Abstract
Alloantibodies can play a key role in acute and chronic allograft rejection. However, relatively little is known of factors that control B cell responses following allograft tolerance induction. Using 3-83 Igi mice expressing an alloreactive B cell receptor (BCR), we recently reported that allograft tolerance was associated with the sustained deletion of the alloreactive B cells at the mature, but not the immature, stage. We have now investigated the basis for the long-term control of alloreactive B cell responses in a non-BCR transgenic model of C57BL/6 cardiac transplantation into BALB/c recipients treated with anti-CD154 and transfusion of donor-specific spleen cells (DST). We demonstrate that the long-term production of alloreactive antibodies by alloreactive B cells is actively regulated in tolerant BALB/c mice through the dominant suppression of T cell help. Deletion of CD25+ cells resulted in a loss of tolerance and an acquisition of the ability to acutely reject allografts. In contrast, the restoration of alloantibody responses required both the deletion of CD25+ cells and the reconstitution of alloreactive B cells. Collectively these data suggest that alloreactive B cell responses in this model of tolerance are controlled by dominant suppression of T cell help as well as the deletion of alloreactive B cells in the periphery.
Keywords: rodent, B cells, costimulation, tolerance/suppression, transplantation
INTRODUCTION
It is widely appreciated that alloantibodies can play a key role in acute and chronic allograft rejection. However, relatively little is known of factors that control B cell responses following allograft tolerance induction. Indeed, most investigations of the induction of allograft tolerance have focused on the regulation of T cell responses (1-4). We hypothesize that control of B cell responses is necessary for stable long-term allograft survival. This hypothesis is based on the notion that alloantibodies and alloreactive B cells can contribute to allograft rejection by at least three independent mechanisms. First, antibodies can directly bind to the allograft and induce injury to the graft endothelium, ultimately causing graft rejection (5). Second, antigen-antibody complexes can bind to activating FcγRs expressed on antigen-presenting cells (APCs) and induce their maturation into immunogenic APCs (6). These mature APCs can then activate alloreactive T cells, thereby preventing allograft tolerance. Third, activated alloreactive B cells can serve as APCs and directly interact with alloreactive T cells (7). Thus, understanding the mechanisms by which B cell responses are controlled may lead to new ways of inducing and maintaining allograft tolerance.
B cell tolerance to allografts, when achieved, will likely exploit the same mechanisms as those maintaining B cell tolerance to self-antigens. A number of critical checkpoints at which self-reactive B cells are controlled have been described (8). These checkpoints include: (i) Clonal deletion and receptor editing of immature self-reactive B cells in the bone marrow (9, 10). In addition, newly formed self-reactive B cells that escape from the bone marrow can be eliminated in the periphery (11), (12). (ii) Clonal anergy of self-reactive B cells that escape deletion or receptor editing in the bone marrow and emerge in the periphery. The anergic state has been associated with the down-regulation of membrane IgM, and diminished receptor signaling through IgD (13, 14). (iii) Absence of T cell help can result in the downstream inhibition of T cell-dependent B cell responses (12, 15). (iv) Immune deviation of T helper cells (Th) cells, resulting in deficiencies of Th1 or Th2 cytokines, may instruct B cells to secrete selected subclasses of IgG that are less pathogenic (16). (iv) Regulatory T cells can inhibit auto-reactive humoral responses either by inhibiting Th cells or B cells themselves (17-19). Collectively, these observations suggest that robust B cell tolerance requires multiple check-points acting within the bone marrow, as well as in the periphery.
Using a B cell receptor transgenic mouse, 3-83 Igi, as recipients, we previously demonstrated that tolerance induction by anti-CD154 and transfusion of donor-specific spleen cells (DST) can result in the long-term peripheral deletion of mature alloreactive B cells (20). In this study, we have used these 3-83 B cells to visualize the fate of alloreactive B cells during tolerance induction in non-transgenic mice. While 3-83 antibodies cannot directly elicit vascular rejection, we here demonstrate that tolerance induced by anti-CD154 and DST in non-transgenic BALB/c mice leads to the long-term deletion of 3-83 B cells and inhibition of alloantibody production that is dependent on the dominant suppression of T cell help.
MATERIALS AND METHODS
Mice and Cardiac Transplantation
Six to ten weeks old BALB/c, BALB/c-scid, BALB/c RAG2-/- mice (Taconic or Jackson Labs) were used as recipients, and C57BL/6, C3H or F1 (C57BL/6 × C3H) mice (Taconic or Jackson Labs) were used as heart donors. Heterotopic mouse hearts were transplanted into the abdomen of recipient mice by anastomosing the donor aorta and recipient aorta and the donor pulmonary artery and recipient inferior vena cava. Second heart grafts were transplanted into the cervical area of the recipient by anastomosing the donor aorta and recipient carotid artery and the donor pulmonary artery and recipient external jugular vein (end-to-side). The heart grafts were monitored daily for the first two weeks, then two times a week until rejection; rejection was defined as complete cessation of pulsation.
The 3-83Igi mice had been backcrossed to BALB/c for eight generations, and express both the 3-83IgH and 3-83Igκ chains (21). For some indicated studies, 3-83 Igi mice were crossed to BALB/c RAG2-/- background. Mice were maintained in specific pathogen-free conditions at the University of Chicago. All experiments were done in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Antibodies and Tolerance Induction
Anti-CD154 monoclonal Abs (MR1 mAbs) were purified from protein-free culture supernatants by 45% ammonium sulfate precipitation and dialyzed in phosphate-buffered saline. Anti-CD154 was administered at a dose of 1 mg/mouse, intravenously on day 0, followed by intraperitoneal injections on day 7 and 14 post-transplant. Donor-specific transfusion (DST) comprised red blood cell-lysed donor-derived spleen cells, which was administered intravenously (2 × 107/recipient) on the day of transplantation.
Purification of Cell Subsets and Cell Transfer
Whole spleen cells from naïve or tolerant mice (2 × 107/recipient) were transferred into BALB/c-scid or BALB/c RAG2-/- on the day of transplantation. For infectious tolerance, the number of naïve spleen cells remained at 2 × 107, while the number of tolerant spleen cells varied as indicated in the Figure legend. 3-83 B cells were purified by negative selection with a B Cell Isolation Kit (Miltenyi Biotec), and 1.5 · 106 enriched B cells (>97% pure) were transferred i.v., on the day of transplantation. CD25+ cells were depleted from naïve or tolerant spleen cells with the anti-CD25 (PC61) antibody followed by incubation with rabbit complement, and the CD25-depleted cells contained <0.2% CD4+CD25+ cells when visualized by flow-cytometry using anti-CD25 (7D4) mAbs (BD Pharmingen). These cells were transferred i.v. into BALB/c-scid mice on the day of heart transplantation. In some indicated experiments, 1 × 106 3-83 B cells (from 3-83 BALB/c RAG2-/-) together with 2-3 × 107 total or CD25-depleted spleen cells from tolerant BALB/c were transferred to BALB/c RAG2-/-.
