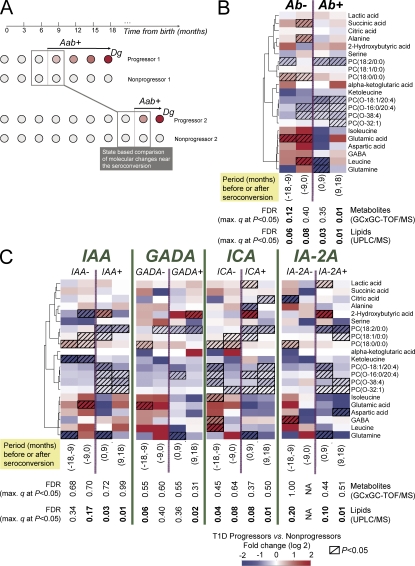
Metabolic changes before emergence of islet autoantibodies and diagnosis of type 1 diabetes
To determine whether the metabolome abnormalities showed association with the emergence of islet autoantibodies, the metabolomes of the progressors and of the nonprogressors were compared using samples obtained from ±18 mo from the emergence of the first autoantibody (Fig. 5 A). The lysoPC PC(18:0/0:0) was increased 1.5-fold within 9–18 mo before seroconversion (P = 0.03) and 1.3-fold within the succeeding 9 mo (P = 0.005; Fig. 5 B).
Figure 5.
Selected metabolite differences between progressors and nonprogressors within 18-mo periods before and after seroconversion to islet autoantibody positivity, divided into four 9-mo periods. (A) Illustrative matching of progressors and nonprogressors for studies of seroconversion-related changes in metabolome. For each progressor's samples near the selected period of seroconversion, the age-matched control samples were selected from the matched nonprogressor. (B) Changes before (Ab−) and after (Ab+) first seroconversion to islet autoantibody positivity. Only one sample from each subject, closest to the time of seroconversion, is included within each time period. Clustering was performed as described in Fig. 4. (C) Changes before and after seroconversion to positivity for each autoantibody. Only one sample from each subject, closest to the time of seroconversion, is included within each time period. Lipids were measured in 56 progressors and 73 nonprogressors, whereas the metabolites were measured in 13 progressors and 26 nonprogressors as shown in Table S1.
Metabolomes in the progressors and nonprogressors showed several unexpected differences around the time when autoantibodies appeared. Glutamic acid was 5.2-fold and 32-fold increased 9–18 mo (P = 0.02) and 0–9 mo before seroconversion (P = 0.02), respectively. Branched chain amino acids (BCAAs) such as leucine and isoleucine were also increased before seroconversion, whereas ketoleucine concentration was diminished.
We also studied whether serum metabolome profiles at the presentation of clinical diabetes resembled those seen before the first autoantibody species emerged. The profiles in the progressors during the last visits before diagnosis of type 1 diabetes revealed no clear differences as compared with the profiles of matched nonprogressors (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20081800/DC1), except for specific phospholipids which were similarly diminished as they had been at an early age and around the time of seroconversion to autoantibody positivity. When we tested whether the concentrations of the key lipids found altered in progressors before autoimmunity correlated with the age at diagnosis or the time interval between the first seroconversion to islet autoimmunity and diagnosis, no significant trends were found (Figs. S6 and S7).
Associations of metabolome with the appearance of specific autoantibodies
The appearance of each autoantibody (insulin autoantibody [IAA], GADA, IA-2A, or islet cell autoantibody [ICA]) was preceded by a relative increase in the lysoPC PC(18:0/0:0) and followed by a transient two to fourfold elevation of 2-hydroxybutyric acid (Fig. 5 C). Within 9–18 mo and 0–9 mo before the appearance of IAA, ketoleucine was 20-fold (P = 0.009) and 2.5-fold (P = 0.02) lower in the progressors than in the nonprogressors, respectively, whereas the BCAAs showed a rising trend before IAA emerged.
