Abstract
Evaporation is a critical problem when handling sub-microliter volumes of fluids. This paper characterizes this problem as it applies to microfluidic cell culture in poly(dimethylsiloxane) (PDMS) devices and provides a practical solution. Evaporation-mediated osmolality shifts through PDMS membranes with varying thicknesses (10, 1, 0.2 or 0.1 mm) were measured over 96 hours. Even in humidified cell culture incubators, evaporation through PDMS and associated shifts in the osmolality of culture media was significant and prevented mouse embryo and human endothelial cell growth and development. A simple diffusion model, where the measured diffusion coefficient for PDMS matches reported values of ~10−9 m2/s, accounts for these evaporation and osmolality shifts. To overcome this problem a PDMS-parylene-PDMS hybrid membrane was developed that greatly suppresses evaporation and osmolality shifts, yet possesses thinness and the flexibility necessary to interface with deformationbased microfluidic actuation systems, maintains the clarity for optical microscopy, and enables the successful development of single cell mouse embryos into blastocysts under static conditions and culture of human endothelial cells underdynamic recirculation of sub-microliter volumes of media. These insights and methods demonstrated specifically for embryo and endothelial cell studies will be generally useful for understanding and overcoming evaporation-associated effects in microfluidic cell cultures.
Microfluidic devices offer the ability to work with nano- to microliter volumes of fluids1-2 and is useful for reducing reagent consumption, creating physiologic cell culture environments that better match the fluid-to-cell-volume ratios in vivo,3-4 as well as performing experiments that take advantage of low Reynolds number phenomenon such as subcellular treatment of cells with multiple laminar streams.5-6 Many microfluidic systems are made of poly(dimethylsiloxane) (PDMS) because of its favorable mechanical properties, optical transparency, biocompatibility,7-8 and its straightforward manufacturing by rapid prototyping.9 A common challenge with PDMS based microfluidic chips, however, is evaporation through PDMS. Evaporation is especially detrimental to cell culture in microfluidic chips because even the slightest amount of evaporation from the small liquid volumes present in microfluidic systems results in a significant increase in osmolality. It is well-documented that elevated osmolality can affect ion balance,10 cellular growth rate,11 metabolism,12-13 antibody production rate,14 signaling,15 and gene expression.14,16 In general, the osmolality of the extracellular environment is normally ~300 mmol/kg. Tolerance to higher osmolalities is cell type dependent. While Chinese Hamster Ovary (CHO) cells and a variety of hardy cell lines tolerate and proliferate under a wide range of osmolality (300-500 mmol/kg),13 more sensitive cells such as mammalian gametes and embryos will undergo a development that is blocked at osmolalities significantly lower or higher than 265 to 285 (mmol/kg).17 Here, we provide a quantitative understanding of the relationship between evaporation and osmolality shift of cell culture media in microfluidic devices constructed of various thicknesses (0.1 to 10 mm) of PDMS and show how devices with thin PDMS layers lead rapidly to large osmolality shifts that result in cell death. Furthermore, we describe methods to greatly suppress such osmolality shifts using membranes with low water permeability and perform two biological demonstrations: 1. Static microfluidic culture of single cell embryos to blastocysts in submicroliter volumes of fluids over 4 days using a humidified incubator; and 2. Dynamic culture of primary human endothelial cells with recirculation of submicroliter volumes of media over 10 hours without media refreshment in a nonhumidified environment
