Abstract
Entry into mitosis of the eukaryotic cell cycle is driven by rising cyclin-dependent kinase (Cdk) activity. During exit from mitosis, Cdk activity must again decline. Cdk downregulation by itself, however, is not able to guide mitotic exit, if not a phosphatase reverses mitotic Cdk phosphorylation events. In budding yeast, this role is played by the Cdc14 phosphatase. We are gaining an increasingly detailed picture of its regulation during anaphase, and of the way it orchestrates ordered progression through mitosis. Much less is known about protein dephosphorylation during mitotic exit in organisms other than budding yeast, but evidence is now mounting for crucial contributions of regulated phosphatases also in metazoan cells.
Introduction
Mitotic exit involves an intricately ordered series of events, leading from the splitting of sister chromatids at anaphase onset to the completion of cell division by cytokinesis. During the course of these events, the mitotic spindle elongates to segregate sister chromatids, the cytokinetic furrow is assembled at the future site of cell division, and once the chromosome segregation is complete, the spindle disassembles again in preparation for cytokinesis. Anaphase onset is triggered when the anaphase promoting complex with its activator Cdc20 (APCCdc20) ubiquitinates, and thereby leads to degradation of securin, an inhibitor of the protease separase that cleaves the cohesive link between sister chromatids. At the same time, APCCdc20 begins to target mitotic cyclins for destruction, thereby initiating Cdk downregulation.
In addition to cyclin destruction, the essential budding yeast Cdc14 phosphatase has long been recognised as a key regulator of mitotic exit. cdc14 mutants arrest in a telophase-like mitotic state, with an elongated mitotic spindle and bilobed anaphase nucleus [1]. Execution point analysis placed CDC14 function after nuclear division but before cytokinesis [2], consistent with a role of the phosphatase in spindle disassembly and cytokinesis during mitotic exit. Budding yeast cells in which mitotic Cdk activity cannot be downregulated during mitotic exit, owing to expression of undegradable mitotic cyclin, display an arrest phenotype reminiscent of cdc14 mutant cells [3]. The first well-characterised role of Cdc14, therefore, became its contribution to Cdk downregulation by dephosphorylation, and thereby activation of the Cdk inhibitor Sic1, its transcription factor Swi5, and a second APC activator Cdh1 [4]. Consistent with its apparent execution point after nuclear division, circumstantial evidence linked activation of the Cdc14 phosphatase to elongation of the anaphase spindle [5,6]. In this way, Cdc14-dependent mitotic exit would not set in before successful chromosome segregation.
Meanwhile it has become clear that Cdc14 is activated already earlier, as soon as sister chromatids split at anaphase onset, and that Cdc14 activation is indeed a prerequisite for successful chromosome segregation. Cdc14 promotes stabilisation of microtubules of the elongating anaphase spindle, assembly of a spindle midzone structure and resolution of late segregating regions of the budding yeast genome, notably of the rDNA locus [7–10,11•,12•]. These roles of Cdc14 during early anaphase had gone largely unnoticed before, probably because they require relatively little Cdc14 activity that was still provided by conditional Cdc14 mutant proteins even at the restrictive temperature. Cdk substrates that are dephosphorylated at this time include the microtubule regulators Ase1, Ask1, Fin1 and Sli15 [7,10,11•,12•]. How relatively little Cdc14 dephosphorylates these early targets at anaphase onset and promotes completion of Cdk downregulation and mitotic exit only afterwards is so far not understood. Cdc14 targets that must be dephosphorylated for cytokinesis are as yet largely elusive.
Here we review recent advances of our understanding about how Cdc14 phosphatase is activated during anaphase. We suggest a quantitative explanation for the existence of at least two pathways, the Cdc Fourteen Early Anaphase Release (FEAR) and the Mitotic Exit Network (MEN) that activate Cdc14 during states of high and low Cdk activity, respectively. We will then compare our knowledge from budding yeast to what is known about Cdk-counteracting phosphatases in other eukaryotes, where exciting discoveries have recently been made. To gain a fuller understanding of regulated mitotic progression, we have to consider the activities of numerous mitotic kinases in addition to Cdk. Likewise, sequential APC-dependent proteolysis of mitotic regulators is likely to make important contributions to ordered mitotic progression [13]. These topics fall outside the reach of this short review, in which we will focus on the regulation of Cdk-counteracting phosphatases during mitotic exit.