High glutamic acid values were associated with subsequent appearance of GADA and IAA. Glutamic acid was increased between 4.1-fold (0–9 mo; P = 0.06) and 4.7-fold (9–18 mo; P = 0.03) before seroconversion to GADA positivity and 8.4-fold (0–9 mo; P = 0.02) before seroconversion to IAA positivity.
DISCUSSION
In this longitudinal study, we compared serum metabolome in children who, during follow up, progressed to type 1 diabetes-associated autoimmunity and, further, to clinical diabetes (progressors) or who remained permanently healthy and autoantibody negative. Our study strongly suggests that metabolic dysregulation precedes overt autoimmunity in type 1 diabetes.
We found that the children who developed type 1 diabetes have reduced serum levels of succinic acid and PC at birth and reduced levels of multiple phospholipids and triglycerides throughout the follow up. As PC is a major source of choline in the body, our data suggest that the progressors are choline-deficient since birth. Besides being an important epigenetic regulator (17), choline also controls the secretion of triglyceride-rich very low-density lipoprotein particles. Choline deficiency leads to lower serum triglyceride levels and their increased accumulation in liver (18). This is consistent with our observation that during infancy and early childhood the progressors have low triglyceride levels. Although diminished choline values were observed in the progressors already at birth, nongenetic regulation cannot be excluded because, for example, choline metabolism is dependent on the composition of the intestinal microbiota (19). Consistent with this, succinic acid, which is also diminished in the progressors at birth, is as well produced by the human intestinal microbiota (20, 21). Interaction of gut microbiota with the innate immune system is an important factor affecting T1D predisposition in the nonobese diabetic mouse (22). Accordingly, it is conceivable that maternal diet or intestinal flora during pregnancy may affect the choline and succinic acid levels in newborn infants and thereby modify systemic energy metabolism and the immune system in the offspring.
Our data show that ether PCs were consistently low in the progressors. The plasmalogens, which are the most abundant ether phospholipids in normal serum, protect cells from oxidative damage (23). Plasmalogen deficiency may also indicate that subjects who are genetically susceptible to type 1 diabetes are particularly prone to specific metabolic stress, which not only leads to plasmalogen deficiency but also triggers progression toward diabetes. One potential pathway linking low phospholipids with the increased risk of type 1 diabetes is the enhanced susceptibility of pancreatic β cells to increased oxidative damage (24). Decreased antioxidant capacity of β cells caused by low ether phospholipids might so enhance their susceptibility to the effects of reactive oxygen species.
The appearance of each islet autoantibody was preceded by elevated serum concentration of lysoPCs, which are reactive lipid byproducts generated in the hydrolysis of PCs by phospholipase A2. These are lipid mediators able to activate a range of proinflammatory molecules (25) that function as natural adjuvants for the immune system (26). The increased levels of lysoPCs observed just a few months before seroconversion to autoantibody positivity may be consistent with the effects of increased oxidative stress associated with, for example, a viral infection (2) or some other proinflammatory event.
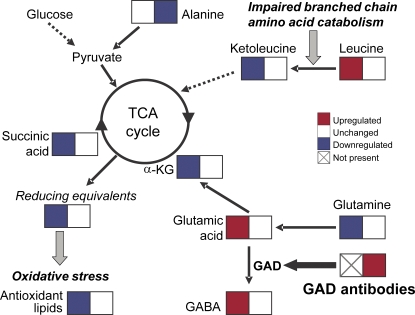
The appearances of autoantibodies against insulin and GADA, an enzyme that decarboxylates glutamic acid to GABA, were preceded by diminished ketoleucine, elevated BCAAs, and elevated glutamic acid. Augmentation of insulin secretion by elevated serum BCAAs is well documented (27) and high blood levels of BCAAs were previously reported in overt type 1 diabetes (28). BCAA and glutamic acid levels are restored to normal after the appearance of GADA or IAA (Fig. 6). This raises an intriguing possibility that the initial autoimmune response is physiological and aimed at restoring the metabolic homeostasis and that the disease may be caused by or be at least partially influenced by a defective response toward the β cell autoantigens (29, 30). A protective role of the diabetes-associated autoantibodies is indirectly supported by the observation that the autoantibody titers frequently decrease before the presentation of clinical diabetes, which can be interpreted as a sign of failing protection (31).
