Abstract
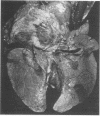
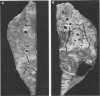
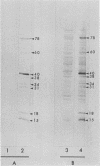
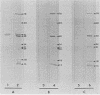
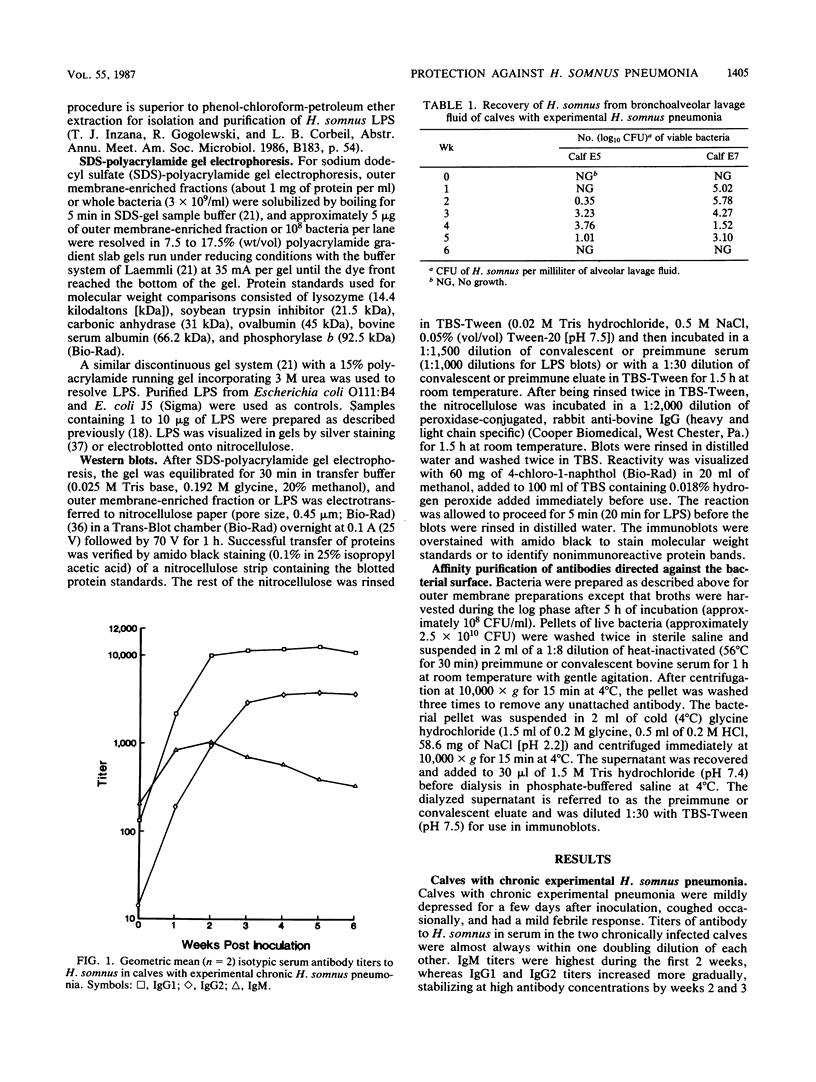
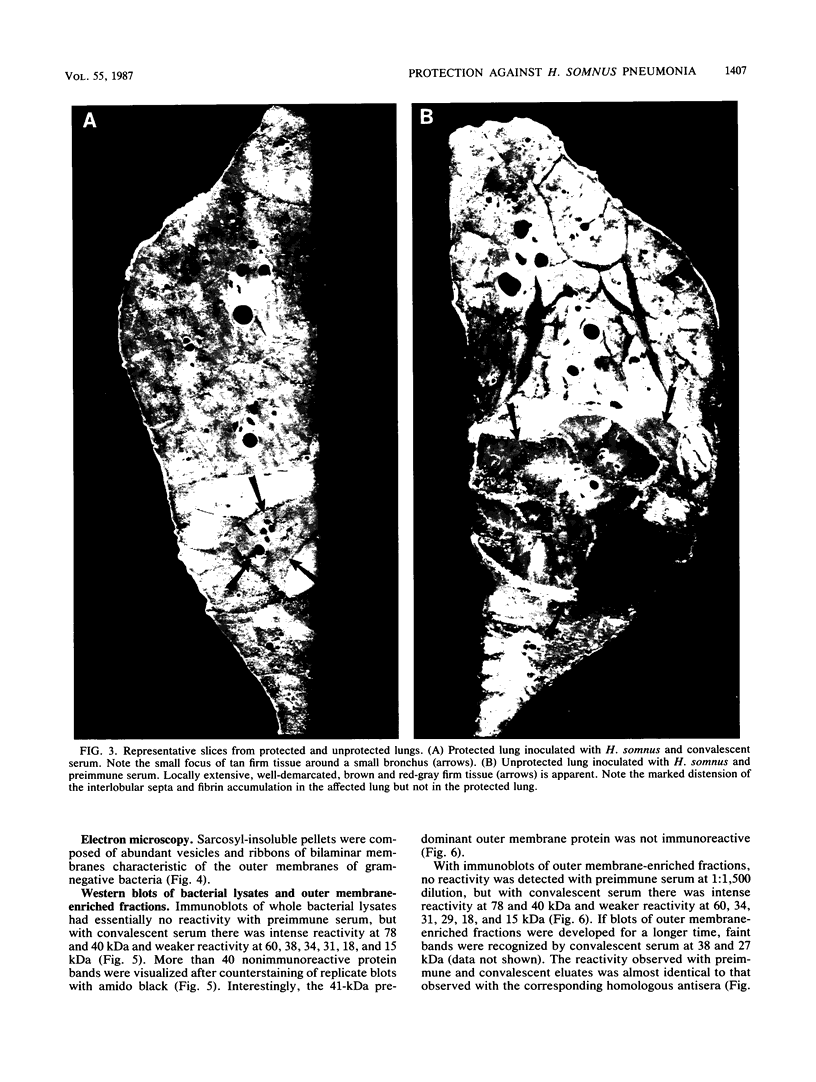
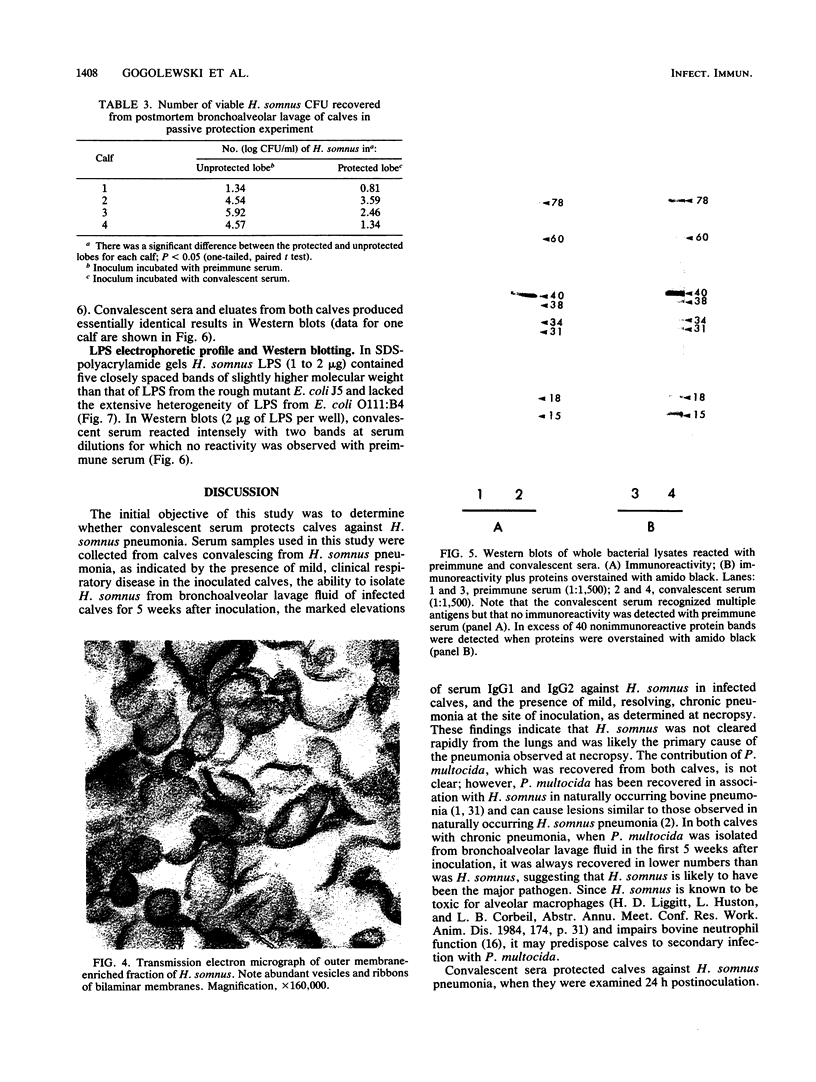
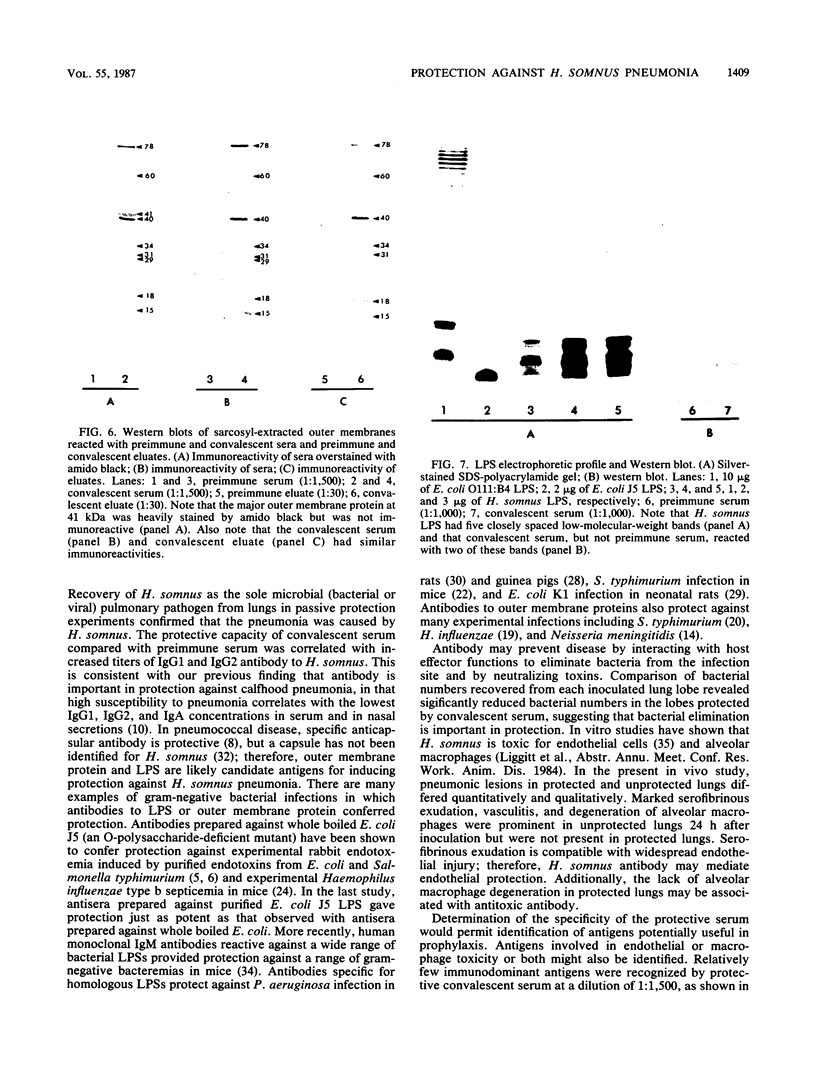
The ability of convalescent serum to passively protect calves against Haemophilus somnus-induced pneumonia was studied. Preimmune and convalescent serum were obtained from calves before or after recovery from experimental chronic H. somnus pneumonia. Passive protection was assessed in another group of calves by intrabronchial inoculation of H. somnus that had been incubated with preimmune or convalescent serum. Each calf was inoculated with each treatment in alternating caudal lung lobes. Twenty-four hours after inoculation almost no pneumonia was present in lungs inoculated with bacteria incubated with convalescent serum, whereas severe pneumonia was present in lungs inoculated with bacteria incubated with preimmune serum. Quantitation of calf pneumonia in both treatment groups indicated a significantly different protective capacity between convalescent serum and preimmune serum (P less than 0.0005). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting of purified H. somnus lipopolysaccharide resulted in intense reactivity with convalescent serum, but no reactivity was detected with preimmune serum. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis of H. somnus outer membrane-enriched fractions, Western blots with convalescent serum gave intense reactions against H. somnus outer membrane antigens with apparent molecular masses of 78 and 40 kilodaltons and weaker reactions with 60-, 34-, 31-, 29-, 18-, and 15-kilodalton outer membrane antigens. No reactivity was detected with preimmune serum. Antibodies eluted from H. somnus after adsorption of convalescent serum reacted almost identically to unadsorbed convalescent serum in Western blots against bacterial outer membrane-enriched fractions. Thus, most of the antigens recognized by convalescent serum are likely to be on the bacterial surface and accessible to antibody. Surface antigens recognized by protective convalescent serum are candidate antigens for a subunit vaccine against H. somnus pneumonia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. J., Anderson T. D., Slife L. N., Stevenson G. W. Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol. 1985 Mar;22(2):131–136. doi: 10.1177/030098588502200206. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Chladek D. W. Bovine abortion associated with Haemophilus somnus. Am J Vet Res. 1975 Jul;36(7):1041–1041. [PubMed] [Google Scholar]
- Chudwin D. S., Artrip S. G., Korenblit A., Schiffman G., Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985 Oct;50(1):213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Blau K., Prieur D. J., Ward A. C. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985 Aug;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Watt B., Corbeil R. R., Betzen T. G., Brownson R. K., Morrill J. L. Immunoglobulin concentrations in serum and nasal secretions of calves at the onset of pneumonia. Am J Vet Res. 1984 Apr;45(4):773–778. [PubMed] [Google Scholar]