There are several remedies against evaporation from PDMS devices such as: placement of water-filled reservoirs on-chip,18-19 submerging the whole chip in water,20 application of PCR-tape to the chip,21-22 use of oil to cover aqueous liquids,23-24 and changing of curing agent to base ratio.25 Although useful and beneficial for many applications, these methods also have drawbacks and limitations such as limiting the environment that the chip can be used in, altering optical access, hindering interfacing to external devices such as Braille display-based microactuator arrays, or simply being messy. In addition, osmolality, a factor particularly important for cell viability. In this paper, we quantify the rate of evaporation and its effect on composition of cell culture media by measuring the rate of osmolality shifts in PDMS chips with different thickness membranes over a 4 day period inside a humidified cell culture incubator. In spite of the humidified environment (typically 85% humidity) of a cell culture incubator, significant evaporation was measured with thinner PDMS membrane devices having a more dramatic shift in osmolality and a correspondingly worse development rate for embryos, where a majority of embryos stop development at the two cell stage or in approximately 24 hours. In heated yet non-humidified environments, such as portable handheld cultures with real-time video microscopy,26 in–channel evaporation is even faster leading to cell death in less than an hour in the absence of media refreshment. Although parylene has been coated onto PDMS previously to prevent evaporation during micro PCR,27 there was little consideration for support of cell growth and viability, mechanical stability, or ability to perform optical microscopy. We describe the use of a “hybrid membrane: PDMS-parylene- PDMS” that can prevent evaporation while maintaining a permissive environment for cell growth and also providing necessary flexibility, mechanical durability against cracking, and thin profile that is required to perform deformation-based microfluidic pumping and valving using piezoelectric pin actuator arrays on refreshable Braille displays.28 The insights and methods described should be broadly useful for advancement of microfluidic cell cultures and assays where prolonged submicroliter cell cultures need to be performed in PDMS devices.
EXPERIMENTAL SECTION
Glass wells and PDMS membrane preparation
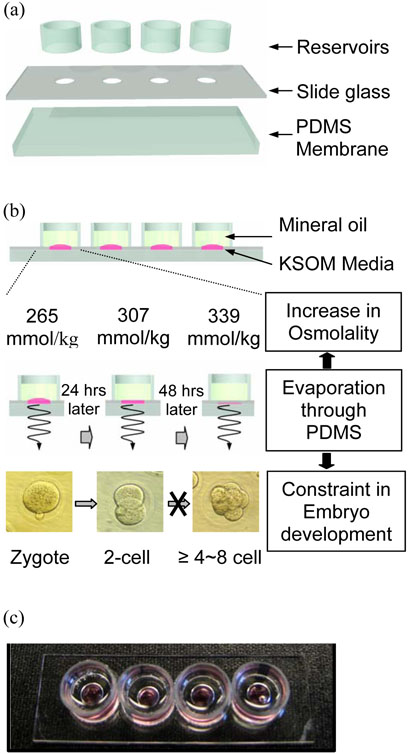
Slide glasses (75×25×1 mm) having 4 holes with a 6.5 mm diameter and placed 1.5 cm apart were used for evaporation and osmolality shift measures as shown in Figure 1 (a). Glass slides were used to avoid contact of oil with PDMS which could potentially lead to unwanted side effects of oil adsorption into the PDMS membrane. Membranes of PDMS with different thickness such as 0.1mm or 0.2mm, 1mm and 10mm were irreversibly bonded to the slide glass after O2 plasma treatment. Additional reservoir structures were attached to each hole to hold mineral oil. In each well, 50μl of Potassium Simplex Optimized Medium (KSOM; Specialty Media, Phillipsburg, NJ) + 0.1% Serum Substitute Supplement (SSS; Irvine Scientific, Santa Ana, CA) was loaded and covered with a layer of 500μl of oil as shown in Figure (b). Conventional microdrop, using 50μl KSOM and covered with oil in a culture dish, was used as control. All prepared devices were placed into a typical humidified cell culture incubator environment (85% humidity) of 5% CO2 in air at 37°C and equilibrated overnight.
Figure 1.
