Abstract
Norepinephrine transporter (NET) and serotonin transporter (SERT) proteins regulate norepinephrine (NE) and serotonin via their reuptake function and are targets of antidepressants action. Several intravenous anesthetics have been shown to inhibit NET and SERT. The interactions between antidepressants and anesthetics on transporter function, however, are not well studied. We examined the effect of different IV anesthetics on NET and SERT function, with and without chronic antidepressant pretreatment, by measuring NE or 5-hydroxytryptamine (5-HT) uptake and determined NET and SERT protein expression via immunoblotting. Both ketamine and propofol inhibited NET dose-dependently (propofol 10−4 M −22% ± 5.6%, and propofol 10−3 M −35% ± 5.7%; ketamine 10−4 M −23% ± 4.1% and ketamine 10−3 M −73% ± 2.9%); and SERT (propofol 10−4 M −11% ± 4.3% and propofol 10−3 M −23% ± 3.8%; ketamine 10−4 M −29% ± 5.2% and ketamine 10−3 M −63% ± 6.4%). Etomidate and thiopental had no effect on either NET or SERT function. Desipramine and fluoxetine, specific inhibitors of NET and SERT, respectively, both enhanced the inhibitory effects of propofol but reduced the inhibitory effects of ketamine on NET and SERT functions. IV anesthetics treatment did not change transporter protein expression in the presence of its respective inhibitor. Our results demonstrate that both ketamine and propofol inhibited SERT and NET function, but the inhibition were differentially modulated by antidepressants. Therefore, in the clinical context, this would suggest that patients receiving antidepressant treatments might have altered response for intravenous anesthetics in an agent-specific manner.
Keywords: Antidepressant medication, Human embryonic kidney 293 cells, HEK-hNET transfected cells, HEK-hSERT transfected cells, Intravenous anesthetics, NET transporter, SERT transporter
1. Introduction
Depression affects between 12% and 17% of the population during the lifetime of most Americans. Pharmacotherapy with antidepressants is commonly used to treat depression and other affective disorders. Although the mechanisms for the action of these antidepressants are not precisely understood, their principal target of action is at the monoamine transporter proteins located at nerve endings. Monoamine neurotransmitter transporters act to terminate synaptic neurotransmission. Serotonin transporter (SERT) and norepinephrine transporter (NET) are two transporter proteins1-3 targeted by clinically prescribed antidepressants. 3-7 They actively take up serotonin and norepinephrine (NE), respectively, following their release and thereby regulate neurotransmitter levels at the neuroeffector junction.2,8-10
Most intravenous anesthetics used to induce general anesthesia exert their effects through stimulation of gamma-aminobutyric acid (GABA) receptors, and others exert their effects through antagonism of the N-methyl-D[ED3]-aspartate (NMDA) receptors11 in the central nervous system (CNS). Propofol and ketamine, two intravenous anesthetics, are GABA agonists or NMDA antagonists, respectively, and they affect NET function and alter neurotransmission.12-15 However, little is known regarding whether IV anesthetics also influence SERT function, the transporter protein that is the target of the newer class of antidepressants, the selective serotonin reuptake inhibitors (SSRI). Moreover, the actions of IV anesthetics on transporter proteins following their chronic inhibition by antidepressant drugs have not been specifically investigated.
In the present study, we compared the effects of different IV anesthetics on both NET and SERT. We also examined the effect of chronic treatment with the antidepressants desipramine and fluoxetine, an NET inhibitor and a SERT inhibitor, respectively, on the effects of IV anesthetics on NET and SERT function and protein expression.
2. Materials and methods
Except where noted, all chemicals were purchased from Sigma (St Louis, MO, USA). Protease inhibitor mixtures were obtained from Roche (Roche Molecular Biochemicals, Indianapolis, IN, USA). Mouse anti-human NET antibody was obtained from Mab Technologies (Stone Mountain, GA, USA). Anti-rat SERT antibody was purchased from Oncogene (Oncogene Research Products, La Jolla, CA, USA). Enhanced-chemiluminescence (ECL) solutions and secondary antibody were obtained from Amersham (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Radioactively labeled norepinephrine ([3H]-NE) and 5-hydroxytryptamine ([3H]-5-HT) were purchased from NEN (New England Nuclear, MA, USA). Precast gels (10% sodium dodecylsulfate, SDS) were obtained from Novex (Carlsbad, CA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal calf serum (dialyzed), G418 (Genitin) were purchased from Invitrogen (Carlsbad, CA, USA). Cell culture plates and other materials used for cell culture were purchased from Fisher (Pittsburgh, PA, USA). Propofol was obtained from AstraZeneca Pharmaceuticals, (Wilmington, DE, USA) and etomidate purchased from Abbott Laboratories (Chicago, IL, USA).