Analysis of Donor-reactive Alloantibody and 3-83 Abs Titers
Donor-reactive Abs were determined by flow cytometry as previously reported (22). Briefly, C57BL/6 or C3H lymph node cells were incubated with 1/10 dilution of mouse serum for 1 hr at 4°C, then the cells were washed and incubated with phycoerythrin-conjugated anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA) or FITC-conjugated anti-mouse IgG (Southern Biotechnology, Birmingham, AL). The mean channel fluorescence of the stained samples was determined by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA).
3-83 IgM and IgG titers in sera were determined by enzyme-linked immunosorbent assay (ELISA) using the anti-idiotypic 54.1 antibody (23). 54.1 mAb-coated plates (Costar, Corning, NY) were blocked with 1% BSA/PBS, then diluted serum (1/10 in 1% BSA/PBS) was added to triplicate wells. After 1 hr, plates were washed, then incubated with horse-radish peroxidase (HRP)-conjugated anti-mouse IgM (BD PharMingen, San Diego, CA) or biotin-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by avdin-HRP (BD PharMingen). The optical densities were determined on an ELISA plate reader (BioRad, Richmond, CA), and the results are presented as mean relative optical densities (OD).
Histology and immunohistochemistry
Heart grafts and spleens were surgically removed and snap-frozen in Tissue-Tek OCT (Sakura Finetek USA, Torrence, CA) using liquid nitrogen. Hearts and spleen were sectioned (5 μm) and stained with hematoxylin and eosin (HE) for histology. Other sections were immunostained, using a standard avidin-biotin peroxidase method (24) or immunofluorescence. A cocktail of biotinylated rat anti-mouse IgG1 (A85-1), IgG2a (R19-15), IgG2b (R12-3), IgG3 (R40-82) was used to detect IgG deposition on cardiac allografts. To detect mononuclear cell infiltration, purified anti-mouse CD8a (53-6.7) was applied as primary antibody, and biotinylated goat anti-rat IgG as secondary antibody. For the identification of B cells and development of germinal centers in the spleen, serial sections of each spleen were stained by immunofluorescence with PE-conjugated rat-anti-mouse B220 (RA3-6B2), and anti-mouse follicular dendritic cell antibody (FDC-M1). All antibodies were from BD Biosciences Pharmingen (San Diego, CA.).
Statistical Analysis
Statistical significance was determined using determined by unpaired t-test or analysis of variance (ANOVA) followed by and post-hoc Dunnett's Multiple Comparison or Student-Newman-Kuels tests (Prism 4 for Macintosh (GraphPad, San Diego, CA) or StatView (Abacus Concepts, Berkeley, CA)). A p value of less than 0.05 was considered statistically significant.
RESULTS
1. Anti-CD154 induces tolerance in BALB/c recipients of C57BL/6 heart transplants
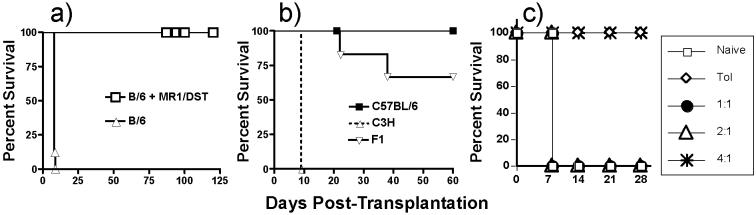
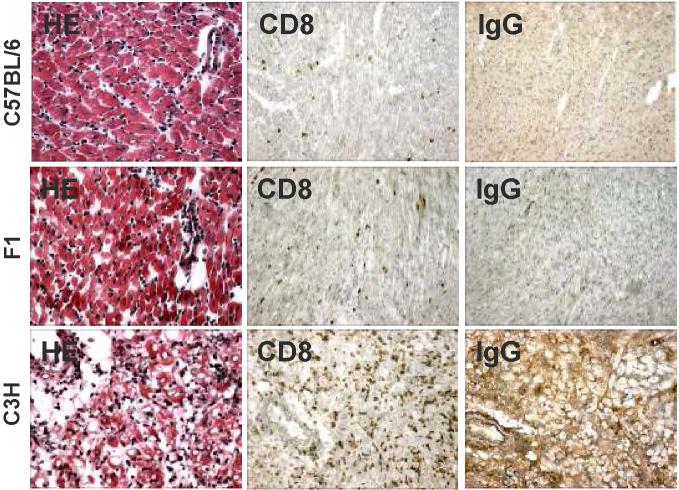
Allogeneic C57BL/6 hearts were rejected in 9 days when transplanted into untreated BALB/c recipients, while treatment with anti-CD154/DST induced long-term allograft survival (Fig 1a). Histological examination of the long-term allografts (>100 days) indicated normal histology of these long-term surviving allografts (Fig 2a). Tolerance was formally demonstrated by the acceptance of a second donor-specific heart graft but not a third-party C3H graft (Fig 1b).
1.
Anti-CD154 plus DST induces dominant allograft tolerance in BALB/c recipients of C57BL/6 heart grafts. (a) Long-term graft survival following treatment with anti-CD154/DST (N=7 for non-tolerant; N≥42 for anti-CD154/DST). (b) Tolerant BALB/c mice accept donor-specific C57BL/6 and F1 (C3H x C57BL/6), but not third-party C3H heart grafts, transplanted on ≥60 days post-first heart transplantation (N=6-8/group) (c) Infectious tolerance is illustrated by the ability of tolerant spleen cells to prevent rejection by naïve/non-tolerant spleen cells (20 × 106/mouse) when administered at a 1:4 (naïve/non-tolerant:tolerant) ratio with tolerant spleen cells into BALB/c-scid mice transplanted with a C57BL/6 heart graft (N=4/group).
2.
A. Histology (HE) and immunohistochemistry (anti-CD8 and anti-mouse IgG deposition) of second, C57BL/6, F1 (C57BL/6 × C3H) or C3H, hearts. The second hearts were transplanted 60-90 days post-first heart transplant, and the C57BL/6 and F1 hearts were harvested on 21 days post-transplant. Positive controls C3H heart grafts that were acutely rejected, and harvested on days 8-9 post-transplant.