Regulation of the Cdc14 phosphatase in budding yeast
Although not all levels of regulation of the Cdc14 phosphatase in budding yeast may yet be understood, its dominant and most striking control is exerted via its sequestration in the nucleolus by its inhibitor Net1 (also called Cfi1) [14,15]. Net1 phosphorylation during anaphase reduces its affinity for Cdc14, thus releasing the active phosphatase [16,17]. After release from the nucleolus, Cdc14 is initially seen distributed all over the nucleus, and shortly thereafter, also spreads throughout the cytoplasm. While much attention has been given to the control of Cdc14 release from the nucleolus, little is known whether and how export of the phosphatase from the nucleus is regulated. Cdc14 can probably reach most of its substrates relevant to cell cycle progression within the nucleus as suggested by largely unaltered cell cycle progression, if Cdc14 nuclear export is prevented by mutation of its nuclear export sequence [18]. In such cells, however, cytokinesis fails. This is indicative of essential Cdc14 targets at the cytokinetic machinery, outside the nucleus, that the phosphatase needs to gain access to.
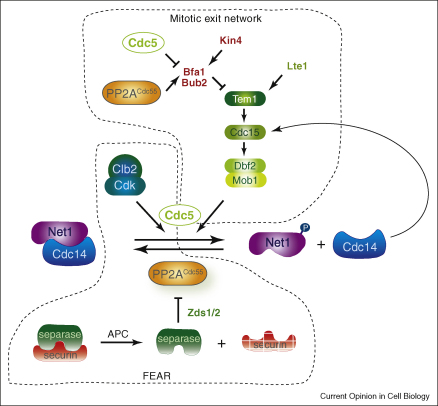
A collection of genes required for mitotic exit has been ordered both genetically and biochemically into the MEN signalling cascade that culminates in the release of the Cdc14 phosphatase from the nucleolus. This includes the Ras-like GTPase Tem1 and its downstream kinases Cdc15 and Mob1/Dbf2 [19–21] (Figure 1). The GTPase at the top of this signalling cascade is thought to be a spatial positioning sensor for mitotic spindle elongation. Tem1 is concentrated at the daughter cell-bound spindle pole body, and elongation of the anaphase spindle into the daughter cell brings the GTPase in proximity to the GDP/GTP nucleotide exchange factor Lte1 that is enriched at the daughter cell cortex [5,6]. Lte1 interacts with Tem1 [22], and while it is tempting to speculate that it thus activates MEN in a spindle elongation-dependent manner, this remains to be demonstrated. Counteracting Tem1 activation is the GTPase activating protein complex Bfa1/Bub2 [23], a potent negative regulator of mitotic exit. Its inhibitory effect on Tem1 is turned off by anaphase-specific phosphorylation of Bfa1 by the Polo-like kinase, Cdc5 (see below) [23,24]. Tem1 needs to interact with the Cdc15 kinase to activate MEN [25], and Cdc15, in turn, is able to activate the Mob1/Dbf2 kinase complex [26]. The components of this signalling cascade are concentrated at the SPB that may act as a catalyst for signal transduction. How Dbf2 finally promotes Cdc14 nucleolar release has not been ascertained. An obvious possibility is that Dbf2 itself phosphorylates Net1 to promote Cdc14 dissociation [27]. Direct evidence for this, or an alternative mechanism, is not yet available.
Figure 1.
A model for mitotic exit in budding yeast. At the centre of the scheme is reversible phosphorylation and dephosphorylation of the Cdc14 phosphatase inhibitor Net1. At anaphase onset, separase-dependent downregulation of PP2ACdc55 promotes Cdk phosphorylation of Net1, amplified by Cdc5. This leads to Cdc14 early anaphase release from nucleolar inhibition by Net1 (FEAR). PP2ACdc55 downregulation, together with Cdc14 and spindle elongation-mediated Tem1 activation by Lte1, contribute to activation of the mitotic exit network (MEN). Kinases of the MEN cascade (e.g. Dbf2) may maintain Cdc14 release by sustaining Cdc5-amplified Net1 phosphorylation.
In cells in which MEN is inactive, Cdc14 does not remain entirely confined to the nucleolus. Instead, a transient release of Cdc14 is observed during early anaphase [28]. This observation led to the discovery of the second Cdc14 activatory FEAR network (Figure 1). At anaphase onset, the release of separase from its inhibitor securin not only leads to proteolytic cleavage of cohesin, but separase at the same time utilises a second, non-proteolytic activity to activate Cdc14 [29]. At this stage, high mitotic Cdk activity itself sets Cdc14 release into motion by phosphorylating at least 6 Cdk recognition sites on Net1 [30]. The link between separase and Cdk-dependent Net1 phosphorylation comes through the PP2A phosphatase in conjunction with its substrate specificity subunit Cdc55 (PP2ACdc55), which until metaphase counteracts Net1 phosphorylation [31•,32]. Separase-dependent downregulation of PP2ACdc55 at anaphase onset now allows Cdk-dependent Net1 phosphorylation. How separase leads to PP2ACdc55 downregulation and what the nature of its second non-proteolytic activity is, are as yet poorly understood. Separase cooperates with two PP2A interactors, Zds1 and Zds2, to promote Cdc14 release [33]. Zds1 and Zds2 (zillion different screens) appear to be ascomycete-specific proteins involved in numerous physiologic pathways. Their ability to regulate PP2ACdc55 suggests that they may act as common PP2A modulators. A number of additional proteins have been found to contribute to Cdc14 regulation during early anaphase. These include the kinetochore protein and separase substrate Slk19, and the Net1 interactor Fob1 as well as its binding partner Spo12 [28,34]. The mechanism by which these proteins contribute to Cdc14 activation is not understood.