Human embryonic kidney 293 cells (HEK-293) were stably transfected with the corresponding cDNA of NET (HEK-hNET) or SERT (HEK-hSERT), a gift from Dr Blakely from Vanderbilt University.7 Cell culture condition and experimental design was referenced from Zhu et al.7 The incubation times for [3H]-NE uptake by HEK-hNET and [3H]-5-HT uptake by HEK-hSERT were from 5 minutes to 60 minutes to establish optimal duration of incubation. HEK-hNET and HEK-hSERT cells were treated with different IV anesthetics from 10−7 M to 10−3 M concentrations at 37°C for 30 minutes.
In order to determine the modulatory effect of antidepressants on IV anesthetics on SERT and NET function, HEK-hSERT and HEK-hNET cells were pretreated for 24 hours with 10−6 M of fluoxetine or desipramine, respectively. The cells were then washed and incubated in medium without any transporter inhibitors before they were exposed to different concentrations of propofol or ketamine. Uptake of [3H]-NE by HEK-hNET or [3H]-5-HT by HEK-hSERT cells was performed at 37°C for 10 minutes in buffer of pH 7.4. The buffer contained: in mM, NaCl 130, KCl 1.3, CaCl2 2.2, MgSO4 1.2, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, KH2PO4 1.2; and dextrose 1.8 g/L, iproniazid 10 μM, troplone 10 μM and L-ascorbic acid 100 μM. Desipramine (10 μM) for NET or fluoxetine (10 μM) for SERT were used for non-specific uptake. The specific uptake was obtained from subtracting non-specific uptake from total uptake.
Immunoblotting is used for detecting protein expression and protocols were modified from Zhu et al.7 The membrane proteins were prepared from HEK-hSERT or HEK-hNET cells after drug treatments using homogenized buffer contained 10 mM Tris, 150 mM NaCl, 1mM ethylene diamine tetraacetic acid (EDTA), 0.1% SDS and 1% Triton X-100 pH 7.4, supplemented with protease inhibitors. Membrane protein (40 μg) was used to determine protein expression following propofol or ketamine treatment in the presence or absence of fluoxetine (10−6 M) for SERT or desipramine (10−6 M) for NET for 24 hours. The proteins were size-fractioned on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel and then electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked in 5% non-fat milk in phosphatebuffered saline (PBS) Tween 20 and then exposed to a polyclonal antibody against either NET or SERT at a 1:1000 dilution overnight at 4°C. Anti-mouse or anti-rabbit secondary antibody was used at a 1:3000 dilution for 1 hour at room temperature. Antibody binding was detected by enhanced chemiluminescence, as recommended by the manufacturer. The same PVDF membranes used for immunoblotting were stripped with an antibody to α-actin to quantify the loading of protein. The signal intensity was quantified using densitometry after X-ray film development. Drug toxicity effects were assessed using trypan blue and no toxicity effects were seen with any drug treatment.
Data were analyzed by analysis of variance (ANOVA) and presented as mean ± standard error. The median effective concentration (EC50) values were calculated with Prism 3.0 (GraphPad Software, San Diego, CA). Individual comparisons between two means were analyzed by student t test. A level of p < 0.05 was considered significant.
