Abstract
The ability to detect errors and adjust behavior accordingly is essential for maneuvering in an uncertain environment. Errors are particularly prone to occur when multiple, conflicting responses are registered in a situation that requires flexible behavioral outputs. Previous studies have provided evidence indicating the importance of the medial cortical brain regions including the cingulate cortex in processing conflicting information. However, conflicting situations can be successfully resolved, or lead to errors, prompting a behavioral change in the observers. In particular, how does the brain use error signals specifically to adjust behavior on the fly? Here we employ a stop signal task (SST) to elicit errors approximately half of the time in high-conflict trials despite constant behavioral adjustment of the observers. Using functional magnetic resonance imaging, we show greater and, sequentially, less activation in the medial cortical regions when observers made an error, compared to when they successfully resolved high-conflict responses. Errors also evoked greater activity in the cuneus, retrosplenial cortex, insula, and subcortical structures including the thalamus and the region of the epithalamus (the habenula). We further showed that the error-related medial cortical activities are not correlated with post-error behavioral adjustment, as indexed by post-error slowing (PES) in go trial reaction time. These results delineate an error-specific pattern of brain activation during the SST. The results also suggest that the relationship between error-related activity and post-error behavioral adjustment may be more complicated than has been conceptualized by the conflict monitoring hypothesis.
Keywords: no-go, performance monitoring, feedback, cognitive control, supplementary motor area
Introduction
We probably all remember skidding when driving on a snowy day and learning to slow down, brake, and turn. A major goal in the study of brain function is to understand how different parts of the brain orchestrate behavioral adjustments in response to errors. To facilitate this goal, investigators have designed cognitive tasks that are intended to elicit errors and require observers to modify their behavior in response to each error. For instance, in a choice reaction time (RT) task observers must quickly press one of two buttons in response to the appearance of a green arrow: right button when the arrow points to the right, and vice versa. Occasionally a red arrow may appear requiring observers to make an incongruent response: left button for a right-pointing arrow and vice versa. The “red” or incongruent condition requires observers to discard or suppress a habitual response and initiate an alternative action. The incongruent trial thus involves greater conflict and incurs more errors. To use our driving analogy again, reaching work on time, but without an accident requires achieving conflicting goals of speed and safety, just like responding quickly, but still accurately, to both green and red arrows. Functional magnetic resonance imaging (fMRI) has been a powerful tool to understand the brain processes underlying these cognitive events. Numerous previous studies have localized conflict processing and error detection to the cingulate cortex, located in the frontal lobe (Botvinick et al., 1999; Carter et al., 1998; Ito et al., 2003; Ridderinkhof et al., 2004; Taylor et al., 2006; Ullsperger and von Cramon, 2004; Van Veen and Carter, 2002). Recent studies have further elucidated the neural processes linking performance monitoring to behavioral adjustments (Durston et al., 2002; Egner et al., 2004; Garavan et al., 2002; Ridderinkhof et al., 2004). In particular, an earlier fMRI study showed that anterior cingulate cortex (ACC) activates during high conflict situations in a color word Stroop task and ACC activation is correlated both with dorsolateral prefrontal activation and with behavioral adjustments during subsequent trials (Kerns et al., 2004). Notably, error-related ACC activity appeared to correlate less stringently with post-error behavioral adjustment and prefrontal activity during such adjustment (i.e., significantly only with region of interest analysis, Kerns et al., 2004). These findings broadly support the hypothesis that ACC implements a conflict-monitoring function and that engagement of this function leads to the recruitment of cognitive control by the prefrontal cortices (MacDonald et al., 2000). On the other hand, it remains open whether distinct, error-specific mechanisms are involved during cognitive control. Although some studies have shown that, compared to successfully resolved trials, errors elicited greater activation in the cingulate cortices, how medial cortical brain regions further differentiate between the two high-conflict situations remains unclear (Braver et al., 2001; Carter et al., 1998; Kiehl et al., 2001; Rushworth et al., 2004; Van Veen and Carter, 2002). Furthermore, subcortical structures such as the thalamus have been shown to be extensively involved in performance monitoring and feedback processing during cognitive and motor control (Christoffels et al., 2007; Hester et al., 2004; Maltby et al., 2005; Ogawa et al., 2006; Rubia et al., 2007). It would be of interest to explore error-specific activity in the subcortical circuits.
We have two specific aims in this study. Our primary goal is to examine whether errors result in a pattern of medial cortical and subcortical activation that differs from the pattern associated with successfully resolved high-conflict situations. Our secondary goal is to assess the relationship between the brain activity during error detection and subsequent behavioral adjustment. To achieve these aims we employed a stop signal task (SST) widely used to study response inhibition and performance monitoring (Li et al., 2006c; Liotti et al., 2005; Logan and Cowan, 1984; Schachar et al., 2004). The SST requires that observers respond quickly to a frequent signal and withhold their response when a less frequent signal appears. We employed a staircase procedure to ensure that the observers continued to make errors throughout the experiment, despite the ongoing behavioral adjustments following errors. This procedure provides an approximately equal number of stop successes and stop errors to be directly contrasted to examine error-specific processes. Furthermore, we have previously identified the neural correlates of post-error behavioral adjustment (Li et al., 2008). Here we assess whether error-related cingulate activity is related to the extent of post-error behavioral adjustment, as posited by the conflict monitoring hypothesis (Kerns et al., 2004; Van Veen and Carter, 2002, Carter and Van Veen, 2007).