A multilayered role is played by the Polo-like kinase Cdc5 during mitotic exit. Cdc5 activity is essential for Cdc14 activation, and it contributes to Cdc14 release at all stages, as part of FEAR, as well as both upstream and downstream of the MEN cascade [19,20,28,29]. The so far best characterised effect of Cdc5 is inhibition of Bfa1, thus contributing to Tem1 activation [23,24]. Bfa1 phosphorylation is counteracted by PP2ACdc55 during metaphase, so that PP2ACdc55 downregulation at anaphase onset not only directly promotes Cdk-dependent Net1 phosphorylation but also activates the MEN cascade [31•]. Another Cdc5 target is probably the Cdc14 inhibitor Net1 itself [16,17], both early and late during mitotic exit. The multiple roles of Cdc5 could be explained by this kinase’s characteristic mode of action. Cdc5 requires a priming phosphorylation event by another kinase to be recruited to its substrates [35]. In this way, Cdc5 would not exert specific regulation onto mitotic exit, but rather act as an essential but generic phosphorylation amplifier. The regulatory priming phosphorylation on Net1 may be under the control of Cdk/PP2ACdc55 in early anaphase, and maybe Dbf2 or another MEN kinase later during exit. In addition, Cdc5 may contribute to the build-up of Cdk activity during mitotic entry, a prerequisite for Cdk-dependent Net1 phosphorylation, as cdc5 mutant cells are deficient in Net1 Cdk phosphorylation [30]. While Cdc5 may, therefore, not be regulating the onset of Cdc14 activation, it remains a limiting factor for Cdc14 release throughout anaphase, and APCCdh1-mediated Cdc5 destruction at the end of mitosis is sufficient to cause Cdc14 resequestration in the nucleolus [36•].
Additional regulators of Cdc14 activation during mitotic exit include the mother cell restricted kinase Kin4 that prevents Bfa1 phosphorylation by Cdc5 if the mitotic spindle fails to enter the daughter cell [37,38]. Less well understood contributions have also been documented for a number of cell polarity effectors and the budding yeast orthologues of the human Chfr tumour suppressor, Dma1 and Dma2 [22,39,40].
A quantitative view of mitotic exit
How do these multiple levels of regulation cooperate to control Cdc14 activity during mitotic exit, and why does the regulation appear so complex? During mitotic exit, a multitude of Cdk phosphorylation events need to be reversed in an ordered series of reactions over the duration of anaphase, cytokinesis and return into G1. This requires that the Cdc14 phosphatase is activated at anaphase onset, exported from the nucleus at the right time, and maintained active throughout mitotic exit as Cdk activity declines and until cells successfully complete cell division. Mitotic exit integrates both temporal and spatial information, and the output may not simply be Cdc14 activation, but quantitatively regulated levels of Cdc14 activity over the course of mitotic exit. It has been argued that budding yeast mitotic exit is a prime example of an evolved complex system [41]. Multiple, distinct, but partly redundant, pathways and regulatory circuits have come together to reinforce each other, selected for the addition of robustness and resistance to noise. While this adds complexity to the overall network, the main reaction pathways and their effects can be relatively succinctly described [31•].
Mitotic exit begins when the APCCdc20 initiates anaphase by causing degradation of the separase inhibitor securin. Sister chromatids split, and the mitotic spindle must be stabilised to successfully segregate chromosomes to daughter cells. This is one of the first mitotic exit tasks, and it requires Cdc14 activation at the time when Cdk activity is still high. It is achieved by separase-dependent PP2ACdc55 downregulation, allowing Cdk-dependent Net1 phosphorylation. Note that early Cdk-dependent Cdc14 activation (FEAR) is based on the fact that complete securin destruction precedes complete cyclin degradation, at least in budding yeast. Possible explanations for selective and rapid securin destruction at anaphase onset have been put forward [13,42•]. The fact that some of the FEAR contributors are non-essential, for example, Spo12, Slk19, or a minimal set of 6 Cdk phosphorylation sites on Net1, has led to the assumption that FEAR is a dispensable pathway during mitotic exit [28,30]. However, cells progressing through anaphase with delayed Cdc14 activation due to FEAR mutations incur a considerable loss in viability, probably due to chromosome segregation defects [8].