3. Results
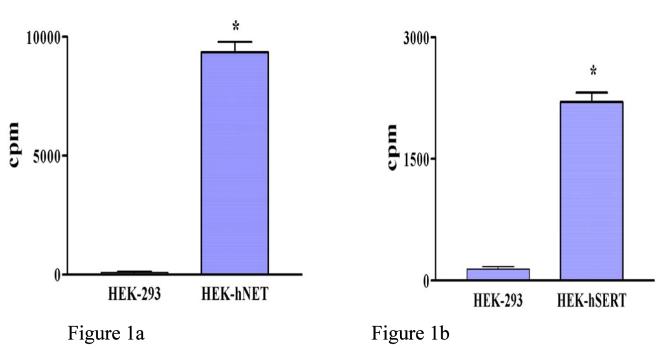
The specificity of uptake of [3H]-NE by HEK-hNET (n = 3) and [3H]-5-HT by HEK-hSERT (n = 3) is illustrated in Figure 1. HEK-293 cells (n = 3) are used as controls.
Fig. 1.
Human embryonic kidney 293 cells (HEK-293) transfected with the corresponding cDNA of norepinephrine transporter (NET) (HEK-hNET) and serotonin transporter (HEK-hSERT) specifically take up (a) labeled norepinephrine ([3H]-NE) (n = 3) or (b) labeled 5-hydroxytryptamine ([3H]-5-HT), respectively, but human embryonic kidney (HEK 293) cells (n = 3) do not. * p < 0.05.
Both propofol and ketamine significantly inhibited NE uptake at concentrations ≥ 10−4 M. Propofol inhibited NE uptake by −22% ± 5.6% (n = 15) at 10−4 M and by −35% ± 5.7% (n = 15) at 10−3 M. Ketamine inhibited uptake by −23% ± 4.1% (n = 15) at 10−4 M and −73% ± 2.9% (n = 14) at 10−3 M (Fig. 2). [3H]-5-HT uptake in HEK-hSERT cells after drug treatment was performed to test the effects of IV anesthetics on SERT function. Similar to the effects on NET function, both ketamine and propofol significantly inhibited [3H]-5HT uptake by HEK-hSERT in a dose-dependent manner. Propofol inhibited 5-HT uptake by −10.4% ± 4.3%, (n = 11) at 10−4 M and by −22.7% ± 3.8% (n = 9) at 10−3 M. Ketamine inhibited uptake by −29.2% ± 5.2% (n = 17) at 10−4 M and −63.2% ± 6.4% (n = 16) at 10−3 M (Fig. 3). Etomidate and thiopental failed to have any effect on either NET or SERT function. The EC50 for NE and 5-HT uptake after different IV anesthetics treatments are shown in Table 1.
Fig. 2.
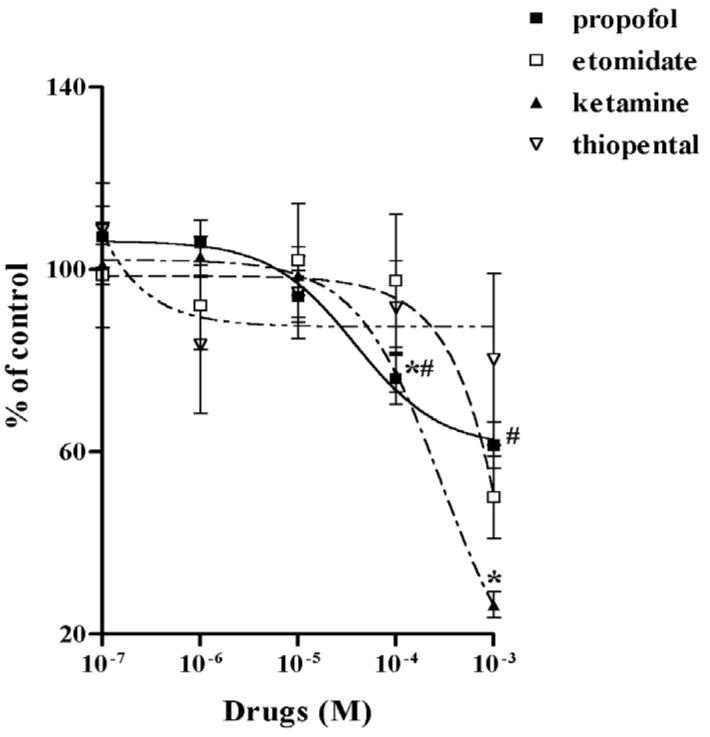
The concentration responses of intravenous anesthetics on labeled norepinephrine ([3H]-NE) uptake in human embryonic kidney cells transfected with the cDNA of norepinephrine transporter (NET) (HEK-hNET) [ED4]transfected cells are shown for ketamine (solid triangle), propofol (solid square), etomidate (open square) and thiopental (open triangle). No evidence of inhibition was seen with etomidate and thiopental. Results are expressed as percentage of uptake in the absence of the drug. # p < 0.05 vs no propofol; * p < 0.05 vs no ketamine.