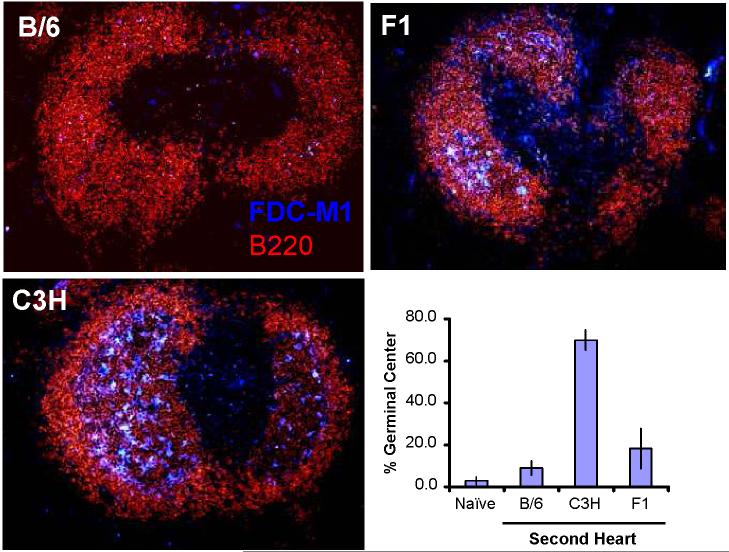
B. Visualization of germinal center formation in tolerant mice challenged with second, C57BL/6 (B/6), F1 (C3H × C57BL/6) or C3H, heart grafts. Spleens were visualized on ≥21 days post-second C57BL/6 or F1 heart transplant, and on 9 days post-C3H heart transplant (N=3-4/group). The negative controls (naïve) were spleens from untransplanted C57BL/6 mice. The percent of B cells with follicles were determined by visualizing 46-90 follicles per spleen (N=3-5/group); and data are presented as the mean percent of follicles with germinal center (Y axis) ± standard error of mean.
Active regulation as the basis for allograft tolerance has been reported in a number of rodent transplantation models (25, 26). However, Bickerstaff et al. (27) reported that BALB/c recipients tolerized to C57BL/6 heart grafts following anti-CD154 therapy do not develop ex vivo delayed type hypersensitivity (DTH) regulatory responses, whereas C57BL/6 mice tolerized to BALB/c heart grafts do. We therefore tested whether regulatory mechanisms develop and mediate allograft tolerance in the BALB/c recipient using more conventional tests of peripheral, dominant tolerance, namely, linked-suppression and infectious tolerance (28, 29). Linked-suppression is defined as the ability to spread tolerance to third-party antigens when those antigens are linked to the tolerant donor antigen on the same antigen-presenting cell. Infectious tolerance refers to the ability of tolerant cells to influence naive or primed cells and induce them to become tolerant. Linked-suppression was assessed by challenging mice tolerant to C57BL/6 hearts with F1 (C57BL/6 x C3H) hearts. As illustrated in Fig 1b, F1 grafts exhibited prolonged survival compared to third-party grafts (p<0.05). Immunohistochemistry showed no CD8+ T cell infiltration and no IgG deposition in the tolerant C57BL/6 or F1 graft. In comparison, there was abundant CD8+ T cell infiltration and IgG deposition in the third-party C3H graft (Fig 2a).
Infectious tolerance was assessed by testing whether spleen cells (2 × 107/mouse) transferred into BALB/c-scid mice from tolerant BALB/c mice could suppress the ability of naïve/non-tolerant BALB/c spleen cells to mediate the rejection of a donor-specific C57BL/6 heart graft. Non-tolerant spleen cells rejected C57BL/6 heart grafts, while spleen cells from tolerant mice did not (Fig 1c). However, spleen cells from tolerant mice were able to reject third-party C3H hearts (data not shown). When tolerant spleen cells were transferred with different numbers of non-tolerant cells into BALB/c-scid mice, allograft rejection was observed at a 1:1 or 2:1 ratio of tolerant:non-tolerant spleen cells. At a 4:1 tolerant-to-non-tolerant spleen cell ratio, no rejection was observed (Fig 1c). While these could reflect either inhibition of homeostatic proliferation, by the tolerant spleen cells, of the naïve T cells that is necessary for acutely rejecting the allograft, collectively the observations of Fig 1b and 1c are consistent with the conclusion that BALB/c recipients develop dominant transplantation tolerance following treatment with anti-CD154/DST.
2. Peripheral control of alloreactive B cell responses in BALB/c recipients
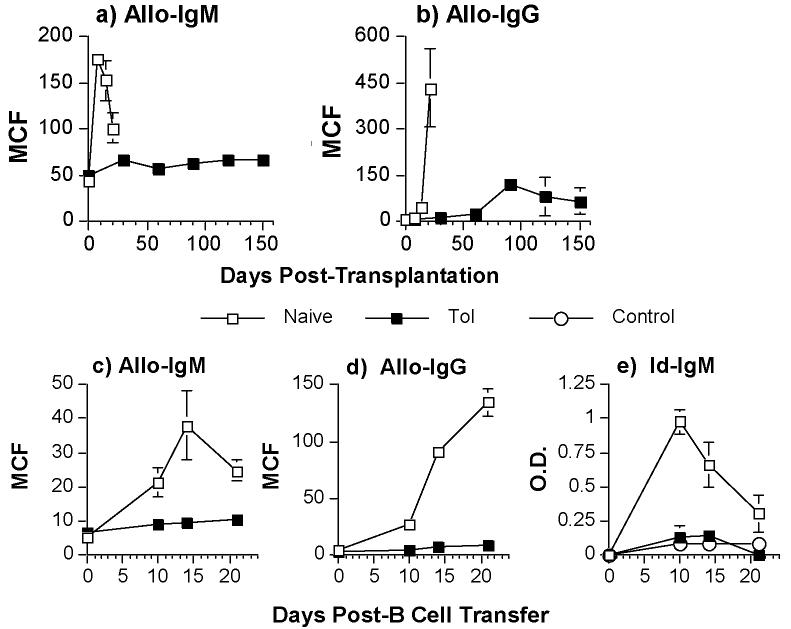
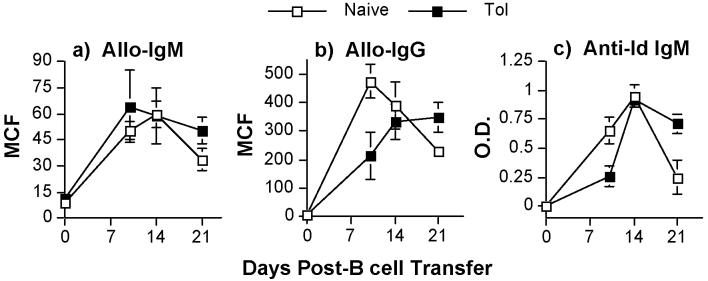
We first demonstrate that anti-CD154 and donor-specific transfusion (DST) after transplant resulted in a significant and prolonged suppression of allo-IgM and allo-IgG responses compared to the untreated non-tolerant group, which demonstrated a 2.5-fold increase in allo-IgM and a 55-fold increase in allo-IgG responses (Fig 3a & 3b; p<0.05). To further assess whether alloreactive B cell function could be maintained by peripheral infectious tolerance mechanisms, we transferred a population of naïve, alloreactive B cells into tolerant BALB/c recipients. This use of non-tolerant alloreactive 3-83 B cells allowed us to confirm that alloreactive B cells can be actively regulated in tolerant recipients. For a source of alloreactive B cells, we used B cells isolated from naive 3-83 Igi mice that have dual anti-H-2Kb/k specificity (21). These 3-83 B cells were transferred into naïve/non-tolerant or tolerant BALB/c mice that received a freshly transplanted C57BL/6 heart graft on the same day. A second heart transplant was performed in the tolerant BABL/c recipient to ensure that both naïve/non-tolerant and tolerant mice received the same allo-antigenic challenge. When purified 3-83 B cells (1.5 × 106/mouse) were transferred into naïve/non-tolerant BALB/c mice that had received a C57BL/6 heart, we observed significant increases in the concentrations of donor-specific allo-IgM and allo-IgG (Fig 3c & 3d; p<0.05) as well as 3-83-idiotypic IgM (Fig 3e; p<0.05). When 3-83 B cells were transferred into tolerant BALB/c mice that received a second donor-specific heart graft, we observed no significant increase in the concentrations of donor-specific allo-IgM and allo-IgG nor of idiotypic IgM responses (Fig 3c-e; p>0.05). Mice that did not receive 3-83 B cells or mice that received 3-83 B cells only, but no C57BL/6 heart grafts, did not exhibit significant increases in idiotypic IgM (data not shown). We could not assess the suppression of the 3-83 IgG response because of the presence of B cells spontaneously secreting 3-83 IgG in the spleens of naïve 3-83 knock-in mice (R. Pelanda, pers. com.). Thus transfer of splenic B cells from naïve 3-83 mice into untransplanted BALB/c recipients resulted in high titers of circulating 3-83 IgG titers, which were unmodified in tolerant recipients (data not shown).