Figure 6.
Biochemical model of metabolic changes in progressors to type 1 diabetes prior and after seroconversion to islet autoimmunity. Detected changes of specific metabolites of citric acid cycle and glutamic acid metabolism before (left boxes) and shortly after (right boxes) the appearance of GADA are shown.
Our findings favor early immunomodulation rather than immunosuppression as a preventive therapy for type 1 diabetes, with the aim of boosting the beneficial components of autoimmunity. However, despite apparent efficacy in animal models of several immunomodulatory agents, success has been poor in human type 1 diabetes prevention trials, including our recent double-blind placebo-controlled nasal insulin study (32). Notably, in that study the intervention was conducted in children who recently had seroconverted to positivity for two or more types of diabetes-associated autoantibodies. Our findings suggest that this period may already be late for such a therapeutic intervention and that the preautoimmune period could be a more suitable window for disease prevention.
Early therapeutic strategies aimed at improving or restoring the metabolic phenotype, e.g., by reestablishing ether phospholipid availability or by modulating gut microbial composition, might also be of value by preventing β cell destruction and delaying progression to overt type 1 diabetes. In support of this, administration of docosahexaenoic acid, which is known to be selectively targeted to plasmalogens (33), has been associated with decreased risk of β cell autoimmunity and type 1 diabetes (34, 35).
One of the strengths of our study is that we used state of the art metabolomics technology to examine our large prospectively collected sample series, which covered the time from birth to overt type 1 diabetes. However, it is essential that these results are validated using other well characterized population cohorts. The German BabyDiab (36), the US-based DAISY (37) and PANDA studies (38), and the multinational TEDDY study (39) will hopefully, in time, be able to confirm or reject our findings. To address the hypotheses derived from our findings and to understand the tissue-specific mechanisms behind the early metabolic disturbances and associated autoimmune changes preceding the disease onset, experimental models reflecting both the metabolic and autoimmune components of early type 1 diabetes will have to be established.
In conclusion, dysregulation of metabolism precedes β cell autoimmunity and overt type 1 diabetes. The factors leading to metabolic stress and autoimmune responses clearly need to be investigated in further studies in the context of autoimmune diseases in general. Our findings also imply that metabolic or immunomodulatory interventions during the preautoimmune period may be used as a potential strategy for prevention of type 1 diabetes.
MATERIALS AND METHODS
DIPP study.
The DIPP project has been performed in three Finnish cities with a combined annual birth rate of 11,000, representing almost 20% of all births in Finland. The project was launched in the city of Turku in November 1994; Oulu joined the study 1 yr later and Tampere 2 yr after that. HLA-DQ–based genetic typing was used to select children positive for DR3-DQ2 and/or DR4-DQ8 risk haplotypes without protective haplotypes (6). By June 6, 2006, 104,111 consecutive newborn infants had been screened, and 8,026 children with genetic risk continued in the follow up. The DIPP study was approved by the local Ethical Committees at the University Hospitals in Turku, Oulu, and Tampere.
In Turku the children were monitored at 3-mo intervals until 2 yr of age and then were monitored twice a year, and in Oulu and Tampere they were monitored at 3, 6, 12, 18, and 24 mo and then annually (40). At each visit, a venous blood sample was collected from the children without fasting. After 30–60 min at room temperature, serum was separated and transferred to −70°C in cryovials within 3 h from the draw. ICA were measured in all samples. If a child seroconverted to ICA positivity, IAA, GADA, and protein tyrosine phosphatase-like antigen IA-2 (IA-2A) were measured in all samples obtained from that child. After seroconversion to autoantibody positivity the visits took place at 3-mo intervals in all clinical centers. As of January 1, 2003, all four autoantibody types, ICA, GADA, IAA, and IA-2A, were measured from all samples drawn as previously described (32). Autoantibodies that were present in cord blood and disappeared before 18 mo of age were regarded as maternal in origin and such values were excluded from the analysis.