Fig. 3.
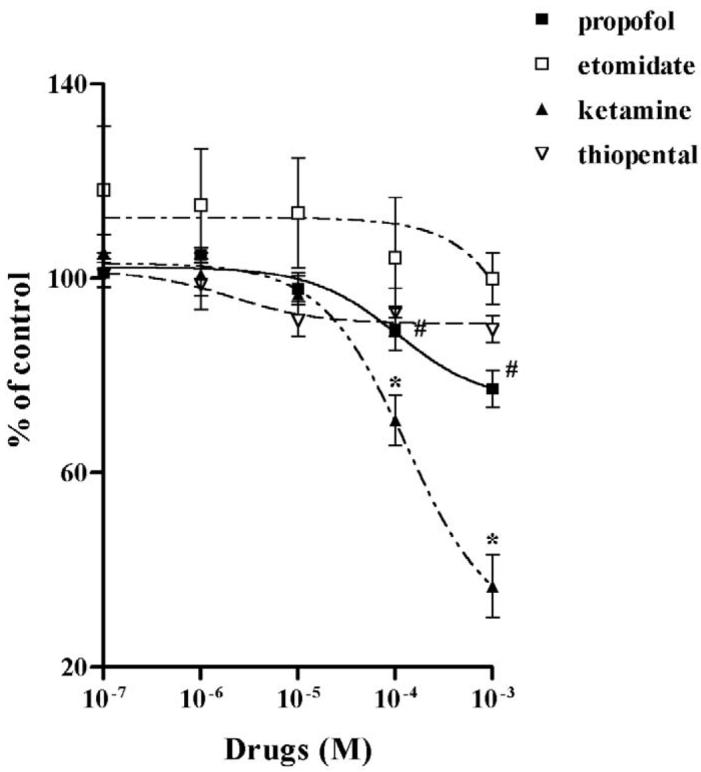
Labeled 5-hydroxytryptamine ([3H]-5-HT) uptake in human embryonic kidney cells transfected with the cDNA of serotonin transporter (HEK-hSERT) is shown for increasing concentrations of ketamine (solid triangle), propofol (solid square), etomidate (open square) and thiopental (open triangle). Inhibition was only evident with ketamine and propofol. # p < 0.05 vs no propofol; * p < 0.05 vs no ketamine.
Table 1.
After exposure to various intravenous anesthetics for 30 minutes, median effective concentrations (EC50) were calculated for labeled norepinephrine ([3H]-NE) uptake in human embryonic kidney 293 cells transfected with the cDNA of norepinephrine (HEK-hNET) and labeled 5-hydroxytryptamine ([3H]-5-HT) uptake in HEK cells transfected with the cDNA of serotonin transporter (HEK-hSERT).
| Treatment | EC50 of [3H]-NE uptake in HEK- hNET cells |
EC50 of [3H]-5-HT uptake in HEK-hSERT cells |
||
|---|---|---|---|---|
| Without desipramine |
With desipramine |
Without fluoxetine |
With Fluoxetine |
|
| Propofol | 41.9 μM | 1.5 μM | 101.3 μM | 4.9 μM |
| Ketamine | 290.7 μM | 1670 μM | 125.2 μM | 2253 μM |
| Etomidate | > 50 mM | NA | >50 mM | NA |
| Thiopental | > 50 mM | NA | >50 mM | NA |
NA = not applicable
Prior to determining the possible modulatory effect of desipramine on the inhibition of NET by propofol and ketamine, we confirmed that desipramine alone inhibited NET uptake function at 10−6 M (75.5% inhibition on [3H]-NE uptake, n = 11). Similarly, fluoxetine alone inhibited SERT function in HEK-hSERT cells (59.4% inhibition on [3H]-5-HT uptake, n = 15). Desipramine potentiated the inhibitory effects of propofol on NET function as evidenced by a significantly lower EC50 compared to propofol alone (Fig. 4A), but attenuated the inhibitory effects of ketamine, with a significantly higher EC50 compared to ketamine alone (Fig. 4B). Following exposure to fluoxetine for 24 hours, the EC50 for propofol inhibition of SERT was reduced from 101.3 μM in non-fluoxetine treated cells to 4.9 μM in HEK-hSERT cells, thus illustrating that chronic fluoxetine treatment enhanced the effect of propofol to inhibit SERT function (Fig. 5A, Table 1). In contrast, after 24 hours of fluoxetine treatment, there was an increase in EC50 for ketamine from 125.2 μM to 2253 μM, thus attenuating the inhibitory effects of ketamine on SERT function (Fig. 5B). There was no change in protein expression after treatment by intravenous anesthetics in the presence or absence of either desipramine or fluoxetine.
