Abstract
Eleven different laboratory strains of Streptococcus mutans representing the various serogroups were found to produce an average of 6.0 +/- 4.8 mM acetoin when grown in glucose-containing medium under aerobic conditions. None of the strains produced detectable acetoin when grown anaerobically. A lactate dehydrogenase-deficient mutant produced acetoin both aerobically and anaerobically and in substantially greater amounts than the wild-type strains did. Substitution of mannitol for glucose resulted in decreased acetoin production by wild-type strains and the lactate dehydrogenase-deficient mutant, indicating a role for NADH2 in the regulation of the acetoin pathway. Pyruvate incorporated into the growth medium of a wild-type strain caused acetoin to be produced anaerobically and stimulated acetoin production aerobically. Cell extracts of a wild-type S. mutans strain were capable of producing acetoin from pyruvate and were (partly) dependent on thiamine PPi. Extracts prepared from aerobically grown cells had approximately twice the acetoin-producing activity as did extracts prepared from anaerobically grown cells. The results indicate that acetoin production by S. mutans may represent an auxiliary reaction of pyruvate dehydrogenase in this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkonyi I., Bolygó E., Gyócsi L., Szabó D. Studies on the formation of acetoin from acetaldehyde by the pyruvate dehydrogenase complex and its regulation. Eur J Biochem. 1976 Jul 15;66(3):551–557. doi: 10.1111/j.1432-1033.1976.tb10581.x. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Ethanol production and alcohol dehydrogenase activity in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):127–131. doi: 10.1016/0003-9969(73)90027-7. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryn K., Ulstrup J. C., Stormer F. C. Effect of acetate upon the formation of acetoin in Klebsiella and Enterobacter and it possible practical application in a rapid voges-proskauer test. Appl Microbiol. 1973 Mar;25(3):511–512. doi: 10.1128/am.25.3.511-512.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Kujala U., Edlund M. B. Pyruvate dehydrogenase activity in Streptococcus mutans. Infect Immun. 1985 Sep;49(3):674–678. doi: 10.1128/iai.49.3.674-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Simplified gas chromatographic procedure for identification of bacterial metabolic products. Appl Microbiol. 1973 Feb;25(2):287–289. doi: 10.1128/am.25.2.287-289.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinan D. F., Robin S., Cogan T. M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl Environ Microbiol. 1976 Apr;31(4):481–486. doi: 10.1128/aem.31.4.481-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. B., Melville T. H. Fermentation end-products of cariogenic and non-cariogenic streptococci. Arch Oral Biol. 1968 May;13(5):565–570. doi: 10.1016/0003-9969(68)90117-9. [DOI] [PubMed] [Google Scholar]
- Gabriel M. A., Jabara H., al-Khalidi U. A. Metabolism of acetoin in mammalian liver slices and extracts. Interconversion with butane-2,3-diol and biacetyl. Biochem J. 1971 Oct;124(4):793–800. doi: 10.1042/bj1240793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Role of citritase in acetoin formation by Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1961 Dec;82:954–959. doi: 10.1128/jb.82.6.954-959.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978 Jul;21(1):206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L., Bryn K., Stormer F. C. Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J Bacteriol. 1975 Sep;123(3):1124–1130. doi: 10.1128/jb.123.3.1124-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
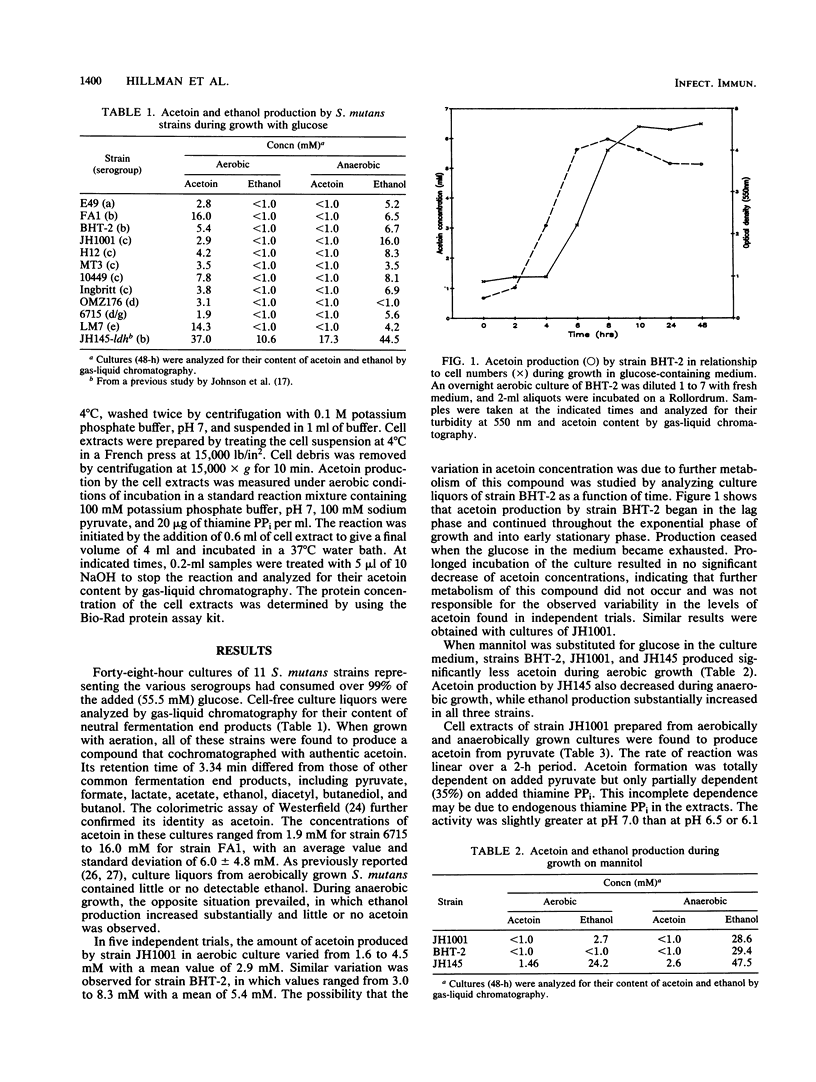
- Johnson C. P., Gross S. M., Hillman J. D. Cariogenic potential in vitro in man and in vivo in the rat of lactate dehydrogenase mutants of Streptococcus mutans. Arch Oral Biol. 1980;25(11-12):707–713. doi: 10.1016/0003-9969(80)90124-7. [DOI] [PubMed] [Google Scholar]
- López J., Thoms B., Fortnagel P. Mutants of Bacillus subtilis blocked in acetoin reductase. Eur J Biochem. 1973 Dec 17;40(2):479–483. doi: 10.1111/j.1432-1033.1973.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Dzink J. L., Smith C. M. Chemically defined medium for oral microorganisms. J Clin Microbiol. 1985 Aug;22(2):303–305. doi: 10.1128/jcm.22.2.303-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer F. C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS Lett. 1968 Nov;2(1):36–38. doi: 10.1016/0014-5793(68)80094-8. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Abbe K., Yamada T. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982 Mar;149(3):1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Takahashi-Abbe S., Abbe K. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1985 Jan;47(1):129–134. doi: 10.1128/iai.47.1.129-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