Quantitative numerical simulations of mitotic exit driven only by the FEAR pathway show that Cdk-dependent Cdc14 activation is not sufficient to complete mitotic exit. This is because Cdk downregulation will lead to Cdc14 resequestration in the nucleolus before mitotic exit is complete, a situation that has been described as transient Cdc14 release in cells lacking MEN function [28,31•]. MEN activity is inhibited by high Cdk levels, and activated by Cdc14 at the level of the Cdc15 kinase, though the biochemical consequences of Cdc15 phosphoregulation remain to be fully understood [28,43]. The essential nature of the MEN ,therefore, stems from its requirement to sustain Cdc14 release as Cdk activity declines during the progression of mitotic exit. Spatial information on the successful elongation of the anaphase spindle is integrated into MEN activation at the level of the Tem1 GTPase on the top of the signalling pathway that may limit Cdc15 activity. Spindle elongation by itself, however, is not sufficient to activate Cdc14, and mitotic exit can proceed in the absence of spindle elongation in cells harbouring proteolytically inactive separase [29]. The relative contributions of spindle elongation, Polo/PP2ACdc55 regulation of Bfa1, Cdc14/Cdk-mediated Cdc15 activation, and possibly additional levels of regulation on the control of MEN activity during anaphase remain to be determined.
Mitotic exit in higher eukaryotes
Cdk substrate dephosphorylation is a hallmark of mitotic exit probably in all eukaryotes. Although most of the relevant substrates whose dephosphorylation drives the execution of mitotic exit remain unknown, persistent Cdk activity prevents mitotic exit and return to interphase in all eukaryotes studied [3,44–46]. Many of the dephosphorylation-dependent anaphase events, described above in budding yeast, also take place in a similar fashion in other eukaryotes. This includes stabilisation of microtubules at anaphase onset, relocalisation of the Aurora B kinase complex, spindle midzone assembly and upon completion of chromosome segregation spindle disassembly, cytokinesis and cell abscission.
A few known examples of Cdk substrates whose dephosphorylation contributes to successful anaphase progression in higher eukaryotes include the C. elegans kinesin ZEN-4 [47], and the human Ase1 orthologue PRC1 [48], both involved in the assembly of the anaphase spindle midzone. The nature of the phosphatase(s) that act to reverse Cdk phosphorylation during mitotic exit in organisms other than budding yeast is not well understood. ZEN-4 dephosphorylation requires the C. elegans Cdc14 orthologue, CDC-14, and this phosphatase has been furthermore reported to play a role during the completion of C. elegans cytokinesis [49]. On the contrary, worms lacking CDC-14 are viable, but show defects in accumulating the Cdk inhibitor CKI-1 during developmentally programmed G1 cell cycle arrest [50]. The latter observation is reminiscent of budding yeast Cdc14’s role in stabilising the Cdk inhibitor Sic1.
Vertebrate genomes encode for two Cdc14 orthologues, Cdc14A and Cdc14B (Figure 2). Their overexpression causes mitotic defects [51,52], but a thorough loss of function analysis of the two paralogues, that may have overlapping substrate specificities, remains to be completed and systematic substrate identification has just begun [53]. Reduced human Cdc14B levels have been reported to lead to centriole amplification [54], while human cells carrying a homozygous disruption of the Cdc14B locus display no apparent cell cycle progression defect, but show an increased incidence of rDNA anaphase bridges [55••]. Introduction of both human Cdc14A and Cdc14B into budding or fission yeast, rescues the respective cdc14 yeast mutant phenotypes, suggesting some functional conservation between these phosphatases [56,57].
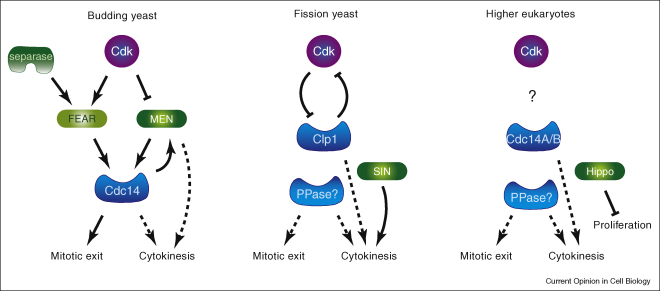
Figure 2.
Comparison of budding yeast mitotic exit regulators with their counterparts in fission yeast and higher eukaryotes. The fission yeast Cdc14 orthologue Clp1 is released from the nucleolus already during mitotic entry, but remains inhibited by Cdk phosphorylation. Its activation is independent of the SIN signalling cascade, made up of the MEN orthologues, but SIN maintains Clp1 activity during completion of cytokinesis. While cytokinesis is linked to mitotic exit, as defined by re-entry into G1, both processes can be uncoupled. SIN is required for cytokinesis, but not for re-entry into the next cell cycle. The Clp1 phosphatase is not essential, raising the question as to the phosphatase(s) that reverse Cdk phosphorylation. In higher eukaryotes, Cdc14 phosphatases contribute to spindle midzone formation and cytokinesis, but are not essential at least in some organisms. Orthologues of the MEN components form part of the Hippo signalling cascade that controls transcription of cell proliferation and apoptosis genes.