Fig. 4.
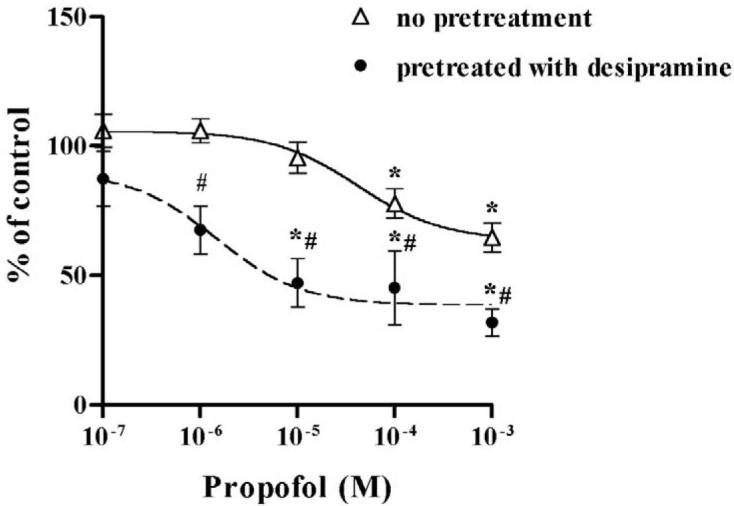
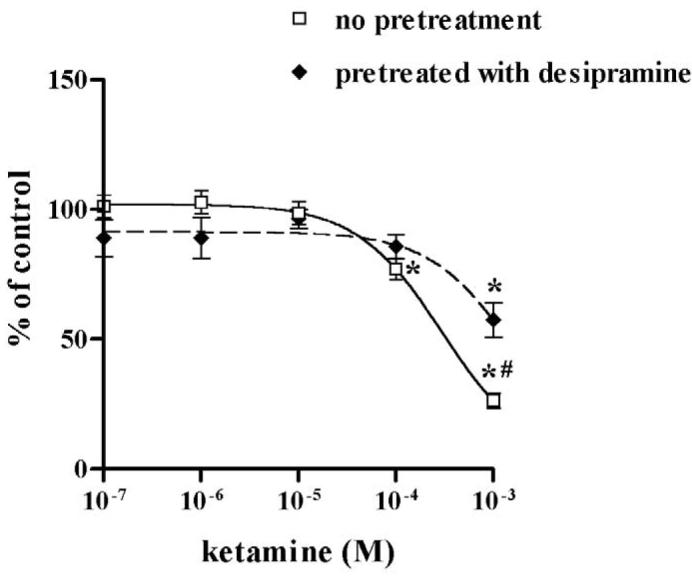
Human embryonic kidney cells transfected with the cDNA of norepinephrine transporter (HEK-hNET) were treated with 1 μM desipramine for 24 hours, and then cells were treated with either propofol or ketamine for 30 minutes at the concentrations indicated. (A) Desipramine exposure enhanced the propofol-mediated inhibitory effects and (B) reduced the inhibitory effect of ketamine on labeled norepinephrine ([3H]-NE) uptake. *p < 0.05 vs no drug treatment; # p < 0.05 vs non-desipramine treated cells.
Fig. 5.