Materials and Methods
Subjects and behavioral task
Forty healthy adults (20 males, 22–42 years of age, all right-handed and using their right hand to respond) were paid to participate in the study. All subjects signed a written consent after details of the study were explained, in accordance to institute guidelines and procedures approved by the Yale Human Investigation Committee. Data of these 40 subjects were examined for the neural correlates of post-error slowing in a previous work (Li et al., 2008).
We employed a simple reaction time task in this stop-signal paradigm (Li et al., 2006; Fig. 1a). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention and eye fixation at the beginning of a go trial. After a randomized time interval (fore-period) anywhere between 1 and 5 s, the dot turned into a circle, which prompted the subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. The remaining one quarter were stop trials. In a stop trial, an additional “X,” the “stop” signal, appeared after and replaced the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. Clearly it would be easier for the subject to withhold the response if the stop signal appeared immediately or early after the go signal, and the reverse applied if the time interval between the stop and the go signals (or the stop-signal delay, SSD) was extended. The SSD started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 64 ms; conversely, if they failed, SSD decreased by 64 ms (Levitt, 1970). There was an inter-trial-interval of 2 s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the fMRI study each subject had a practice session outside the scanner. In the scanner each subject completed four 10-min runs of the task with the SSD updated manually across runs. Depending on the actual stimulus timing (trial varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure we anticipated that the subjects succeeded in withholding their response in approximately half of the stop trials.
FIGURE 1.
(a) stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in “stop” trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP, uniform distribution) following the appearance of the fixation point. The stop signal followed the go signal by a time delay – the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success and stop error trial, respectively. We distinguished go success (G, 97.1 ± 2.7%, mean ± s.d.) and go error (F, 2.9%), and stop success (SS, 50.3 ± 2.6%) and stop error (SE, 49.7%) trials during the task. (b) Go successes were further distinguished by their preceding trial; thus G trials preceded by a G, SS, and SE trial were indicated by pG, pSS, and pSE trials, respectively. Depending on whether they increased or did not increase in RT, compared to the mean RT of all preceding pG trials, pSS and pSE trials were further grouped into pSSi and pSSni, and pSEi and pSEni trials, respectively (not shown here; see Materials and Methods).
To report the general performance of the subjects in the stop signal task, we computed the critical stop signal delay (SSD) and stop signal reaction time (SSR) for individual subjects. Specifically, SSDs across trials were grouped into runs, with each run being defined as a monotonically increasing or decreasing series. We derived a mid-run estimate by taking the middle SSD (or average of two middle SSDs if there was an even number of SSDs) of every second run. The critical SSD was computed by taking the mean of all mid-run SSDs. It was reported that, except for experiments with a small number of trials (less than 30), the mid-run estimate was close to the maximum likelihood estimate of X50 (50% positive response; i.e., 50% SS in the SST, Wetherill et al., 1966). The stop signal reaction time or SSRT was computed for individual subjects by subtracting the critical SSD from the median go trial RT, on the basis of the race model of the stop signal task (Levitt, 1970; Logan, 1994). Briefly, one way to understand the stop signal task is in terms of a horse race model with a go process and a stop process racing toward a finishing line (Logan, 1994). The go process prepares and generates the movement while the stop process inhibits movement initiation: whichever process finishes first determines whether a response will be initiated or not. Importantly, the go and stop processes race toward the activation threshold independently. Thus, the time required for the stop signal to be processed so a response is withheld (i.e., stop signal reaction time or SSRT) can be computed on the basis of the go trial RT distribution and the odds of successful inhibits for different time delays between go and stop signals. This is done by estimating the critical SSD at which a response can be correctly stopped in approximately 50% of the stop trials. Generally speaking, the SSRT is the time required for a subject to cancel the movement after seeing the stop signal. A longer SSRT indicates poor response inhibition (Li et al., 2006a).
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4mm and no gap. Three hundred images were acquired in each run for a total of 4 runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping version 2 (SPM2, Welcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995a). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 10 mm at Full Width at Half Maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
We constructed two statistical models. In the first model, four main types of trial outcome were first distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial (Fig. 1b). An SS or SE trial involves incongruent goals between the prepotency to respond and the motor intention to withhold the response, and thus is “high-conflict,” compared to a G trial. For the evaluation of post-conflict/error processes, G trials were further divided into those that followed a G (pG), SS (pSS) and SE (pSE) trial. A general linear model (GLM) was constructed for each individual subject, with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al., 1995b). Because each G trial was associated with a different reaction time (RT), we entered a column of G trial onset parametrically modulated by its corresponding RT as a regressor in the model. Likewise, because SS and SE trials came with different stop-signal delays (SSD), we entered columns of SS and SE trial onsets parametrically modulated by SSD in the model. Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation was corrected by a first-degree autoregressive or AR(1) model. The GLM estimated the component of variance that could be explained by each of the regressors. In the first-level analysis, we constructed for each individual subject the following statistical contrasts: SS vs. G; SE vs. G; SS vs. SE; pSS vs. pG; pSE vs. pG; pSS vs. pSE.