3.
Control of allo-IgM (a) or allo-IgG (b) responses in BALB/c recipients receiving C57BL/6 heart grafts and treated with anti-CD154 plus DST (filled squares) or untreated (empty squares). Sera were harvested on the indicated days posttransplantation. (c-e) Tolerant BALB/c mice (>day 60 post-first transplant) were challenged with second C57BL/6 hearts and transfused with 1.5 × 106 3-83 B cells (Tol). A group of naive BALB/c mice receiving the first C57BL/6 heart and 3-83 B cells served as positive controls (Naïve), while untransplanted BALB/c mice receiving 3-83 B cells only served as negative controls (Control). Sera were harvested on the indicated days post-second heart transplant and infusion of 3-83 B cells (c-e). The levels of anti-C57BL/6 IgM (a,c) and IgG (b,d) were quantified at a 1:10 serum dilution by flow cytometry, and the 3-83 idiotypic (Id) IgM levels (diluted 1:10) were determined with an anti-idiotypic ELISA (e). Data are presented as the mean channel fluorescence (MCF) (a-d) or mean optical density (O.D.) (e) ± standard error of mean (N=8/group).
The presence of germinal center development in the spleen and peripheral lymphoid structures is considered evidence of in vivo T-dependent B cell priming and activation. We observed that the reduced antibody titers following challenge with a second donor-specific graft was associated with minimal germinal center formation in tolerant mice (Fig 2b). This is in contrast to third-party grafts, which induced a robust germinal center formation (Fig 2b). Collectively, these observations suggest that peripheral mechanisms prevent the production of alloantibody by transferred 3-83 B cells in BALB/c mice made tolerant to C57BL/6 cardiac grafts by the treatment of anti-CD154/DST.
Inhibition of Alloantibody production is due to the absence of T cell help
Both allo-IgM and IgG responses are dependent on T cell help, as anti-CD154/DST treatment or the absence of CD4 cells in CD4-/- mice results in significantly reduced alloantibody production (Fig 3a and data not shown). Thus it is possible that the inability of tolerant mice to produce donor-specific antibodies, and naïve 3-83 B cells transferred to produce 3-83 IgM antibodies when transferred into tolerant mice, was due to the absence of T cell help. To test this possibility, we took advantage of the dual specificity of the 3-83 BCR, which binds Kk with the highest affinity and Kb with intermediate affinity (10). We transferred 3-83 B cells into mice tolerant to C57BL/6 hearts, then transplanted these tolerant recipients with a third-party C3H heart graft. As indicated in Fig 1b, these grafts were acutely rejected. We reasoned that C3H-specific, self-restricted T cells in these tolerant mice should not be tolerant, and would be primed by the indirect pathway by recipient APC presenting C3H antigens. These T cells could be able to interact with alloreactive B cells to provide cognate T cell help. Indeed we observed C3H-reactive B cells produced C3H-reactive IgM and IgG alloantibodies, in both non-tolerant and C57BL/6-tolerant BALB/c (Fig 4a & 4b). We also assessed the ability of naïve 3-83 B cells to become activated and to secrete 3-83 IgM in non-tolerant and C57BL/6-tolerant recipients. We observed comparable production of 3-83 IgM titers in both non-tolerant and C57BL/6-tolerant recipients (Fig 4c; p<0.05).
4.
No suppression of alloantibody responses in tolerant BALB/c recipients when third-party heart grafts are transplanted. Tolerant BALB/c mice (>day 60 post-first transplant) were challenged with C3H hearts and transfused with 1.5 × 106 3-83 B cells (Tol). The positive control was naive BALB/c mice receiving C3H hearts and 3-83 B cells (Naive). Sera were harvested on the indicated days post-C3H heart transplant and infusion of 3-83 B cells. The levels of anti-C3H IgM (a), and IgG (b) were quantified at a 1:10 serum dilution by flow cytometry, and the 3-83 idiotypic (Id) IgM (c) levels (diluted 1:10) were determined with an anti-idiotypic ELISA. Data are presented as the mean channel fluorescence (MCF) (a,b) or mean optical density (O.D.) (c) ± standard error of mean (N=6/group).
We also observed the development of germinal centers in the spleens of C57BL/6-tolerant mice challenged with C3H heart grafts (Fig 2b), consistent with the increased titers of anti-C3H alloantibody and 3-83 IgM. Thus, a major mechanism by which long-term alloantibody production is controlled in mice made tolerant with anti-CD154/DST treatment is through the sustained absence of T cell help.
3. Long-term absence of T cell help is actively regulated through linked-suppression
An important feature of active T cell regulation is linked suppression, in which tolerance to a third party antigen can be elicited if the third party antigen is presented in conjunction with the donor-specific antigen. While this has been demonstrated for allograft rejection, it is not known whether linked-suppression can also occur for alloantibody responses. We therefore tested whether linked-suppression of 3-83 B cell responses can be observed following the challenge of F1 (C57BL/6 × C3H) hearts in naïve/non-tolerant or tolerant BALB/c mice.