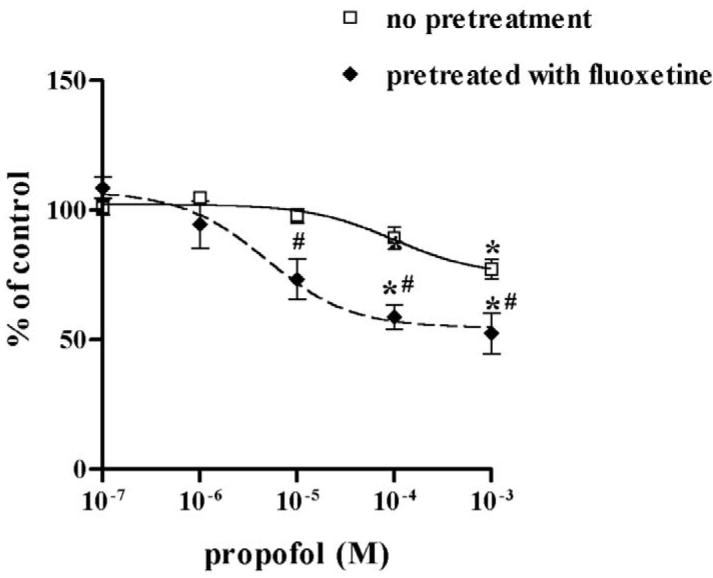
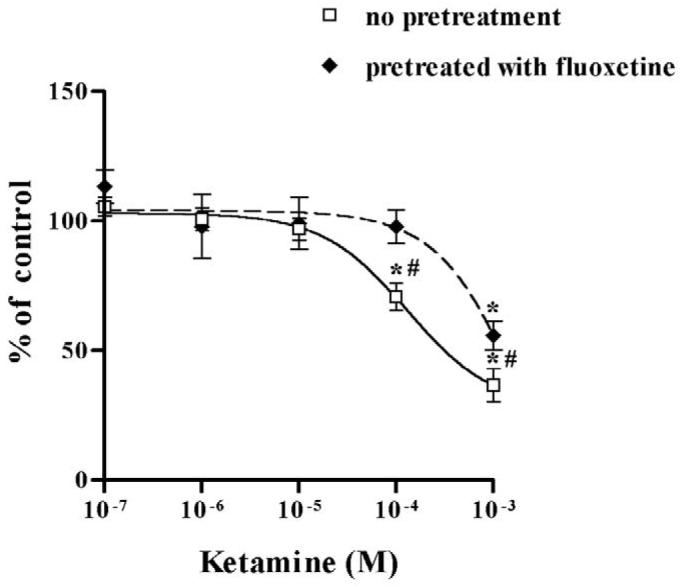
Human embryonic kidney cells transfected with the cDNA of serotonin transporter (HEK-hSERT) were pretreated with fluoxetine 1 μM for 24 hours before exposure to propofol or ketamine for 30 minutes. (A) Fluoxetine significantly shifted the dose response of labeled 5-hydroxytryptamine ([3H]-5-HT) uptake to enhance propofol-mediated inhibition and (B) to reduce ketaminemediated inhibition. * p < 0.05 vs no drug treatment. # p < 0.05 vs non-fluoxetine pretreated cells.
4. Discussion
We examined the effects of four commonly used intravenous anesthetics on both NET and SERT expression and function. We demonstrated that NET and SERT functions were selectively inhibited by propofol and ketamine, but not by thiopental or etomidate. The EC50 for NET or SERT inhibition by propofol and ketamine in the current study was comparable to other reports.12,14 We also demonstrated that fluoxetine and desipramine, two clinically prescribed antidepressants, modulated the inhibitory effects of IV anesthetics on NET and SERT function, respectively.
Anesthetics have been shown to interact with specific neurotransmitter transporters to modulate synaptic neurotransmission16, but most of these effects occur at relatively high concentrations that induced clinically significant hemodynamic alterations.17 Our results confirm both propofol and ketamine were inhibitory at higher than clinically relevant concentrations.14,15 However, the chronic treatment of antidepressant drugs known to inhibit specific transporter proteins significantly shifted the dose response curves. This occurred particularly for the intravenous anesthetic propofol. In the presence of both desipramine and fluoxetine, the concentrations of propofol that inhibited NET and SERT functions were significantly lowered to within the clinically relevant concentration range. In contrast, chronic desipramine and fluoxetine treatment had the opposite effect on interaction of ketamine with NET and SERT. Desipramine and fluoxetine pretreatment effectively eliminated the inhibitory effect of ketamine on SERT and NET function. These findings indicate that antidepressants differentially modulated intravenous anesthetics regulation on NET and SERT.