- Corbeil L. B., Widders P. R., Gogolewski R., Arthur J., Inzana T. J., Ward A. C. Haemophilus somnus: Bovine Reproductive and Respiratory Disease. Can Vet J. 1986 Feb;27(2):90–93. [PMC free article] [PubMed] [Google Scholar]
- Crandell R. A., Smith A. R., Kissil M. Colonization and transmission of Haemophilus somnus in cattle. Am J Vet Res. 1977 Nov;38(11):1749–1751. [PubMed] [Google Scholar]
- Gogolewski R. P., Leathers C. W., Liggitt H. D., Corbeil L. B. Experimental Haemophilus somnus pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol. 1987 May;24(3):250–256. doi: 10.1177/030098588702400309. [DOI] [PubMed] [Google Scholar]
- Hall R. F., Williams J. M., Smith G. L. Field evaluation of Haemophilus somnus bacterin. Vet Med Small Anim Clin. 1977 Aug;72(8):1368–1370. [PubMed] [Google Scholar]
- Hubbard R. D., Kaeberle M. L., Roth J. A., Chiang Y. W. Haemophilus somnus-induced interference with bovine neutrophil functions. Vet Microbiol. 1986 Jun;12(1):77–85. doi: 10.1016/0378-1135(86)90043-x. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Acres S., Janzen E., Willson P., Allen B. A field trial of preshipment vaccination of calves. Can Vet J. 1984 Mar;25(3):145–147. [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Small G. J., Lostrom M. E., Pier G. B. Polyclonal and monoclonal antibody therapy for experimental Pseudomonas aeruginosa pneumonia. Infect Immun. 1986 Oct;54(1):239–244. doi: 10.1128/iai.54.1.239-244.