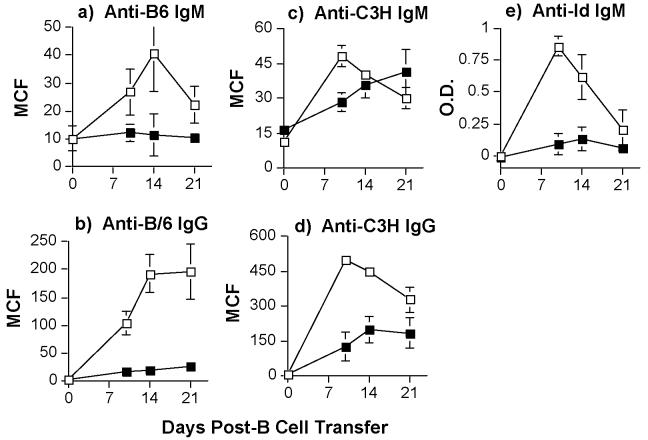
We observed the most efficient suppression of anti-C57BL/6 IgM and IgG production in BALB/c mice initially tolerized to C57BL/6 hearts and then challenged with F1 (C57BL/6 × C3H) grafts (Fig 5a & 5b). We also observed that the 3-83 IgM response was significantly suppressed in tolerant mice challenged with F1 hearts (Fig 5e). The anti-C3H IgG responses also remained significantly suppressed (p<0.05), while the anti-C3H IgM response was transiently delayed, but eventually reached the levels of naïve/non-tolerant BALB/c recipients that rejected C3H grafts (Fig 5c & 5d; p>0.05). Thus, we are able to demonstrate linked-suppression of B cells responses in tolerant mice, although the linked-suppression of IgG responses was more effective than that of IgM responses. We reason that this may be due, at least in part, to allo-IgG production being more T cell-dependent than IgM responses. Examination of the spleens of these recipient mice reveal an inhibition of germinal center formation that was not significantly different from that of the spleens of mice receiving donor-specific heart grafts (p>0.05), but that was significantly different from those receiving third-party heart grafts (Fig 2b; p<0.05). Collectively, these data are consistent with a model of active regulation of T cell help that results in a sustained suppression of alloreactive B cell responses.
5.
Linked-suppression of alloantibody responses in tolerant BALB/c recipients. Tolerant BALB/c mice (>day 60 post-first transplant; filled squares) were challenged with second F1 (C3H × C57BL/6) hearts and transfused with 1.5 × 106 3-83 B cells (black squares). The positive control was naive BALB/c mice receiving F1 hearts and 3-83 B cells (empty squares). Sera were harvested on the indicated days post-F1 heart transplant and infusion of 3-83 B cells. The levels of anti-B6 IgM (a) and IgG (b), anti-C3H IgM (c) and anti-C3H-IgG (d), were quantified at a 1:10 serum dilution by flow cytometry and of anti-3-83 idiotypic (Id) IgM (e) levels (diluted 1:10) by an anti-idiotypic ELISA. Data are presented as the mean channel fluorescence (MCF) (a-d) or mean optical density (O.D.) (e) ± standard error of mean (N=6/group).
4. The role of CD25+ T cells in the regulation of alloreactive B cell responses
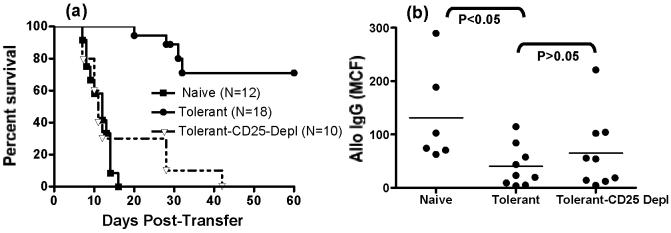
A number of reports have suggested an important role for CD4+CD25+ regulatory T cells in the suppression of alloreactive T cell responses and in the maintenance of allograft tolerance (30) (31). We therefore tested whether depletion of CD25+ cells would reverse tolerance and restore alloreactive B cell responses. Depletion of CD25+ cells in the intact tolerant BALB/c mouse did not reverse tolerance (data not shown) leading us to use a model of spleen cell transfer into immune-deficient BALB/c-scid or BALB/c RAG2-/- mice. In this model, the transfer of naïve spleen cells (107/mouse) into BALB/c-scid recipients resulted in the acute rejection of C57BL/6 heart grafts, but that the transfer of tolerant spleen cells resulted in delayed or no rejection (Fig 1c & 6a). To avoid problems that could arise as a result of the immunosuppressive effects of anti-CD25 in vivo, we depleted CD25+ cells from the spleen cells from tolerant mice in vitro. These CD25-depleted splenocytes, when transferred into BALB/c-scid recipients of C57BL/6 heart grafts, were able to induce the rejection of C57BL/6 heart grafts, confirming previous reports of a role of CD25+ T cells in the prevention of acute allograft rejection (Fig 6a; (32)).
6.
Role of CD25+ cells in allograft tolerance and the suppression of alloantibody production. Naïve/non-tolerant, and whole tolerant or CD25-depleted tolerant spleen cells (2 × 107/mouse) were transferred into BALB/c-scid mice transplanted with a C57BL/6 heart graft. The survival of the heart allograft (a) and the titers of allo-IgG on day 28 post-transplant (b) are presented.
We quantified the levels of allo-IgM and IgG in the BALB/c-scid mice receiving naïve/non-tolerant, tolerant and CD25-depleted spleen cells. No significant increase in allo-IgM was detected (data not shown), but increased titers of allo-IgG were observed in BALB/c-scid mice receiving naïve/non-tolerant spleen cells and C57BL/6 heart grafts (Fig 6b). There was a significantly reduction in the titer of allo-IgG in BALB/c-scid mice receiving tolerant spleen cells and C57BL/6 heart grafts (p<0.05). Surprisingly, depletion of CD25+ cells from the tolerant spleen did not result in a statistically significant increase in allo-IgG titers (p>0.05), despite a restoration of allograft rejection. We hypothesized that this is due to alloreactive B cells being deleted in the periphery of tolerant mice (20), and reasoned that removal of the regulatory T cells alone would not able to restore the alloantibody response.