Between 1997 and 2007, study children with two or more types of autoantibodies detected in at least two consecutive serum samples have been recruited into a randomized double-blind placebo-controlled trial testing the efficacy of nasal insulin for preventing type 1 diabetes (http://clinicaltrials.gov, identifier: NCT00223613). The study showed that daily nasal insulin administration in an ∼1 U/kg dose failed to delay diabetes development in children with genetic risk and two or more types of autoantibodies (32). There were no meaningful differences in serum metabolome components in the enrolled children, who comprised roughly half of those eligible, compared with those who did not participate in the prevention trial.
Lipidomic analysis.
10-μl serum samples diluted with 10 μl of 0.15 M NaCl and spiked with a standard mixture containing 10 lipid species were extracted with 100 μl of a 2:1 mixture of chloroform and methanol. The extraction time was 0.5 h and the lower organic phase was separated by centrifuging at 10,000 rpm for 3 min. Another standard mixture containing three labeled lipid species was added to the extracts and the lipids were analyzed on a mass spectrometer (Q-Tof Premier; Waters) combined with an Acquity UPLC (Waters). The column, kept at 50°C, was an Acquity UPLC BEH C18, 50 mm, with 1.7-μm particles (Waters). The solvent system included water (1% 1 M ammonium acetate and 0.1% HCOOH) and a mixture of acetonitrile and 2-propanol (5:2; 1% 1 M NH4Ac and 0.1% HCOOH). The flow rate was 0.2 ml/min and the total run time, including column reequilibration, was 18 min.
Raw centroid data from the LC/MS instrument was converted to netCDF files, which were processed with MZmine software version 0.60 (41, 42). Calibration was performed as follows: all monoacyl lipids except cholesterol esters, such as monoacylglycerols and monoacyl glycerophospholipids, were calibrated with the lysoPC PC(17:0/0:0) as internal standard. All diacyl lipids except phosphatidylethanolamines were calibrated with the PC PC(17:0/17:0), the phosphatidylethanolamines with PE(17:0/17:0), and the triacylglycerols and cholesterol esters with the triacylglycerol TG(17:0/17:0/17:0). Calibration-based concentrations are expressed in μmol/liter unless otherwise noted.
The samples were analyzed in four separate runs within 12 mo and the data were processed for each analytical run separately (Table S1, available at http://www.jem.org/cgi/content/full/jem.20081800/DC1). For the final combined dataset consisting of data from all four analytical runs, we matched the lipids across different runs based on the following criteria: lipid identity is the same across all runs; lipid is detected in all four analytical runs (this stringent criterion is the main reason for relatively low number of lipids in the final dataset and, thus, only the most abundant lipids within each class are included); starting from the first analytical run as reference (Turku DIPP series), retention time deviation is <2%; and starting from the first analytical run as reference (Turku DIPP series), deviation of median intensity (calculated within the analytical run) between analytical runs is <50%.
Metabolomic analysis using the GCxGC-TOF/MS platform.
10 μl of an internal standard-labeled palmitic acid (16:0-16,16,16d3; 500 mg/l) and 400 μl of methanol solvent were added to 20 μl of the sample. After vortexing for 1 min and incubating for 30 min at room temperature, the supernatant was separated by centrifugation at 10,000 rpm for 5 min at room temperature. The sample was dried under constant flow of nitrogen gas. 25 μl MOX (2% methoxyamine HCl in pyridine) was added to the dried sample. The mixture was then incubated at 45°C for 1 h and derivatized with 25 μl N-methyl-N-(trimethylsilyl)trifluoroacetamide by incubating at 45°C for 1 h. 10 μl of retention index standard mixture with five alkanes at 800 ppm was added to the metabolite mixture. Sample order for analysis was established by randomization.