In a second GLM, G, F, SS, SE trials were first distinguished. We further sub-grouped SS and SE trials according to whether the following go trial (i.e., pSS and pSE, respectively) increased in RT (SSi and SEi) or did not increase in RT (SSni and SEni). To determine whether a pSS/pSE trial increased or did not increase in RT, it was compared to the pG trials that preceded it in time during each session. The pG trials that followed the pSS or pSE trial were not included for comparison because cognitive processes that “trailed behind” could not have a causal effect on the trial in question. Thus, SSi trials were stop successes followed by a pSS trial with an RT > mean RT of all preceding pG trials; and SEni trials were stop errors followed by a pSE trial with an RT ≤ mean RT of all preceding pG trials, and so forth. We obtained “SS vs. SE” (for verification and comparison with the results obtained from the first GLM) and two additional contrasts for this GLM: SSi vs. SSni, and SEi vs. SEni. The latter two contrasts examined for regional activations during stop trial processing that is associated with post-stop trial performance on a within-subject basis (please see Results and Discussion). This second GLM thus provides an alternative model to address the neural correlates of post-error behavioral adjustment.
The con or contrast (difference in β) images of the first-level analysis were then used for the second-level group statistics (random effect analysis; Penny and Holmes, 2004). Brain regions were identified using an atlas (Mai et al., 2003). In Region of interest (ROI) analysis, we used MarsBaR (Brett et al., 2002; http://marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity change for the ROIs. All voxel activations are presented in MNI coordinates. To examine for evidence in support of the conflict monitoring hypothesis, we correlated across all subjects activity of ROIs during errors with post-error slowing in RT. Although power analysis for a new study could be complicated, we have performed a power analysis to estimate the approximate sample size required to detect a linear correlation between the effect size of activity change in an ROI with the adjustment in RT (Kraemer et al., 2006). This power analysis was based on the data collected in a previous study of the correlation between prefrontal activity and stop signal reaction time (Li et al., 2006a). At an alpha=0.05, a sample size of 10, 13, 19, 23, 29, 47, 85 is required to detect a linear correlation (two-sided) at an r of 0.8, 0.7, 0.6, 0.55, 0.5, 0.4, and 0.3, respectively, with a power of 0.8 (Hsieh et al., 1998). Thus, with 40 participants the current study is powered to observe an r of 0.5.
Results
General behavioral performance
Behavioral measures of the stop signal task have been presented in details previously (Li et al., In Press). Briefly, our 40 observers achieved a go trial success rate of 97.1 ± 2.7% (mean ± s.d.), with a mean go trial RT of 547 ± 115 ms, and a stop trial success rate of 50.3 ± 2.6%. The critical stop signal delay and stop signal reaction time as computed by the race model was 332 ± 124 ms and 216 ± 32 ms, respectively. Furthermore, the RT and SSD of stop error trials were highly correlated (0.58<R’s<0.94, all P’s<0.0001, Pearson regression), providing more evidence for the success of the tracking procedure. Our observers also showed a robust post-stop trial slowing in go trial RT: (pSE) 570 ± 127 ms and (pSS) 562 ± 126 ms, compared to (pG) 528 ± 121 ms (all values are mean ± s.d.; p<0.001, paired t test, both for pSE vs. pG and pSS vs. pG).
Error-specific cortical and subcortical processes
Unless otherwise noted, we imposed a threshold on our results by using a p<0.05, corrected for multiple comparisons for the whole brain (family-wise error or FWE computed on the basis of the random field theory, Brett et al., 2004). Both SS and SE activated the anterior cingulate cortices, compared to the low-conflict G trials, corroborating the role of the ACC in conflict/error processing (x=8 mm, y=36 mm, z=32 mm, Z=6.89, 18,368 mm3, and x=0 mm, y=28 mm, z=40 mm, Z=10.10, 39,616 mm3, respectively; Fig. 2a). Compared to SS trials, the SE trials showed greater activation in the dorsal cingulate cortex extending to a medial frontal region (two peaks: x=−4 mm, y=16 mm, z=44 mm, Z=9.92, and x=4 mm, y=8 mm, z=54 mm, Z=8.58, 23,040 mm3; Fig. 2b). Thus, compared to a successfully resolved high-conflict situation, errors appear to engage the cingulate and medial frontal cortices for additional processing.
FIGURE 2.