To test the hypothesis that B cell deletion as well as dominant suppression of T cell help were responsible for controlling B cells responses in long-term tolerant recipients, we co-transferred naïve 3-83 B cells together with spleen cells from tolerant BALB/c, without or without CD25-depletion. To avoid transferring contaminating naïve T cells and 3-83 IgG secreting plasma cells, we generated 3-83 Igi on RAG2-/- background and isolated 3-83 B cells from these mice. Of the five BALB/c RAG2-/-recipients that received spleen cells from tolerant BALB/c without CD25-depletion, three had long-term graft survival (>60 days) and showed suppressed 3-83 IgG secretion (Fig 7). The other two whose grafts were rejected showed comparable 3-83 IgG to the CD25-depleted group (data not shown). Four of the five BALB/c RAG2-/- recipients (transplanted with C57BL/6 heart grafts) receiving CD25-depleted spleen cells together with 3-83 B cells rejected the heart allograft. All five showed a significant increase in 3-83 IgG titers. In all groups, no 3-83 IgM titers were detected (data not shown). We conclude from these experiments that the removal of CD25+ regulatory T cells from the spleen cells of tolerant BALB/c restored their ability to reject cardiac allografts and permitted the activation of alloreactive T helper cells capable of providing help to alloreactive B cells. However, alloreactive B cells are deleted in long-term tolerant mice and the restoration of alloantibody responses required both the reconstitution of alloreactive B cells as well as the restoration of T cell help.
7.
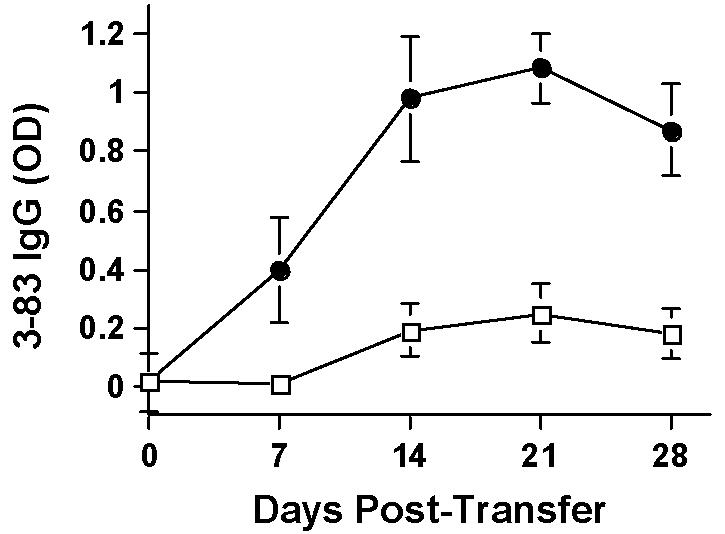
3-83 IgG titers are shown for BALB/c RAG2-/- mice transplanted with C57BL/6 hearts and receiving (1 × 106/mouse) 3-38 B cells together with (2~3 × 107/mouse) whole (open squares; N=3) or CD25-depleted spleen cells (filled circles; N=5) from tolerant BALB/c mice.
DISCUSSION
In the absence of immunosuppression, transplantation of solid organs across histocompatibility barriers is invariably followed by acute allograft rejection. Non-specific immunosuppressive agents that inhibit all T cells, also leave the host vulnerable to severe infections and cancers (33). Therefore, a major goal in transplantation immunology is to induce donor-specific tolerance by designing short-term immunosuppressive therapies that will result in extended suppression of the immune responses specific for the allograft, but that will leave the rest of the immune system competent. Therapies that target the costimulatory molecules CD40-CD154, in particular, have been reported to induce long-term allograft survival in mice and in non-human primates (34). Different mechanisms directed at T cells have been identified to explain the tolerigenic effects of anti-CD154 mAbs (35). However, most reports currently support a model of dominant suppression by regulatory CD4+ T cells as the most important basis for tolerance induced by anti-CD154 mAbs (25, 26).
The effect of anti-CD154 on B cell responses is less well understood. Early studies indicated that anti-CD154 treatment could suppress B cell responses, but not induce B cell tolerance (36). In contrast, we have reported that anti-CD154 therapy, in the context of allograft tolerance models, can induce the long-term suppression of alloantibody and anti-Gal antibody production (37). To address the different effects of anti-CD154 on B cell responses, we have examined the basis for how alloreactive B cell responses are controlled in a mouse heart allograft transplant model. Using a B cell transfer approach, we focused on defining the peripheral mechanisms of B cell tolerance. To circumvent possible contributions of B cell intrinsic mechanisms of tolerance that may have developed during the induction phase of tolerance, we transferred naïve alloreactive B cells into tolerant or non-tolerant mice that received an allograft on the day of B cell transfer. Using this model, we formally demonstrated that naïve alloreactive B cells are constrained from maturing into antibody-secreting cells by the long-term absence of T cell help in mice made tolerant to C57BL/6 allografts. If T cell help was provided in the form of a third-party graft, then alloreactive naïve 3-83 B cells were able to mature into antibody-secreting cells. Based on these observations, and previous reports that regulatory T cells can control B cell responses (17-19), we postulate that the alloreactive-specific B cell responses are inhibited by regulatory T cells acting to constrain T cell help.
T cell help to B cells producing donor-reactive antibodies is invoked, in this situation, by T cells stimulated by indirect antigen presentation. While direct antigen presentation to alloreactive T cells can certainly play an important role in graft rejection, in terms of T cell help to B cells, the helper alloreactive T cells have to interact with host B cells presenting donor MHC peptides for alloantibodies to be produced. Certainly helper alloreactive T cells can interact with donor B cells presenting alloantigen by the direct pathway, but such B cells would not be able to secrete graft-reactive antibodies, as the allograft would be ‘self’ relative to donor B cells directly presenting antigen to recipient alloreacti T cells. Therefore, T cells providing help to B cells producing alloantibodies must have been activated by the indirect pathway (by host B cells presenting allopeptide).