The instrument used was a GCxGC-TOF mass spectrometer (Pegasus 4D; Leco) with an autosampler (6890N GC and Combi PAL;Agilent Technologies). The instrument parameters were as follows: 1-μl split injection 1:20; first column, RTX-5, 10 m × 180 μm × 0.20 μm; second column, BPX-50, 1.50 m × 100 μm × 0.10 μm; helium 39.6 psig constant pressure; temperature programs, primary oven, Initial 50°C, 1 min→295°C, 7°C/min, 3 min, and secondary oven, +20°C above primary oven temperature; second dimension separation time 5 s; MS measurement 45–700 amu, 100 spectra/s. Raw data were processed using ChromaTOF software (Leco) followed by alignment and normalization using the in house–developed software. Unwanted background peaks were eliminated using the classifications feature of ChromaTOF software. In house–developed software was used to perform additional filtering using compound identifications by ChromaTOF.
The metabolites were identified using an in house reference compound library as well as by searching the reference mass spectral library. Mass spectra from the GCxGC-TOF/MS analysis were searched against The Palisade Complete Mass Spectral Library, 600K Edition (Palisade Mass Spectrometry), which includes all spectra available from the NIST 2002 and Wiley registry collections as well as 150,000 other spectra. The matches to reference spectra are based on a weighted dot product of the two spectra, with higher m/z peaks having more weight than the lower. A similarity value is assigned between 0 and 999, with 999 being a perfect match and 750 generally considered as a reasonable match. We used the conservative cut-off criterion of 850 for identification. Response curves are shown for selected metabolites of relevance to the study in Figs. S8 and S9 (available at http://www.jem.org/cgi/content/full/jem.20081800/DC1).
Statistical methods.
R statistical software (http://www.r-project.org/) was used for data analyses and visualization. The concentrations were compared using the Wilcoxon rank-sum test, with p-values <0.05 considered significant. To account for multiple comparisons, false discovery rate was estimated as the maximum q-value (43) in the set of significant differences for each dataset (i.e., lipidomics or metabolomics) and the family of hypotheses tested (e.g., differences between progressors and nonprogressors in a specific age cohort). False discovery rates were computed using the R package qvalue. Lipid class concentration differences between the mean longitudinal profiles of progressors and nonprogressors were tested using linear mixed effects models implemented with the lme function in the R package nlme. The fixed effects were modeled as constant levels for each group, i.e., for progressors and nonprogressors, and the random effects were modeled as constant deviations from these constant group level trajectories. The significance of the group differences was evaluated by the p-value for the fixed effect parameter estimate of group differences.
The fold difference was calculated by dividing the median concentration in progressors by the median concentration in nonprogressors. If this number was less than one, the negative is listed (e.g., 0.75, or a drop of 25% vs. nonprogressors, is reported as a −1.3-fold difference).
Online supplemental material.
Fig. S1 shows lysoPC levels across all 1,196 samples measured as a function of sample storage time. Fig. S2 shows the total PC concentration in cord serum of progressors and nonprogressors. Fig. S3 compares the lipidomes between children with the high-risk HLA genotype and those with moderate-risk HLA genotypes. Fig. S4 shows the concentrations of ethanolamine plasmalogen detected in a subset of progressors and nonprogressors. Differences in selected metabolite concentrations between the progressors to type 1 diabetes and nonprogressors within the four 9-mo intervals before disease diagnosis in the progressors are shown in Fig. S5. Associations of lysoPC and ether PC with the rate of progression to type 1 diabetes are shown in Figs. S6 and S7, respectively. Figs. S8 and S9 show the selected characteristics of the GCxGC-TOF/MS analytical platform. Table S1 provides an overview of the four analytical runs used to generate the data for this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081800/DC1.
Supplementary Material
Acknowledgments
We thank the dedicated personnel of the DIPP study in Turku, Oulu, and Tampere and the DIPP families for their support. We acknowledge the technical support of Leena Öhrnberg, Anna-Liisa Ruskeepää, Jarkko Miettinen, Airi Hyrkäs, and Jing Tang. We thank Antonio Vidal-Puig, Fredrik Bäckhed, and Kari Alitalo for discussions and comments.