(a) The anterior cingulate cortex (ACC) showed greater activation both during stop success (SS) and stop error (SE) compared to go (G) trials, consistent with its role in conflict including error monitoring. Paracentral lobule and occipital and parahippocampal gyri showed less activation in SS, and mid-cingulate region and thalamus showed greater activation, compared to G trials, in this midline section. (b) SE trials showed greater activation in the dorsal ACC and medial frontal cortex including the pre-supplementary motor area, compared to SS trials. (c) pSS trials continued to show greater activation (though diminished in magnitude and extent) in the dorsal cingulate while pSE trials showed “deactivation,” compared to pG trials. The “deactivated” areas include medial cortical and paracentral structures, posterior cingulate/precuneus and nucleus accumbens. (d) As a result, compared to pSS trials, pSE trials demonstrated “deactivated” medial cortical regions, at a location posterior to but overlapping those that showed greater activation during SE compared to SS trials. BOLD signals are shown on a single mid-sagittal slice (x=0 mm) of a smoothed structural image. Color bar represents voxel T value.
We then compared pSS and pSE each with pG trials in order to examine brain activations to post-conflict/error processing. Compared to pG trials, pSS trials involved greater activation in the dorsal medial cortical region (x=0 mm, y=16 mm, z=52 mm, Z=4.68, 576 mm3, and x=−8 mm, y=−4 mm, z=52 mm, Z=4.63, 640 mm3; Fig. 2c). Compared to pG trials, pSE trials evoked less activation of a medial cortical cluster involving paracentral lobules (x=8 mm, y=−28 mm, z=60 mm, Z=6.12, 36,224 mm3; Fig. 2c). Thus, in contrast to the greater cingulate and medial frontal activation observed during SE compared to SS trials, the medial cortices “deactivated” during pSE compared to pSS trials (x=−4 mm, y=−8 mm, z=56 mm, Z=8.50, 68,672 mm3; Fig. 2d). The medial brain regions that “deactivated” during pSE trials were located more posteriorly from those that activated during SE trials and encompassed the paracentral lobules. There was significant spatial overlap between the regions showing activation and the regions showing delayed deactivation. Overall, the anterior medial cortex showed greater activation immediately at stop errors, compared to stop successes, and the more posterior region showed less activation following stop errors, compared to the process following stop successes. A medial frontal locus, located in the pre-supplementary motor area or Brodmann’s area 6 (BA 6), showed greater activation on SE trials compared to SS trials but less activation on pSE trials compared to pSS trials. Thus, compared to stop successes, stop errors involved greater and less activation sequentially in the medial cortical regions.
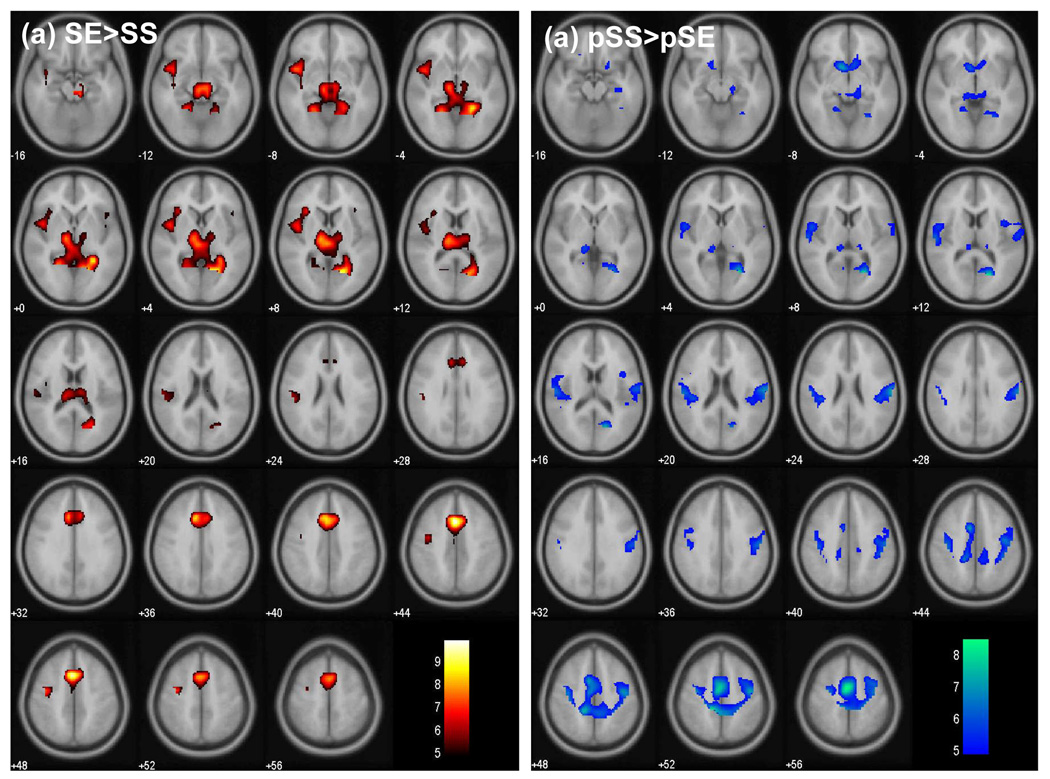
Compared to SS, SE also evoked greater activity in other cortical structures including the retrosplenial (posterior cingulate) cortex, cuneus, insula, and subcortical structures including the thalamus, a region encompassing the epithalamus (the habenular complex), and the midbrain at the same statistical threshold (p<0.05, FWE corrected). This error-specific activation in the thalamus is fairly extensive and appears to involve the pulvinar and all major thalamic subnuclei. These activities are shown in Figure 3a and summarized in Table 1. Furthermore, compared to post-SS go (i.e., pSS) trials, post-SE go (i.e., pSE) trials showed less activation in a number of other cortical and subcortical structures, which included cuneus and retrosplenial cortex, the bottom of caudate head which probably also involved the ventral striatum, some thalamic nuclei, and hippocampus (Figure 3b, Table 2). Notably, thus, similar to the supplementary motor area, the cuneus and retrosplenial cortex along with part of the thalamus also showed sequential greater and less activity following an error.