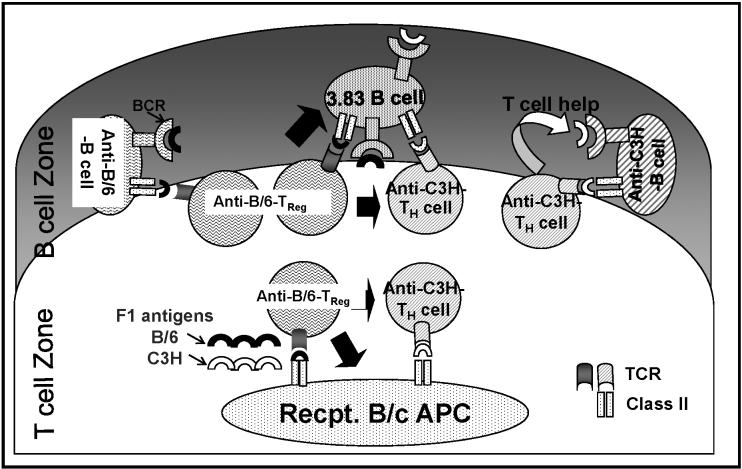
Dominant suppression of alloreactive T cell responses in vivo have been demonstrated by infectious tolerance and linked-suppression (28, 29). Subsequent studies have revealed that this dominant suppression is mediated by CD4+, and, in many cases, by CD4+CD25+, regulatory T cells (30, 31). Here we demonstrate linked-suppression of B cell-responses in which the generation of antibody responses to a third-party antigen was constrained when the antigen was presented in conjunction with the tolerizing donor-antigen. We note that the control of anti-C57BL/6 IgM and IgG, as well as 3-83 IgM, were better controlled than the anti-C3H alloantibody response following the challenge with an F1 graft. We speculate that this may be due, at least in part, to allo-IgG production's being more T cell-dependent than IgM responses. In addition, the weaker control of C3H responses, compared to the C57BL/6-specific responses, following transplantation of an F1 graft can be explained by the efficacy of infections tolerance (see Fig 8). We postulate that regulatory T cells inhibit the activation of T helper cells by modifying the capacity of antigen-presenting cells (APCs) to simulate conventional T helper cells, as well as by directly suppressing T helper cells when both T cell subsets engage with the same alloreactive B cell at the T-B cell interface. Thus, C57BL/6-specific regulatory T cells can function at both the APC and B cell levels when anti-C57BL/6-specific B cells present C57BL/6-derived peptides or when 3-83 B cells present both C57BL/6-derived and C3H-derived peptides to effector or regulatory T cells. However, C57BL/6-specific regulatory T cells can only function at the level of the APCs presenting both C57BL/6-derived and C3H-derived peptides to inhibit C3H-specific T helper cells, but are unable to do so when C3H-specific T helper cells interact with anti-C3H-specific B cells.
8.
Model of linked-suppression of B cell responses in mice tolerant to C57BL/6 hearts, and challenged with F1 (C3H × C57BL/6) hearts. The responses of anti-C57BL/6, anti-C3H and 3-83 B cells in the presence of C57BL/6-specific regulatory T cells (Treg) are illustrated. Solid black arrows indicate suppression; APC refers to antigen presenting cells, Recpt. refers to recipient.
An important question in the field of allograft tolerance is where and how regulatory T cells exert their function in vivo. Early studies on regulatory T cells have focused on their ability to inhibit T cell activation and proliferation in vitro (38). These in vitro studies are supported by recent observations that regulatory T cells can inhibit priming events in the lymph-node (39, 40). Using intravital two-photon microscopy to visualize the effect of regulatory T cells in vivo, both Tang et al. (39) and Tadokoro et al. (39) reported that the antigen-driven interaction between conventional T cells (T conv) and APCs was significantly diminished in the presence of antigen-specific regulatory T cells. The consequence of the diminished interactions between conventional T cells and APCs is reduced priming and generation of pathogenic effector cells in vivo (39).
In contrast to the notion that regulatory T cells inhibit the priming of conventional CD25- T cells, an increasing number of reports suggest that regulatory T cells may in fact mediate tolerance by inhibiting effector T cells in the non-lymphoid tissue sites (41). Chen et al. (41) reported that FoxP3+ regulatory T cells had no effect on the priming of diabetogenic T cells in the pancreatic lymph node, but prevented diabetes by inhibiting the function of effector T cells in the pancreatic islets. In transplantation models, the presence of cells capable of functional regulation was demonstrated by the ability of tolerant skin, to confer donor-specific suppression when retransplanted into Rag-/- mice (25). Subsequently, higher levels of FoxP3 mRNA, a marker of regulatory T cells, were observed in tolerized skin or heart grafts than in rejecting graft or isograft controls (42, 43). This was more recently confirmed by observations by Chen et al. (44) of increased numbers of CD4+FoxP3+ T cells in the tolerant compared to rejected heart grafts. Lee et al. (42) reported that inhibiting the accumulation of regulatory T cells in the graft prevents the development of allograft acceptance. Finally, Waldman and colleagues reported that alloreactive TCR-Tg CD8+ T cells proliferate normally but their ability to induce allograft rejection was associated with the censoring of immune effector function by regulatory T cells (45). Collectively, these data are consistent with the notion that the maintenance of long-term allograft tolerance is dependent on CD4+CD25+FoxP3+ regulatory T cells that inhibit the effector function of conventional T cells within the allograft.
While T cells are primed in the secondary lymphoid organs and then migrate to the allograft to mediate rejection, optimal alloreactive B cell priming, class-switching and somatic hypermutation normally occurs in secondary lymphoid organs--specifically, in the germinal center (46). Antibody-secreting cells and plasma cells remain in the spleen, or migrate to the bone marrow, but generally do not have to infiltrate the allograft to facilitate graft rejection (47). We here provide histological evidence that the suppression of T cell help, and ultimately B cell responses, occurs within the spleen. The regulation of B cell responses can be visualized by the inhibition of the germinal center development in tolerant mice challenged with donor-specific or F1 heart grafts. In contrast, a vigorous development of germinal centers was observed following challenge with third-party grafts. Based on these observations, we hypothesize that a subset of regulatory T cells within the secondary lymphoid organs inhibits the development of donor-specific T cell help in mice tolerant to allografts following treatment with anti-CD154/DST. This hypothesis is consistent with the in vitro observations by Lim et al. (19) that regulatory CD4+CD25+ T cells can migrate to B cell follicles and regulate T helper cells that facilitate germinal center reactions and B cell responses, and with the report of Fields et al. (17) that regulatory CD4+CD25+ T cells can inhibit autoreactive anti-chromatin B cell antibody production, and that this inhibition is associated with inhibition of Th cell cytokine production of IL-10 and IFN-γ.
A unique feature of alloreactive B cell tolerance induced by anti-CD154/DST treatment is the restoration of acute allograft rejection following the removal of CD4+CD25+ T cells, but not the restoration of alloantibody responses. We interpret these observations as consistent with our recent report that alloreactive B cells are deleted in tolerant recipients (20). In those studies using 3-83 Igi mice as recipients of C57BL/6 heart grafts, we observed that the deletion of alloreactive B cells was at the mature B cell stage. This is in contrast to other models of B cell tolerance to self-antigens, which results in the deletion of self-reactive B cells at the immature/transitional stage in the periphery (48, 49). A number of caveats are associated with the use of the 3-83 Igi mice, with the high frequency of alloreactive B cells being the most critical. We were also unable to demonstrate changes in the titers of 3-83 antibodies in naïve 3-83 mice because of the spontaneously high titers of 3-83 IgG (unpublished data and pers comm. Dr. Roberta Pelanda). The basis for the high titers of 3-83 IgG titers is not currently understood, but may reflect the presence of plasma cells that secrete 3-83 IgG independently of T cell help.