The study was funded by Juvenile Diabetes Research Foundation International (4-1999-731 and 4-2001-435 to O. Simell), Tekes FinnWell Program (M. Orešič, R. Lahesmaa, and O. Simell), DIAPREPP project of the Seventh Framework Program of the European Community (HEALTH-F2-2008-202013 to M. Orešič and O. Simell), Academy of Finland, Sigrid Juselius Foundation, Päivikki and Sakari Sohlberg Foundation, Signe and Ane Gyllenberg Foundation, Diabetes Research Foundation, Finland, and Special Federal Funds to the Turku, Oulu, and Tampere University Hospitals.
The authors have no conflicting financial interests.
Abbreviations used: BCAA, branched chain amino acids; DIPP, Type 1 Diabetes prediction and prevention study; GABA, γ-aminobutyric acid; GADA, glutamic acid decarboxylase antibody; GCxGC-TOF/MS, two-dimensional gas chromatography coupled to time-of-flight mass spectrometry; IAA, insulin autoantibody; ICA, islet cell autoantibody; PC, phosphatidylcholine; STRIP, Special Turku Coronary Risk Factor Intervention Project for Children.
S. Simell, M. Sysi-Aho, K. Näntö-Salonen, T. Seppänen-Laakso, V. Parikka, and M. Katajamaa contributed equally to this paper.
References
- 1.Gale, E.A.M. 2002. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 51:3353–3361. [DOI] [PubMed] [Google Scholar]
- 2.Knip, M., R. Veijola, S.M. Virtanen, H. Hyöty, O. Vaarala, and H.K. Åkerblom. 2005. Environmental triggers and determinants of type 1 diabetes. Diabetes. 54:S125–S136. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto, J., M. Karvonen, J. Pitkäniemi, E. Virtala, K. Kohtamäki, L. Toivanen, and E. Tuomilehto-Wolf. 1999. Record-high incidence of type 1 (insulin-dependent) diabetes mellitus in Finnish children. Diabetologia. 42:655–660. [DOI] [PubMed] [Google Scholar]
- 4.Harjutsalo, V., L. Sjöberg, and J. Tuomilehto. 2008. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 371:1777–1782. [DOI] [PubMed] [Google Scholar]
- 5.Achenbach, P., E. Bonifacio, K. Koczwara, and A.-G. Ziegler. 2005. Natural history of type 1 diabetes. Diabetes. 54:S25–S31. [DOI] [PubMed] [Google Scholar]
- 6.Kupila, A., P. Muona, T. Simell, P. Arvilommi, H. Savolainen, A.-M. Hämäläinen, S. Korhonen, T. Kimpimäki, M. Sjåroos, J. Ilonen, et al. 2001. Feasibility of genetic and immunological prediction of type 1 diabetes in a population-based birth cohort. Diabetologia. 44:290–297. [DOI] [PubMed] [Google Scholar]
- 7.Orešič, M., A. Vidal-Puig, and V. Hänninen. 2006. Metabolomic approaches to phenotype characterization and applications to complex diseases. Expert Rev. Mol. Diagn. 6:575–585. [DOI] [PubMed] [Google Scholar]
- 8.Nikkilä, J., M. Sysi-Aho, A. Ermolov, T. Seppänen-Laakso, O. Simell, S. Kaski, and M. Orešič. 2008. Gender dependent progression of systemic metabolic states in early childhood. Mol. Syst. Biol. 4:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz, E.M., J. Bright, I.D. Wilson, A. Hughes, J. Morrisson, H. Lindberg, and A. Lockton. 2004. Metabonomics, dietary influences and cultural differences: a 1H NMR-based study of urine samples obtained from healthy British and Swedish subjects. J. Pharm. Biomed. Anal. 36:841–849. [DOI] [PubMed] [Google Scholar]
- 10.Bäckhed, F., H. Ding, T. Wang, L.V. Hooper, G.Y. Koh, A. Nagy, C.F. Semenkovich, and J.I. Gordon. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman, P.D., M.A. Hanson, C. Cooper, and K.L. Thornburg. 2008. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simell, O., H. Niinikoski, T. Rönnemaa, H. Lapinleimu, T. Routi, H. Lagström, P. Salo, E. Jokinen, and J. Viikari. 2000. Special Turku Coronary Risk Factor Intervention Project for Babies (STRIP). Am. J. Clin. Nutr. 72:1316S–1331S. [DOI] [PubMed] [Google Scholar]