FIGURE 3.
(a) Regional brain activation during stop error (SE) trials, as compared to stop success (SS) trials. (b) Regional brain activation during post-SS go (pSS) trials, as compared to post-SE go (pSE) trials. BOLD signals are overlaid on a smoothed structural image in axial slices, from z=−16 to z=+56, with adjacent slices 4mm apart. Color bars represented voxel t values. Activated peaks are summarized in Table 1 (SE>SS) and Table 2 (pSS>pSE).
Table 1.
Brain regions showing greater activation during SE, compared to SS
| cluster size (voxels) | voxel Z value | MNI coordinate (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 360 | 6.97 | −4 | 16 | 44 | L | dorsal anterior cingulate cortex |
| 6.39 | 4 | 8 | 64 | R | supplementary motor area | |
| 6.35 | −8 | 20 | 36 | L | dorsal anterior cingulate cortex | |
| 767 | 6.69 | 16 | −64 | 8 | R | Cuneus/retrosplenial cortex |
| 6.43 | 28 | −48 | 0 | R | Retrosplenial cortex | |
| 6.09 | −4 | −24 | 8 | L | Cuneus/retrosplenial cortex | |
| 187 | 5.93 | −48 | 8 | −8 | L | Insula |
| 5.60 | −44 | 4 | 4 | L | Insula/probably inferior frontal G | |
| 5.39 | −32 | 24 | 8 | L | Insula/probably inferior frontal G | |
| 34 | 5.75 | −36 | −8 | 52 | L | Superior frontal/precentral G |
| 36 | 5.07 | −48 | −28 | 24 | L | Superior temporal G |
| 7 | 4.47 | 44 | 16 | 0 | R | Insula |
Note: p<0.05, FWE corrected, and 5 voxels in extent of activation; all peak activations 8mm apart are shown; G=gyrus
Table 2.
Regions showing greater activation during post-SS go, compared to post-SE go trials.
| cluster size (voxels) | voxel Z value | MNI coordinate (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1073 | 6.36 | -4 | -8 | 56 | L | Supplementary motor area |
| 6.20 | -16 | -36 | 52 | L | Paracentral lobules | |
| 5.75 | -36 | -12 | 52 | L | Paracentral lobules | |
| 92 | 6.21 | 16 | -68 | 12 | R | Cuneus/Retrosplenial cortex |
| 6.14 | 24 | -64 | 4 | R | Cuneus/Retrosplenial cortex | |
| 4.74 | 16 | -56 | -4 | R | Cuneus | |
| 56 | 5.64 | -12 | 12 | -8 | L | Caudate head/ventral striatum |
| 4.95 | 8 | 12 | -8 | R | Caudate head/ventral striatum | |
| 4.33 | 12 | 28 | -8 | R | Caudate head | |
| 59 | 4.91 | -16 | -32 | 0 | L | Pulvinar |
| 4.79 | -12 | -28 | 8 | L | Pulvinar | |
| 4.73 | 16 | -20 | -12 | R | Midbrain/ventroposterior lateral thalamus | |
| 6 | 4.80 | 20 | 12 | -16 | R | Hippocampus |
| 11 | 4.74 | -16 | -52 | -4 | L | Cuneus |
| 15 | 4.67 | 40 | 4 | 12 | R | Postcentral G |
| 13 | 4.66 | 28 | -20 | -28 | R | Hippocampus |
| 5 | 4.57 | 16 | -28 | 8 | R | Pulvinar |
Note: p<0.05, FWE corrected, and 5 voxels in extent of activation; all peak activations 8mm apart are shown; G=gyrus
Relating error processing to post-error behavioral adjustment – a between-subject approach
We have identified a pattern of cingulate/medial cortical activity specific to error processing. Our earlier work identified activation in the ventrolateral prefrontal cortex (VLPFC) during post-error slowing in go trial reaction time (Li et al., 2008). A logical next step is to examine whether the cingulate/medial cortical conflict/error monitoring process is related to behavioral adjustment carried out by the prefrontal cortices? Previous studies have addressed this question by correlating cingulate/medial frontal activity during conflict/error processing with subsequent behavioral adjustment (Debener et al., 2005; Gehring and Fencsik, 2001; Hajcak et al., 2003; Kerns et al., 2004; Murphy et al., 2006; Riba et al., 2005; Ridderinkhof et al., 2002; Tsai et al., 2005; see also Discussion). We thus implemented the same analysis. The results showed that the effect size of the activity change of the dorsal anterior cingulate cluster during SE>G or SS>G (Fig. 2a) did not correlate with the pSE or pSS slowing in RT (r=0.163, p=0.315 and r=0.043, p=0.793, respectively, Pearson regression).