The fission yeast Cdc14 homologue Clp1 (Flp1) is not essential for normal cell cycle progression. Like human Cdc14B, Clp1 is nucleolar for much of the cell cycle but, unlike budding yeast Cdc14, these phosphatases are released from the nucleolus already as cells enter mitosis [58,59]. At this stage Clp1 remains inhibited by Cdk phosphorylation, and Cdk downregulation during mitotic exit is thought to initiate Clp1 activation by self-catalysed dephosphorylation [60•] (Figure 2). Clp1, in turn, contributes to Cdk downregulation by dephosphorylation and destabilisation of the Cdk activating phosphatase Cdc25, a mechanism that may be also at work in higher eukaryotes, but used to control mitotic entry more than exit [57,61]. It is noteworthy that fission yeast cells possess a signalling cascade of remarkable conservation to the budding yeast MEN, the Septation Initiation Network (SIN). The SIN controls cytokinesis, but its downstream effectors are not yet known. The SIN is not required for Clp1 activation, but contributes to sustain Clp1 nucleolar release until the completion of cytokinesis [59]. Orthologues of the MEN kinases Cdc15 and Mob1/Dbf2 are also found in higher eukaryotes. They form part of the Hippo pathway that has been best characterised in Drosophila where it controls cell proliferation by regulating the transcription factor Yorkie [62]. Whether this pathway involves a Cdc14 phosphatase or whether it also impinges on mitotic regulation is not known. No Cdc14 orthologues have been found encoded in the genomes of higher plants [63].
If in several, if not most, eukaryotes Cdc14 is dispensable for mitotic exit, which are the phosphatases that reverse mitotic Cdk phosphorylation? One possibility is that a range of constitutive, broad-specificity phosphatases continuously counteract Cdk phosphorylation in these organisms. In this case mitotic exit would be driven solely by downregulation of Cdk activity, with substrate dephosphorylation as the consequence. While this scenario is possible, it is difficult to explain how some Cdk substrates may be dephosphorylated more rapidly at anaphase onset than the decline of Cdk activity itself. Recent observations using chemical Cdk inhibition in human mitotic cells have confirmed that phosphatase activity directed against Cdk substrates is largely quiescent during metaphase but activated during mitotic exit [64•]. The nature of these phosphatase(s) is not yet known, but it was shown to be sensitive to the phosphatase inhibitor Okadaic acid, a compound that only marginally affects Cdc14. Another striking example is the upregulation of the calcium-dependent phosphatase calcineurin during exit of Xenopus oocytes from metaphase of meiosis II. An increase of cytoplasmic calcium upon fertilisation triggers meiosis II exit in these oocytes, which involves calmodulin-activated kinase-dependent activation of the APC. The APC, however, is not sufficient to drive Cdk substrate dephosphorylation and meiosis II exit, if at the same time calcineurin is not activated [65••,66••]. Once meiosis II exit is complete, calcineurin is no longer required during the mitotic cell cycles. Instead a second, again Okadaic acid sensitive, phosphatase activity appears in a cyclic fashion during interphase that is downregulated again in mitosis [65••]. A survey of phosphatase contributions to cell cycle progression in Drosophila has failed to identify a specific candidate for a mitotic exit phosphatase [67], suggesting that more than one phosphatase may act redundantly, or that its involvement in mitotic exit is not the only function of the phosphatase.
Conclusions
It is becoming increasingly clear that in addition to the downregulation of Cdk activity, control of mitotic phosphatases plays an important role in regulating mitotic exit. The identity of mitotic exit phosphatase(s) in most organisms is not yet known. Once identified, the elucidation of their quantitative regulation will have important implications. Simple antagonistic feedback between Cdk and a phosphatase, as observed with fission yeast Clp1, could provide sharpness to mitotic exit processes. A Cdk-independent input to phosphatase activation, as exemplified by separase-induced Cdc14 activation at budding yeast anaphase onset, opens the opportunity for additional time-resolved regulation that is probably important for ordered progression of mitotic exit. We are just at the beginning of understanding the regulatory pathways that control the faithful completion of chromosome segregation into newborn daughter cells.
References and recommended reading
Papers of particular interest published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We apologise to our colleagues for the omission of many seminal contributions to the field, and their references, owing to space constraints. We thank Céline Bouchoux, Sandra López-Avilés and Béla Novák for discussion and comments on the manuscript. Work in our laboratories is supported by the Ramón y Cajal programme (EQ), and by Cancer Research UK, EMBO, the European Commission and the Human Frontier Science Program (FU).
Contributor Information
Ethel Queralt, Email: equeralt@iconcologia.net.
Frank Uhlmann, Email: frank.uhlmann@cancer.org.uk.