NET and SERT are major targets of drug action for antidepressants that downregulate the function of NET or SERT in vivo and in vitro.7,18 Previous studies demonstrated that heterologous uptake occurs among different monoamines transporters important in the therapeutic effects of antidepressants.19 Because NET and SERT regulate the synaptic concentrations of NE and 5-HT by reuptake of up to 80% of the amount released from presynaptic terminals2,20,21, even a modest inhibition of NET and SERT activity could lead to significant changes in postsynaptic effects. In the present study, our results indicate that chronic treatment with inhibitors of NET and SERT modulated the inhibitory effects of IV anesthetics on the uptake function of both NET and SERT. Since there were no changes in protein expression in response to chronic desipramine or fluoxetine treatment, this is therefore not the mechanism for the observed altered response to IV anesthetics. Either direct or indirect action of antidepressant on IV anesthetic binding might be an alternative mechanism. Ketamine and propofol may bind to the transporter proteins at separate binding sites that are differentially modified after chronic inhibitor treatment and thereby lead to their distinct responses to chronic antidepressant treatment. Studies of NET and SERT structure indicate they contain multiple binding sites that could induce either non-competitive or competitive inhibition. Ketamine is known to competitively inhibit NET and SERT by direct drug binding to the NE and 5-HT binding sites.12,14,22 Because propofol has been reported to significantly reduce Vmax value of NET without changing Km12, it appears that propofol inhibits NET in a non-competitive manner. They are further consistent with binding of propofol to a site apart from the recognition site for NE on NET protein12,23 The specific site of propofol action on SERT, however, remains to be determined.
The common clinical use of antidepressants in many affective and depressive conditions underscores the importance and relevance of understanding the effect of antidepressant exposure on drug action. Our studies suggest that chronic antidepressant treatment may modify the effects of IV anesthetics on monoamine transporters in patients receiving antidepressant therapy. We found that chronic antidepressant exposure potentiated the inhibitory effects of propofol on NET and SERT such that inhibition was evident at clinically relevant concentrations. In contrast, the inhibitory effects of ketamine on both NET and SERT were attenuated so that following chronic antidepressant exposure, ketamine had no effect on either transporter protein at clinically relevant concentrations.
In conclusion, propofol and ketamine inhibited NET and SERT transporters. The results from these in vitro studies indicate that further studies in animal models and human populations are required to determine the significance of chronic antidepressant exposure on the effect of intravenous anesthetics on monoamine transporters. Although transporter proteins may not be the sites of action for the hypnotic or analgesic effects of the intravenous anesthetics, they are probably important in the manifestation of autonomic signs and possibly nociceptive neurotransmission. Therefore, our results suggest that chronic antidepressant treatment may have important clinical effects. Propofol may have a more noticeable clinical effect, whereas ketamine may have less pronounced side effects attributable to its action.
Acknowledgements
The authors thank Dr Charles Emala for invaluable advice and gift of HEK-293 cells used for the experiments. We would also like to thank Dr Randy Blakely for the gift of hNET-HEK and hSERT-HEK cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
[ED2] The study was supported in part by DHHS, KO8 (YZ) and NIDA RO1 DA12962 (LSS).