Relating error processing to post-error behavioral adjustment – a within-subject approach
The second GLM examined whether a “within-subject” design would reveal the error processes that are specifically associated with post-error behavioral adjustment. The results showed that SEi and SEni trials did not differ in regional brain activation even at a p<0.01, uncorrected and 5 voxels in the extent of activation. Compared to SSni trials, SSi trials involved greater activation in two small clusters: one in the orbital part of the right inferior frontal gyrus (IFG, x=32mm, y=24mm, z=−8mm, 704 mm3, Z=3.57) and the other in the right posterior superior temporal gyrus (STS, x=56mm, y=−44mm, z=8mm, 448 mm3, Z=3.35), at a p<0.001, uncorrected and 5 voxels in the extent of activation. Activity in neither cluster is significantly correlated with pSS slowing (r=0.077, p=0.635, IFG; r=0.046, p=0.776, STS; Pearson regressions). No brain regions showed greater activation during SSni, compared to SSi trials (p<0.01, uncorrected, 5 voxels).
Discussion
Cortical and subcortical processing of errors
By contrasting stop error and stop success trials, and post-error and post-success trials, respectively, the current study isolates cortical and subcortical regions that show greater and less activation sequentially following an error. Thus for our first aim, we have identified a specific pattern of regional brain activation during error trials, in contrast to successfully resolved high conflict trials. The greater activation of the dorsal anterior cingulate cortex (ACC) during errors, compared to successfully resolved stop trials, replicated previous imaging findings obtained with different behavioral tasks (Braver et al., 2001; Carter et al., 1998; Kiehl et al., 2001). For instance, examining the ubiquity of ACC activation during low-frequency responding, irrespective of task conditions, Braver and colleagues observed greater ACC activation to errors for both the low-frequency and equal-frequency conditions, as well as to low-frequency errors, compared to low-frequency correct trials (Braver et al., 2001). Although the voxel of peak activation (x=−1 mm, y=21 mm, z=27 mm, Talairach coordinate) appeared to be spatially inferior to what we observed here (x=−4 mm, y=16 mm, z= 44 mm), the two cingulate clusters overlapped. The findings of greater ACC activity during both SS and SE as compared to go trials were also consistent with recent literature of a role of ACC in predicting error likelihood (Brown and Braver, 2005; Brown and Braver, 2007). It is not unlikely that, as with motor brain regions that activate to both motor response and motor preparation, the ACC activates both in anticipation and during the occurrence of an error.
Compared to pG trials, pSS trials activated the supplementary motor area (SMA)/pre-SMA region, perhaps suggesting greater attention to action as a result of the alerting effect of the (stop) signal (Lau et al., 2004). In contrast, brain regions including the paracentral lobules “deactivated” during pSE, compared to pG trials. Thus, a novel finding concerns “deactivation” of the medial cortical regions during post-stop error, compared to post-stop success processing. This error-specific pattern of brain activation is also observed in the cuneus and retrosplenial cortex, both located in the midline of the brain. These midline cortical regions are part of the “default mode” circuitry which deactivates during a cognitive task, compared to a relaxed, awake mental state (Greicius and Menon, 2004; Raichle et al., 2001; Shulman et al., 1997; Tomasi et al., 2006). The transition from activation to deactivation thus appears to signal a switch in mental state in preparation for subsequent trials after participants make an error.
We observed greater activity in the thalamus, insula, and retrosplenial cortex during SE, compared to SS, replicating earlier work implicating these structures in error detection and feedback processing (Christoffels et al., 2007; Hester et al., 2004; Tsukamoto et al., 2006; Wrase et al., 2007). Greater activity in the insula and retrosplenial cortex might reflect differential affective responses during errors, as compared to successfully resolved conflicts (Critchley, 2005; Maddock, 1999; Phan et al., 2004). In particular, a region in the epithalamus involving the habenular complex is engaged in this error-specific processing. The habenula is a diencephalic structure located dorsomedial to the caudal thalamus and, via its regulation of monoaminergic and cholinergic neurotransmission throughout the forebrain, is implicated in various aspects of cognition (see Lecourtier and Kelly, 2007 for a review). Both animal and human studies implicated habenula in processing negative reinforcement or error feedback (Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007; Shepard et al., 2006; Ullsperger et al., 2003).
A medial cortical – prefrontal circuitry for cognitive control?