References
- 1.Culotti J., Hartwell L.H. Genetic control of the cell division cycle in yeast. III. Seven genes controlling nuclear division. Exp Cell Res. 1971;67:389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- 2.Wood J.S., Hartwell L.H. A dependent pathway of gene functions leading to chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1982;94:718–726. doi: 10.1083/jcb.94.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surana U., Amon A., Dowzer C., McGrew J.T., Byers B., Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visintin R., Craig K., Hwang E.S., Prinz S., Tyers M., Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 5.Bardin A.J., Visintin R., Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. doi: 10.1016/s0092-8674(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 6.Pereira G., Höfken T., Grindlay J., Manson C., Schiebel E. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10. [PubMed] [Google Scholar]
- 7.Pereira G., Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 8.D’Amours D., Stegmeier F., Amon A. Cdc14 and condensin control the dissolution of cohesin-independent linkages at repeated DNA. Cell. 2004;117:455–469. doi: 10.1016/s0092-8674(04)00413-1. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan M., Higuchi T., Katis V.L., Uhlmann F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 2004;117:471–482. doi: 10.1016/s0092-8674(04)00415-5. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T., Uhlmann F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 2005;433:171–176. doi: 10.1038/nature03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Khmelinskii A., Lawrence C., Roostalu J., Schiebel E. Cdc14-regulated midzone assembly controls anaphase B. J Cell Biol. 2007;177:981–993. doi: 10.1083/jcb.200702145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of how Cdc14-dependent Ase1 dephosphorylation contributes to anaphase by assembly of a stable spindle midzone.
- 12•.Woodbury E.L., Morgan D.O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat Cell Biol. 2007;9:106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]; Identification of Fin1 as a Cdc14 target, whose dephosphorylation at anaphase onset is crucial for anaphase spindle stabilisation.
- 13.Rape M., Reddy S.K., Kirschner M.W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Shou W., Seol J.H., Shevchenko A., Baskerville C., Moazed D., Chen Z.W.S., Jang J., Shevchenko A., Charbonneau H., Deshaies R.J. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 15.Visintin R., Hwang E.S., Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- 16.Shou W., Azzam R., Chen S.L., Huddleston M.J., Baskerville C., Charbonneau H., Annan R.S., Carr S.A., Deshaies R.J. Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex. BMC Mol Biol. 2002;3:3. doi: 10.1186/1471-2199-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida S., Toh-e A. Budding yeast Cdc5 phosphorylates Net1 and assists Cdc14 release from the nucleolus. Biochem Biophys Res Commun. 2002;294:687–691. doi: 10.1016/S0006-291X(02)00544-2. [DOI] [PubMed] [Google Scholar]
- 18.Bambenek J., Kang J., Kurischko C., Li B., Raab J.R., Belanger K.D., Luca F.C., Yu H. Crm1-mediated nuclear export of Cdc14 is required for the completion of cytokinesis in budding yeast. Cell Cycle. 2005;4:961–971. doi: 10.4161/cc.4.7.1798. [DOI] [PubMed] [Google Scholar]
- 19.Jaspersen S.L., Charles J.F., Tinker-Kulberg R.L., Morgan D.O. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.E., Frenz L.M., Wells N.J., Johnson A.L., Johnston L.H. Order of function of the budding yeast mitotic exit-network proteins Tem1, Cdc15, Mob1, Dbf2, and Cdc5. Curr Biol. 2001;11:784–788. doi: 10.1016/s0960-9822(01)00228-7. [DOI] [PubMed] [Google Scholar]
- 21.Visintin R., Amon A. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol Biol Cell. 2001;12:2961–2974. doi: 10.1091/mbc.12.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höfken T., Schiebel E. A role for cell polarity proteins in mitotic exit. EMBO J. 2002;21:4851–4862. doi: 10.1093/emboj/cdf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geymonat M., Spanos A., Walker P.A., Johnston L.H., Sedgwick S.G. In vitro regulation of budding yeast Bfa1/Bub2 GAP activity by Cdc5. J Biol Chem. 2003;278:14591–14594. doi: 10.1074/jbc.C300059200. [DOI] [PubMed] [Google Scholar]
- 24.Hu F., Wang Y., Liu D., Li Y., Qin J., Elledge S.J. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell. 2001;107:655–665. doi: 10.1016/s0092-8674(01)00580-3. [DOI] [PubMed] [Google Scholar]
- 25.Asakawa K., Yoshida S., Otake F., Toh-e A. A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae. Genetics. 2001;157:1437–1450. doi: 10.1093/genetics/157.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mah A.S., Jang J., Deshaies R.J. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc Natl Acad Sci U S A. 2001;98:7325–7330. doi: 10.1073/pnas.141098998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mah A.S., Elia A.E.H., Devgan G., Ptacek J., Schutkowski M., Snyder M., Yaffe M.B., Deshaies R.J. Substrate specificity analysis of protein kinase complex Dbf2-Mob1 by peptide library and proteome array screening. BMC Biochem. 2005;6:22. doi: 10.1186/1471-2091-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegmeier F., Visintin R., Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M., Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzam R., Chen S.L., Shou W., Mah A.S., Alexandru G., Nasmyth K., Annan R.S., Carr S.A., Deshaies R.J. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305:516–519. doi: 10.1126/science.1099402. [DOI] [PubMed] [Google Scholar]
- 31•.Queralt E., Lehane C., Novak B., Uhlmann F. Downregulation of PP2ACdc55 phosphatase by separase initiates mitotic exit in budding yeast. Cell. 2006;125:719–732. doi: 10.1016/j.cell.2006.03.038. [DOI] [PubMed] [Google Scholar]; Description of PP2ACdc55 downregulation as trigger for Cdc14 release in early anaphase and development of a quantitative model to describe the contributions of FEAR and MEN to mitotic exit.