- 13.Watson, A.D. 2006. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J. Lipid Res. 47:2101–2111. [DOI] [PubMed] [Google Scholar]
- 14.Orešič, M., V.A. Hänninen, and A. Vidal-Puig. 2008. Lipidomics: a new window to biomedical frontiers. Trends Biotechnol. 26:647–652. [DOI] [PubMed] [Google Scholar]
- 15.Laaksonen, R., M. Katajamaa, H. Päivä, M. Sysi-Aho, L. Saarinen, P. Junni, D. Lütjohann, J. Smet, R.V. Coster, T. Seppänen-Laakso, et al. 2006. A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin induced changes in muscle. PLoS ONE. 1:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welthagen, W., R.A. Shellie, J. Spranger, M. Ristow, R. Zimmermann, and O. Fiehn. 2005. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC— GC-TOF) for high resolution metabolomics: biomarker discovery on spleen tissue extracts of obese NZO compared to lean C57BL/6 mice. Metabolomics. 1:65–73. [Google Scholar]
- 17.Niculescu, M.D., C.N. Craciunescu, and S.H. Zeisel. 2006. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 20:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao, Z.M., and D. Vance. 1988. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 263:2998–3004. [PubMed] [Google Scholar]
- 19.Dumas, M.-E., R.H. Barton, A. Toye, O. Cloarec, C. Blancher, A. Rothwell, J. Fearnside, R. Tatoud, V. Blanc, J.C. Lindon, et al. 2006. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA. 103:12511–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macy, J.M., L.G. Ljungdahl, and G. Gottschalk. 1978. Pathway of succinate and propionate formation in Bacteroides fragilis. J. Bacteriol. 134:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill, S.R., M. Pop, R.T. DeBoy, P.B. Eckburg, P.J. Turnbaugh, B.S. Samuel, J.I. Gordon, D.A. Relman, C.M. Fraser-Liggett, and K.E. Nelson. 2006. Metagenomic analysis of the human distal gut microbiome. Science. 312:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen, L., R.E. Ley, P.Y. Volchkov, P.B. Stranges, L. Avanesyan, A.C. Stonebraker, C. Hu, F.S. Wong, G.L. Szot, J.A. Bluestone, et al. 2008. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 455:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoeller, R.A., T.J. Grazia, P. LaCamera, J. Park, D.P. Gaposchkin, and H.W. Farber. 2002. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am. J. Physiol. Heart Circ. Physiol. 283:H671–H679. [DOI] [PubMed] [Google Scholar]
- 24.Lenzen, S., J. Drinkgern, and M. Tiedge. 1996. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20:463–466. [DOI] [PubMed] [Google Scholar]
- 25.Mehta, D. 2005. Lysophosphatidylcholine: an enigmatic lysolipid. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L174–L175. [DOI] [PubMed] [Google Scholar]
- 26.Kabarowski, J.H.S., Y. Xu, and O.N. Witte. 2002. Lysophosphatidylcholine as a ligand for immunoregulation. Biochem. Pharmacol. 64:161–167. [DOI] [PubMed] [Google Scholar]
- 27.Floyd, J.C.J., S.S. Fajans, R.F. Knopf, and J.W. Conn. 1963. Evidence that insulin release is the mechanism for experimentally induced leucine hypoglycemia in man. J. Clin. Invest. 42:1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marliss, E.B., A.F. Nakhooda, P. Poussier, and A.A. Sima. 1982. The diabetic syndrome of the ‘BB’ Wistar rat: possible relevance to type 1 (insulin-dependent) diabetes in man. Diabetologia. 22:225–232. [DOI] [PubMed] [Google Scholar]