We extend our previous observations by demonstrating here that the long-term inhibition of alloreactive B cell responses following the induction of allograft tolerance in wild-type BALB/c recipients with anti-CD154/DST treatment is maintained by the dominant suppression of T cell help as well as the deletion of alloreactive B cells in the periphery. In intact tolerant mice, the provision of T cell help permits new alloreactive B cell emigrants from the bone marrow to mature into antibody-secreting cells. In contrast, in the spleen cell transfer experiments into BALB/c RAG2-/- recipients, the depletion of CD25+ regulatory T cells was able to restore T cell alloreactivity and allograft rejection, but not alloantibody production. We reasoned that this was because alloreactive B cells had been deleted in the periphery and that no new alloreactive B cells could be reconstituted by the BALB/c RAG2-/- recipients. Tolerant spleen cells were able to suppress antibody production by co-transferred 3-83 naïve alloreactive B cells, while the depletion of CD25+ regulatory T cells from the tolerant spleen cell population resulted in productive alloantibody responses. These observations are therefore consistent with the conclusion that the long-term inhibition of alloreactive B cell responses following the induction of allograft tolerance through anti-CD154/DST treatment is through dominant inhibition of T cell help and the deletion of alloreactive B cells in the periphery.
In conclusion, we report that active regulation and peripheral deletion of alloreactive B cells contribute to the control of alloantibody production in recipients made tolerant to cardiac allografts by treatment with anti-CD154/DST. We speculate that the absence of T cell help in the presence of allograft facilitates the deletion of mature alloreactive B cells in the periphery. The reduced frequency of alloreactive B cells, likewise, ensures a tight control of antibody responses in the periphery and reduces the probability of the inadvertent activation of alloreactive B cells responses by T-independent mechanisms.
ACKNOWLEDGEMENTS
We thank Dr. Roberta Pelanda (Department of Immunology, National Jewish Medical and Research Center, Denver) for generously providing 3-83 Igi mice, the anti-idiotype 54.1 mAb and for technical advice. We also thank Ms. Jamie Kim, Lucy Deriy and Ting-ting Zhou for their technical assistance, and Dr. Ian Boussy for help in preparation of the manuscript.
This work was supported by an NIH grant 2R56AI043631-07A2 to ASC and a research grant from the American Society of Transplantation to YL.
REFERENCES
- 1.Chai JG, Vendetti S, Amofah E, Dyson J, Lechler R. CD152 ligation by CD80 on T cells is required for the induction of unresponsiveness by costimulation-deficient antigen presentation. J Immunol. 2000;165:3037–3042. doi: 10.4049/jimmunol.165.6.3037. [DOI] [PubMed] [Google Scholar]
- 2.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810. [PubMed] [Google Scholar]
- 3.Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994;24:2383–2392. doi: 10.1002/eji.1830241019. [DOI] [PubMed] [Google Scholar]
- 4.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, Swenson KG, Zhao G, Sykes M. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non- myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 5.Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, Sanfilippo F, Baldwin WM., 3rd Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 6.Fanger NA, Voigtlaender D, Liu C, Swink S, Wardwell K, Fisher J, Graziano RF, Pfefferkorn LC, Guyre PM. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fc gamma RI (CD64) expressed on human blood dendritic cells. J Immunol. 1997;158:3090–3098. [PubMed] [Google Scholar]
- 7.Noorchashm H, Greeley SA, Naji A. The role of t/b lymphocyte collaboration in the regulation of autoimmune and alloimmune responses. Immunol Res. 2003;27:443–450. doi: 10.1385/IR:27:2-3:443. [DOI] [PubMed] [Google Scholar]
- 8.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 9.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 12.Rathmell JC, Fournier S, Weintraub BC, Allison JP, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion by CD4(+) T cells. J Exp Med. 1998;188:651–659. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang W, Weintraub BC, Dunlap B, Garside P, Pape KA, Jenkins MK, Goodnow CC, Mueller DL, Behrens TW. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- 14.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 15.Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC. A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity. 1997;7:255–271. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 17.Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- 18.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 19.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci U S A. 2007;104:12093–12098. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 22.Yin D, Ma L, Zeng H, Shen J, Chong AS. Allograft tolernace induced by intact active bone and anti-CD40L mAb therapy. Transplantation. 2002;74:345–354. doi: 10.1097/00007890-200208150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci U S A. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin D, Ma L, Blinder L, Shen J, Sankary H, Williams J, Chong A-F. Induction of species-specific host accommodation in the hamster-to-rat xenotransplantation model. J. Immunol. 1998;161:2044–2051. [PubMed] [Google Scholar]
- 25.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165:4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 26.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 27.Bickerstaff AA, Wang JJ, Xia D, Orosz CG. Allograft acceptance despite differential strain-specific induction of TGF-beta/IL-10-mediated immunoregulation. Am J Transplant. 2002;2:819–827. doi: 10.1034/j.1600-6143.2002.20903.x. [DOI] [PubMed] [Google Scholar]
- 28.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 29.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607. [PubMed] [Google Scholar]
- 30.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–126. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Wood KJ, Ushigome H, Karim M, Bushell A, Hori S, Sakaguchi S. Regulatory cells in transplantation. Novartis Found Symp. 2003;252:177–188. discussion 188-193, 203-110. [PubMed] [Google Scholar]
- 32.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25(+)CD4(+) Regulatory T Cells Prevent Graft Rejection: CTLA-4- and IL-10-Dependent Immunoregulation of Alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 33.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Yamada And A, Sayegh MH. The CD154-CD40 costimulatory pathway in transplantation. Transplantation. 2002;73:S36–39. doi: 10.1097/00007890-200201151-00012. [DOI] [PubMed] [Google Scholar]
- 35.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 36.Foy TM, Shepherd DM, Durie FH, Aruffo A, Ledbetter JA, Noelle RJ. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin D, Ma L, Varghese A, Shen J, Chong AS. Intact active bone transplantation synergizes with anti-CD40L therapy to induce B cell tolerance. J Immunol. 2002;168:5352–5358. doi: 10.4049/jimmunol.168.10.5352. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, Akira S, Wang CR, Fairchild RL, Alegre ML, Chong A. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002;3:1208–1213. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 46.van den Eertwegh AJ, Laman JD, Noelle RJ, Boersma WJ, Claassen E. In vivo T-B cell interactions and cytokine-production in the spleen. Semin Immunol. 1994;6:327–336. doi: 10.1006/smim.1994.1041. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro-Shelef M, Calame K. 2005. Regulation of plasma-cell development. Nat Rev Immunol. 5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 48.Cornall RJ, Goodnow CC, Cyster JG. The regulation of self-reactive B cells. Curr Opin Immunol. 1995;7:804–811. doi: 10.1016/0952-7915(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 49.Goodnow CC, Sprent J, de St Groth B. Fazekas, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]