- 32.Wang Y., Ng T.-Y. Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:80–89. doi: 10.1091/mbc.E04-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queralt E., Uhlmann F. Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase. J Cell Biol. 2008;182:873–883. doi: 10.1083/jcb.200801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stegmeier F., Huang J., Rahal R., Zmolik J., Moazed D., Amon A. The replication fork block protein Fob1 functions as a negative regulator of the FEAR network. Curr Biol. 2004;14:467–480. doi: 10.1016/j.cub.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Elia A.E., Cantley L.C., Yaffe M.B. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 36•.Visintin C., Tomson B.N., Rahal R., Paulson J., Cohen M., Taunton J., Amon A., Visintin R. APC/C-Cdh1-mediated degradation of the Polo kinase Cdc5 promotes the return of Cdc14 into the nucleolus. Genes Dev. 2008;22:79–90. doi: 10.1101/gad.1601308. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration that Cdc5 is the limiting factor for Cdc14 release throughout mitotic exit, and that APCCdh1-dependent Cdc5 destruction is responsible for the return of Cdc14 into the nucleolus in G1.
- 37.D’Aquino K.E., Monje-Casas F., Paulson J., Reiser V., Charles G.M., Lai L., Shokat K.M., Amon A. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell. 2005;19:223–234. doi: 10.1016/j.molcel.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Maekawa H., Priest C., Lechner J., Pereira G., Schiebel E. The yeast centrosome translates the positional information of the anaphase spindle into a cell cycle signal. J Cell Biol. 2007;179:423–436. doi: 10.1083/jcb.200705197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiroli E., Fraschini R., Beretta A., Tonelli M., Lucchini G., Piatti S. Budding yeast PAK kinases regulate mitotic exit by two different mechanisms. J Cell Biol. 2003;106:857–874. doi: 10.1083/jcb.200209097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraschini R., Bilotta D., Lucchini G., Piatti S. Functional characterization of Dma1 and Dma2, the budding yeast homologues of Schizosaccharomyces pombe Dma1 and human Chfr. Mol Biol Cell. 2004;15:3796–3810. doi: 10.1091/mbc.E04-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosl W.J., Li R. Mitotic-exit control as an evolved complex system. Cell. 2005;121:325–333. doi: 10.1016/j.cell.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 42•.Holt L.J., Krutchinsky A.N., Morgan D.O. Positive feedback sharpens the anaphase switch. Nature. 2008;454:353–357. doi: 10.1038/nature07050. [DOI] [PMC free article] [PubMed] [Google Scholar]; The requirement of Cdc14-dependent securin dephosphorylation for its APCCdc20-dependent destruction creates a positive feedback loop that adds abruptness to separase activation at anaphase onset.