- 29.Nevo, U., I. Golding, A.U. Neumann, M. Schwartz, and S. Akselrod. 2004. Autoimmunity as an immune defense against degenerative processes: a primary mathematical model illustrating the bright side of autoimmunity. J. Theor. Biol. 227:583–592. [DOI] [PubMed] [Google Scholar]
- 30.Hauben, E., M.G. Roncarolo, U. Nevo, and M. Schwartz. 2005. Beneficial autoimmunity in type 1 diabetes mellitus. Trends Immunol. 26:248–253. [DOI] [PubMed] [Google Scholar]
- 31.Kimpimaki, T., P. Kulmala, K. Savola, A. Kupila, S. Korhonen, T. Simell, J. Ilonen, O. Simell, and M. Knip. 2002. Natural history of {beta}-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J. Clin. Endocrinol. Metab. 87:4572–4579. [DOI] [PubMed] [Google Scholar]
- 32.Näntö-Salonen, K., A. Kupila, S. Simell, H. Siljander, T. Salonsaari, A. Hekkala, S. Korhonen, R. Erkkola, J.I. Sipilä, L. Haavisto, et al. 2008. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 372:1746–1755. [DOI] [PubMed] [Google Scholar]
- 33.Gaposchkin, D.P., and R.A. Zoeller. 1999. Plasmalogen status influences docosahexaenoic acid levels in a macrophage cell line: insights using ether lipid-deficient variants. J. Lipid Res. 40:495–503. [PubMed] [Google Scholar]
- 34.Stene, L.C., and G. Joner, and the Norwegian Childhood Diabetes Study Group. 2003. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am. J. Clin. Nutr. 78:1128–1134. [DOI] [PubMed] [Google Scholar]
- 35.Norris, J.M., X. Yin, M.M. Lamb, K. Barriga, J. Seifert, M. Hoffman, H.D. Orton, A.E. Baron, M. Clare-Salzler, H.P. Chase, et al. 2007. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 298:1420–1428. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach, P., K. Koczwara, A. Knopff, H. Naserke, A.-G. Ziegler, and E. Bonifacio. 2004. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J. Clin. Invest. 114:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baschal, E.E., T.A. Aly, S.R. Babu, M.S. Fernando, L. Yu, D. Miao, K.J. Barriga, J.M. Norris, J.A. Noble, H.A. Erlich, et al. 2007. HLA-DPB1*0402 protects against type 1A diabetic autoimmunity in the highest risk DR3-DQB1*0201/DR4-DQB1*0302 DAISY population. Diabetes. 56:2405–2409. [DOI] [PubMed] [Google Scholar]
- 38.Carmichael, S.K., S.B. Johnson, A. Baughcum, K. North, D. Hopkins, M.G. Dukes, J.X. She, and D.A. Schatz. 2003. Prospective assessment in newborns of diabetes autoimmunity (PANDA): maternal understanding of infant diabetes risk. Genet. Med. 5:77–83. [DOI] [PubMed] [Google Scholar]
- 39.Hagopian, W.A., A. Lernmark, M.J. Rewers, O.G. Simell, J.-X. She, A.G. Ziegler, J.P. Krischer, and B. Akolkar. 2006. TEDDY - the environmental determinants of diabetes in the young: an observational clinical trial. Ann. N. Y. Acad. Sci. 1079:320–326. [DOI] [PubMed] [Google Scholar]
- 40.Kupila, A., P. Keskinen, T. Simell, S. Erkkilä, P. Arvilommi, S. Korhonen, T. Kimpimäki, M. Sjöroos, M. Ronkainen, J. Ilonen, et al. 2002. Genetic risk determines the emergence of diabetes-associated autoantibodies in young children. Diabetes. 51:646–651. [DOI] [PubMed] [Google Scholar]
- 41.Katajamaa, M., and M. Orešič. 2005. Processing methods for differential analysis of LC/MS profile data. BMC Bioinformatics. 6:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katajamaa, M., J. Miettinen, and M. Orešič. 2006. MZmine: toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics. 22:634–636. [DOI] [PubMed] [Google Scholar]
- 43.Storey, J.D. 2002. A direct approach to false discovery rates. Journal of the Royal Statistical Society Series B. 64:479–498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.