References
- 1.Amara SG, Arriza JL. Neurotransmitter transporter:three distinct gene families. Curr Opin Neurobiol. 1993;3:337–344. doi: 10.1016/0959-4388(93)90126-j. [DOI] [PubMed] [Google Scholar]
- 2.Amara SG, Kuhar MJ. Neurotransmitter transporters:recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 3.Barker EL, Blackly RD. Norepinephrine and serotonin transporters. Molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. A Fourth Generation of Progress. Raven Press; New York: 1995. pp. 321–33. [Google Scholar]
- 4.Blakely R. Physiological genomics of antidepressant targets: keeping the periphery in mind. J Neurosci. 2000;21:8319–23. doi: 10.1523/JNEUROSCI.21-21-08319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucki I, O’Leary OF. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J Clin Psychiatry. 2004;65:11–24. [PubMed] [Google Scholar]
- 6.Ren ZG, Porzgen P, Zhang JM, et al. Autocrine regulation of norepinephrine transporter expression. Mol Cell Neuroscience. 2000;17:539–50. doi: 10.1006/mcne.2000.0946. [DOI] [PubMed] [Google Scholar]
- 7.Zhu MY, Blakely RD, Apparsundaram S, et al. Down-regulation of the human norepinephrine transporter in intact 293-hNET cells exposed to desipramine. J Neurochem. 1998;70:1547–55. doi: 10.1046/j.1471-4159.1998.70041547.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT, Axelrod J. Development of the uptake and storage of L-[3H]-norepinephrine in the rat brain. J Neurochemistry. 1971;18:2061–75. doi: 10.1111/j.1471-4159.1971.tb05065.x. [DOI] [PubMed] [Google Scholar]
- 9.Horschitz S, Hummerich E, Schloss P. Functional coupling of serotonin and nordrenaline transporters. J Neurochemistry. 2003;86:958–65. doi: 10.1046/j.1471-4159.2003.01899.x. [DOI] [PubMed] [Google Scholar]
- 10.Ordway GA, Stockmeier CA, Cason GW, et al. Pharmacology and distribution of norepinephrine transporter in the human locus coeruleus and raphe nuclei. J Neuroscience. 1997;17:1710–19. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoelting RT. 3th Lippincott Williams and Wilkins; Philadelphia: 1999. Pharmacology and Physiology in Anesthetic Practice; pp. 140–58. [Google Scholar]
- 12.Hara K, Yanagihara N, Minami K, et al. Ketamine interacts with the noradrenaline transporter at a site partly overlapping the desipramine binding site. Naunyn—Schmiedeberg’s Arch Pharmacol. 1998;358:328–33. doi: 10.1007/pl00005261. [DOI] [PubMed] [Google Scholar]
- 13.Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochemistry. 2006;97:310–33. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura M, Sato K, Okada T, et al. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–74. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Shahani S, Lingamaneni R, Hemmings HC. General anesthetic actions on norepinephrine, dopamine, and γ-aminobutyric acid transporters in stable transfected cells. Anesth Analg. 2002;95:893–9. doi: 10.1097/00000539-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Richard C. What the actions of anesthetics on fast synaptic transmission reveal about the molecular mechanisms of anesthesia. Toxicol Let. 1998;100:41–50. doi: 10.1016/s0378-4274(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 17.Gottschling S, Meyer S, Krenn T, et al. Propofol versus midazolam/ketamine for procedural sedation in pediatric oncology. J Pediatr Hemotol Oncol. 2005;27:471–6. doi: 10.1097/01.mph.0000179238.37647.91. [DOI] [PubMed] [Google Scholar]
- 18.Weinshenker D, White S, Javors M, et al. Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Research. 2002;946:239–46. doi: 10.1016/s0006-8993(02)02889-5. [DOI] [PubMed] [Google Scholar]
- 19.Vizi ES, Zsilla G, Caron MG. Uptake and release of norepinephrine by serotonergic terminals inn norepinephrine transporter knock-out mice:implications for the action of selective serotonin reuptake inhibitors. J Neuroscience. 2004;24:7888–94. doi: 10.1523/JNEUROSCI.1506-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–9. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 21.Montanez S, Daws LC, Gould GG, Frazer A. Serotonin (5-HT) transporter (SERT) function after graded destruction of serotonergic neurons. J Neurochem. 2003;87:861–7. doi: 10.1046/j.1471-4159.2003.02032.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin DC, Introna RP, Aronstam RS. Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett. 1990;112:99–103. doi: 10.1016/0304-3940(90)90329-8. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura R, Yanagihara N, Hara K, et al. Inhibitory effects of clonzapine and other antipsychotic drugs on noradrenaline transporter in cultured bovine adrenal medullary cells. Psychophamacology. 2000;149:17–3. doi: 10.1007/s002139900339. [DOI] [PubMed] [Google Scholar]