- 43.Jaspersen S.L., Morgan D.O. Cdc14 activates Cdc15 to promote mitotic exit in budding yeast. Curr Biol. 2000;10:615–618. doi: 10.1016/s0960-9822(00)00491-7. [DOI] [PubMed] [Google Scholar]
- 44.Murray A.W., Solomon M.J., Kirschner M.W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 45.Yamano H., Gannon J., Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- 46.Parry D.H., O’Farrell P.H. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr Biol. 2001;11:671–683. doi: 10.1016/s0960-9822(01)00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishima M., Pavicic V., Grüneberg U., Nigg E.A., Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 48.Zhu C., Lau E., Schwarzenbacher R., Bossy-Wetzel E., Jiang W. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc Natl Acad Sci U S A. 2006;103:6196–6201. doi: 10.1073/pnas.0506926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruneberg U., Glotzer M., Gartner A., Nigg E.A. The CeCDC-14 phosphatase is required for cytokinesis in the Caenorhabditis elegans embryo. J Cell Biol. 2002;158:901–914. doi: 10.1083/jcb.200202054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito R.M., Perreault A., Peach B., Satterlee J.S., van den Heuvel S. The CDC-14 phosphatase controls developmental cell-cycle arrest in C. elegans. Nat Cell Biol. 2004;6:777–783. doi: 10.1038/ncb1154. [DOI] [PubMed] [Google Scholar]
- 51.Mailand N., Lukas C., Kaiser B.K., Jackson P.K., Bartek J., Lukas J. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol. 2002;4:317–322. doi: 10.1038/ncb777. [DOI] [PubMed] [Google Scholar]
- 52.Krasinska L., de Bettignies G., Fisher D., Abrieu A., Fesquet D., Morin N. Regulation of multiple cell cycle events by Cdc14 homologues in vertebrates. Exp Cell Res. 2007;313:1225–1239. doi: 10.1016/j.yexcr.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 53.Lanzetti L., Margaria V., Melander F., Virgili L., Lee M.-H., Bartek J., Jensen S. Regulation of the Rab5 GTPase-activating protein RN-tre by the dual specificity phosphatase Cdc14A in human cells. J Biol Chem. 2007;282:15258–15270. doi: 10.1074/jbc.M700914200. [DOI] [PubMed] [Google Scholar]
- 54.Wu J., Cho H.P., Rhee D.B., Johnson D.K., Dunlap J., Liu Y., Wang Y. Cdc14B depletion leads to centriole amplification, and its overexpression prevents unscheduled centriole duplication. J Cell Biol. 2008;181:475–483. doi: 10.1083/jcb.200710127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Berdougo E., Nachury M., Jackson P.K., Jallepalli P.V. The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells. Cell Cycle. 2008;7:1184–1190. doi: 10.4161/cc.7.9.5792. [DOI] [PubMed] [Google Scholar]; Creation of a targeted transgenic human cell line in which both Cdc14B alleles have been disrupted. A method that is by far superior to most methods of gene dosage downregulation in tissue culture cells, and that should allow conclusive experiments as to the role of Cdc14 in human cells once combined with Cdc14A targeting.
- 56.Li L., Ernsting B.R., Wishart M.J., Lohse D.L., Dixon J.E. A family of putative tumor suppressors is structrually and functionally conserved in humans and yeast. J Biol Chem. 1997;272:29403–29406. doi: 10.1074/jbc.272.47.29403. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Novelle M.D., Esteban V., Bueno A., Sacristan M.P. Functional homology among human and fission yeast Cdc14 phosphatases. J Biol Chem. 2005;280:29144–29150. doi: 10.1074/jbc.M413328200. [DOI] [PubMed] [Google Scholar]
- 58.Cueille N., Salimova E., Esteban V., Blanco M., Moreno S., Bueno A., Simanis V. Flp1, a fission yeast orthologue of the S. cerevisiae CDC14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J Cell Sci. 2001;114:2649–2664. doi: 10.1242/jcs.114.14.2649. [DOI] [PubMed] [Google Scholar]
- 59.Trautmann S., Wolfe B.A., Jorgensen P., Tyers M., Gould K.L., McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 60•.Wolfe B.A., McDonald W.H., Yates J.R., III, Gould K.L. Phospho-regulation of the Cdc14/Clp1 phosphatase delays late mitotic events in S. pombe. Dev Cell. 2006;11:423–430. doi: 10.1016/j.devcel.2006.07.016. [DOI] [PubMed] [Google Scholar]; Description of the Cdk phosphorylation-dependent regulation of fission yeast Clp1 phosphatase activity that contrasts it from the distinct regulation of budding yeast Cdc14.
- 61.Wolfe B.A., Gould K.L. Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 2004;23:919–929. doi: 10.1038/sj.emboj.7600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hergovich A., Stegert M.R., Schmitz D., Hemmings B.A. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- 63.Kerk D., Templeton G., Moorhead G.B.G. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae and higher plants. Plant Physiol. 2008;146:351–367. doi: 10.1104/pp.107.111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Skoufias D.A., Indorato R.-L., Lacroix F., Panopoulos A., Margolis R.L. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J Cell Biol. 2007;179:671–685. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration that Cdk downregulation is not sufficient for mitotic exit in human cells as long as an unidentified phosphatase is also not activated.
- 65••.Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]; Discovery that calcium-dependent activation of calcineurin phosphatase is required in addition to Cdk downregulation for meiosis II exit. Upon entry into mitotic cycle progression, the presence of a second cyclic phosphatase activity is observed.
- 66••.Nishiyama T., Yoshizaki N., Kishimoto T., Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]; Discovery of calcineurin as a calcium-activated transient phosphatase activity required for meiosis II exit and entry into vertebrate somatic development.
- 67.Chen F., Archambault V., Kar A., Lio P., D’Avino P.P., Sinka R., Lilley K., Laue E.D., Deak P., Capalbo L. Multiple protein phosphatases are required for mitosis in Drosophila. Curr Biol. 2007;17:293–303. doi: 10.1016/j.cub.2007.01.068. [DOI] [PubMed] [Google Scholar]