The medial frontal including the anterior cingulate cortex (ACC) has been implicated in conflict/error monitoring and cognitive control. An important thesis of this hypothesis is that the cingulate activity during conflict/error processing will predict changes in behavioral performance following high conflict situations and errors. However, previous imaging including ERP studies that correlated the magnitude of error-related electrical signals and post-error behavioral adjustments have yielded less than consistent results (Debener et al., 2005; Gehring and Fencsik, 2001; Hajcak et al., 2003; Murphy et al., 2006; Riba et al., 2005; Ridderinkhof et al., 2002; Tsai et al., 2005; see also Carter and Van Veen, 2007 for a review). For instance, sleep deprivation impaired post-error slowing during a RT task with decreased ERN in one study but not another (Murphy et al., 2006; Tsai et al., 2005); although alcohol consumption led to decreased ERN amplitude and eliminated post-error reduction of interference, alprazolam appeared to show a similar effect on ERN without influencing post-error slowing in flanker tasks (Riba et al., 2005; Ridderinkhof et al., 2002). In the current study we have not observed a significant correlation between the extent of cingulate/medial frontal activation and pSE or PSS slowing. This finding is thus at odds with some previous reports (Debener et al., 2005; Kerns et al., 2004; Ridderinkhof et al., 2002; Tsai et al., 2005) but consistent with others (Gehring and Fencsik, 2001; Hajcak et al., 2003; Murphy et al., 2006; Riba et al., 2005). Also of interest here is a recent neuropsychological study showing that dorsal ACC lesions did not impair post-error slowing in the Stroop and go/no-go task in humans (Fellows and Farah, 2005).
One possibility is that the between-subject analyses might not have been ideal to isolate the neural substrates whose activities are to be correlated with behavioral performance of individual subjects. Our second model based on a within-subject design thus represented a useful alternative approach. At a liberal threshold, this within-subject analysis similarly did not yield cingulate activation prior to post-stop slowing in RT. Note that the finding of ventrolateral prefrontal activity during post-error slowing in Li et al., 2008 and the lack of finding of error-related regional activation in the current study are by no means inconsistent as they involved contrasts at two distinct time points. Taken together, these results fail to provide evidence for conflict/error-related activity preceding behavioral adjustment in the stop signal task. Importantly, one should be cautioned that the current as well as the previous studies are only powered to detect moderate associations. Furthermore, the analyses presented here are by no means exhaustive and the results do not nullify a role of the medical cortical – prefrontal circuitry in cognitive control.
The discrepancy between these results could also reflect important differences in behavioral paradigm and experimental condition. For instance, in the fMRI study of Kerns and colleagues, participants made relatively fewer errors and theoretically participants could perform the task without making any errors (Kerns et al., 2004, Supporting Online Material). In our tracking stop signal task, on the other hand, observers were bound to make mistakes as a result of the tracking procedure (50% of stop trials). Thus, error as well as successfully resolved high-conflict trials could evoke very different psychological processes in the two task conditions. The exact location of the regional brain activation could also be a source of disparity. Compared to low conflict (G) trials, our high-conflict (SS) and error (SE) trials appeared to involve activation extending to the medial frontal region including the supplementary motor area (SMA), in contrast to the more restricted dorsal anterior cingulate activation of Kerns et al. (Compare Fig. 2 with Figure 2 of Kerns et al., 2004). Further studies would be required to investigate whether distinct cognitive control mechanisms are implemented under different behavioral conditions.
Another issue is that behavioral adjustment in different “cognitive control” tasks may involve very different outcome measures. In the current as well as many of the aforementioned studies, behavioral adjustment is defined solely in terms of RT change following an error or high-conflict situation. However, many other studies required adjustment by, for instance, attending to a different stimulus-response rule, which entailed behavioral outcomes that could not be as readily captured by RT changes (Crone et al., 2006; Liston et al., 2006; Walton et al., 2004). Thus, although performance monitoring during these diverse cognitive tasks invariably involves medical cortical activity, whether and how this medial cortical activity relates to cognitive control needs to be examined with respect to the specificity of task requirement.
Relevance to macaque studies on cognitive control
Unit recordings in behaving primates have also investigated the role of medial cortical structures in conflict monitoring, error processing and cognitive control (Amiez et al., 2005; Ito et al., 2003; Kennerley et al., 2006; Nakamura et al., 2005; Shima and Tanji, 1998; Stuphorn and Schall, 2006). Of particular interest are the studies showing that the ACC may not be crucial for updating behaviors but keeps track of the local history of reinforcement during decision making (Amiez et al., 2005; Kennerley et al., 2006; Shima and Tanji, 1998). For instance, ACC lesions did not impair monkeys’ post-error performance but hamper their ability to make optimal decisions in a dynamic foraging task, in which risk and reward were to be integrated to guide actions (Kennerley et al., 2006). Our current results showed that the magnitude of ACC activity during stop trials did not appear to be correlated with the RT adjustment of post-stop trials, thus in agreement with the latter recording studies.
Conclusions and implications
We have identified an error-specific pattern of medial cortical and subcortical activity during a visuomotor task. The results extend our understanding of cognitive control by delineating the neural processes underlying error processing, but the mechanisms linking error processing and post-error behavioral adjustment need to be further examined. The medial cortical regions have been shown to respond to a wide range of human behaviors, including social exchanges, in which error processing assumes a critical role (Somerville et al., 2006; Tomlin et al., 2006). The unique pattern of medial cortical activity that we have identified may thus have implications that go beyond the current cognitive task. Furthermore, activity related to decision making and other goal-directed operations have been located in the medial frontal cortices (Matsumoto and Tanaka, 2004; Ridderinkhof et al., 2004; Rushworth et al., 2004). It would be of interest to explore whether the sequential medial frontal activation and “deactivation” reflects processes other than those merely to “get ready.” Finally, errors occur in the stop signal paradigm as a result of observers’ own action, but they also occur in many circumstances as a result of external feedback. Further studies could examine whether distinct pathways mediate these two forms of error-specific cognitive control (Walton et al., 2004).