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Antibodies to O-antigen of lipopolysaccharide are protective against neonatal infection with Escherichia coli K1. Infect Immun. 1985 Aug;49(2):365–370. doi: 10.1128/iai.49.2.365-370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer B., Ward G. E., Moffatt R. E. Correlation of microbiological and histological findings in bovine fibrinous pneumonia. Vet Pathol. 1978 May;15(3):313–321. doi: 10.1177/030098587801500305. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Little P. B. Ultrastructure of Haemophilus somnus, causative agent of bovine infectious thromboembolic meningoencephalitis. Am J Vet Res. 1981 Sep;42(9):1638–1640. [PubMed] [Google Scholar]
- Stephens L. R., Little P. B., Wilkie B. N., Barnum D. A. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981 Feb 15;178(4):378–384. [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. G., Little P. B. Effect of Haemophilus somnus on bovine endothelial cell in organ culture. Am J Vet Res. 1981 May;42(5):748–754. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Widders P. R., Paisley L. G., Gogolewski R. P., Evermann J. F., Smith J. W., Corbeil L. B. Experimental abortion and the systemic immune response to "Haemophilus somnus" in cattle. Infect Immun. 1986 Nov;54(2):555–560. doi: 10.1128/iai.54.2.555-560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikse S. E. Feedlot cattle pneumonia. Vet Clin North Am Food Anim Pract. 1985 Jul;1(2):289–310. doi: 10.1016/s0749-0720(15)31328-1. [DOI] [PubMed] [Google Scholar]