Acknowledgements
This study was supported by the Yale Interdisciplinary Women's Health Research Scholar Program on Women and Drug Abuse (Mazure), funded by the NIH Office of Research on Women's Health and the National Institute on Drug Abuse, a Clinician Scientist Award in Substance Abuse Research (Rounsaville), NIH grants (Sinha; Li), the Alcoholic Beverage Medical Research Foundation (Li), and a Clinical Translational Science Award (Sherwin), to Yale University. We thank Mark Laubach, Daeyeol Lee, James Mazer, Nandakumar Narayanan, Camillo Padoa-Schioppa, and Maolin Qiu for their most helpful discussions and comments on earlier versions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. [Google Scholar]
- Brett M, Penny W, Kiebel S. Introduction to random field theory. In: Frackowiak, et al., editors. Human Brain Function. Second Edition. San Diego, California: Elsevier/Academic Press; 2004. pp. 867–879. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver T, Barch D, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of Right Inferior Frontal Gyrus during Response Inhibition across Response Modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Christoffels IK, Formisano E, Schiller NO. Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Hum Brain Mapp. 2007;28:868–879. doi: 10.1002/hbm.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16:908–920. doi: 10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. NeuroImage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage. 2004;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Electroencephalogr. Effects of cross-modal divided attention on late ERP components: II. Error processing in choice reaction time tasks. Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Neural correlates of error detection and error correction: is there a common neuroanatomical substrate? Eur J Neurosci. 2004;19:3081–3087. doi: 10.1111/j.0953-816X.2004.03414.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiol. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating Attentional Control Using Event-Related fMRI. Cereb Cortex. doi: 10.1093/cercor/bhj116. In Press. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hsieh F, Bloch D, Larsen M. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiol. 2000;37:216–223. [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable T, Sinha R. Imaging response inhibition in a stop signal task – neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable T, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006b;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural Correlates of Posterror Slowing during a Stop Signal Task: A Functional Magnetic Resonance Imaging Study. J Cogn Neurosci. doi: 10.1162/jocn.2008.20071. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006c;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Multimodal mechanisms of attention related to rates of spatial shifting in vision and touch. Exp Brain Res. 2001;137:445–454. doi: 10.1007/s002210000656. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Asheuer JK. Atlas of the Human Brain. Second Edition. New York, NY: Academic Press; 2003. [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol. 2004;14:178–185. doi: 10.1016/j.conb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Murphy TI, Richard M, Masaki H, Segalowitz SJ. The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? J Sleep Res. 2006;15:15–21. doi: 10.1111/j.1365-2869.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res Cogn Brain Res. 2001;12:225–231. doi: 10.1016/s0926-6410(01)00053-2. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Inui T, Sugio T. Separating brain regions involved in internally guided and visual feedback control of moving effectors: an event-related fMRI study. Neuroimage. 2006;32:1760–1770. doi: 10.1016/j.neuroimage.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP. Random-effects analysis. In: Frackowiak, et al., editors. Human Brain Function. San Diego: Elsevier; 2004. pp. 843–850. [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Pierno AC, Becchio C, Wall MB, Smith AT, Turella L, Castiello U. When gaze turns into grasp. J Cogn Neurosci. 2006;18:2130–2137. doi: 10.1162/jocn.2006.18.12.2130. [DOI] [PubMed] [Google Scholar]
- Rabbit PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Münte TF, Barbanoj MJ. A neurophysiological study of the detrimental effects of alprazolam on human action monitoring. Cogn Brain Res. 2005;25:554–565. doi: 10.1016/j.cogbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GPH. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes, Schizophr. Bull. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RI, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. NeuroImage. 2005;25:600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cognit Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J. Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin D, Kayali MA, King-Casas B, Anen C, Camerer CF, Quartz SR, Montague PR. Agent-specific responses in the cingulate cortex during economic exchanges. Science. 2006;312:1047–1050. doi: 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- Tsai L-L, Young H-Y, Hsieh S, Lee C-S. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28:707–713. doi: 10.1093/sleep/28.6.707. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Kotani Y, Ohgami Y, Omura K, Inoue Y, Aihara Y. Activation of insular cortex and subcortical regions related to feedback stimuli in a time estimation task: an fMRI study. Neurosci Lett. 2006;399:39–44. doi: 10.1016/j.neulet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cognit Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wetherill GB, Chen H, Vasudeva RB. Sequential estimation of quantal response curves: A new method of estimation. Biometrika. 1966;53:439–454. [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS. Sustained neural activity associated with cognitive control during temporally extended decision making. Brain Res Cogn Brain Res. 2005;23:71–84. doi: 10.1016/j.cogbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]