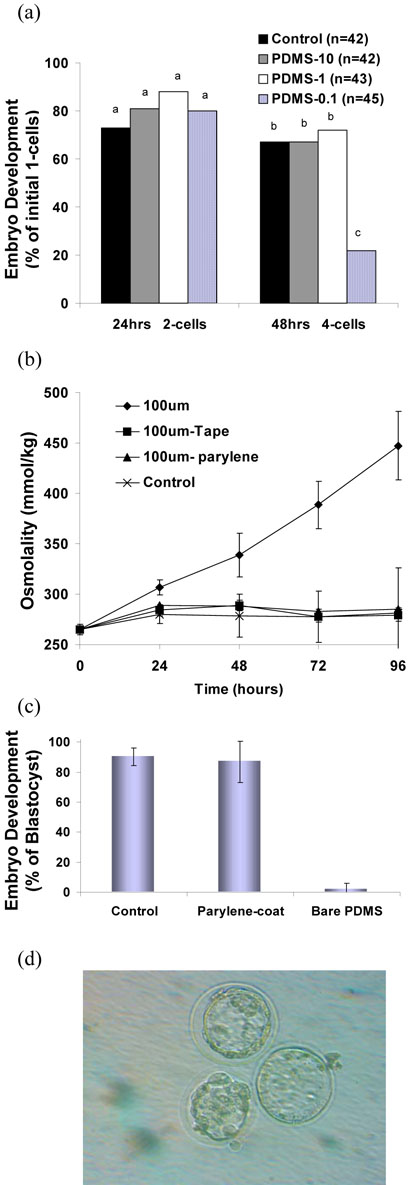
(a) Schematic design of slide glass with 6.5 mm diameter holes attached to PDMS membranes of 10, 1.0 or 0.1mm thickness to form wells. (b) A cross-section showing the glass-PDMS hybrid structure filled media and overlayed with mineral oil. Evaporation through PDMS increases osmolality in media and constrain embryo development. The displayed values are the measured average osmolality in PDMS-0.1 at 24hours intervals. At 48 hours the osmolality shifts from 265 mmol/kg to 339mmol/kg, and embryos development is blocked accordingly at the 4~8 cell stage. (c) A photograph of an actual device filled with media and mineral oil.
Embryo preparation
Six- to eight-week-old B6C3F1 female mice (Charles River) were superovulated by an intraperitoneal administration of 10 IU of pregnant mare serum gonadotropin (PMSG; Sigma Chemical Co.) by injection, followed 44h later with an injection of 10 IU human chorionic gonadatropin (hCG; Sigma Chemical Co.). Presumptive zygotes were collected 18 h later by dissecting oviducts in Hepes-buffered Human Tubal Fluid medium (HTFH; Irvine Scientific) supplemented with 0.1% (w/v) hyaluronidase to remove surrounding cumulus cells. All procedures performed on animals were approved by the University of Michigan Animal Care and Use Committee. Zygotes were placed into 50μl of KSOM + 0.1% SSS overlaid with mineral oil in either organ culture dishes (control), or glass-wells with PDMS membranes of 10 (PDMS-10), 1.0 (PDMS-1), or 0.1 (PDMS-0.1) mm thick. Zygotes and culture devices were placed into a humidified environment of 5% CO2 in air at 37°C. Embryos were assessed at 24hr intervals. Nonparametric measures of timeappropriate development to ≥ 2-cells at 24hrs and ≥ 4-cells at 48hr were compared by chi-square analysis. Differences were considered significant at P<0.05.
Osmolality measurement
Wescor's Vapor Pressure Osmometer, VAPRO (Wescor Inc, Utah, USA) was used to measure the osmolality. Each glass-PDMS well was filled with 50μl KSOM and overlaid with oil. Change in osmolality of the KSOM media in the wells was measured at 24hr intervals over a total of 96 hour period. To measure osmolality, 10μl of KSOM was taken from each well and placed on sample loading area in VAPRO. For each run VAPRO was calibrated using Wescor's Optimol® osmolality standards. Statistics were performed using mixed linear regression and differences considered significant at P<0.05.
Parylene deposition
For parylene coated PDMS, 2.5 or 5μm thick layer of parylene C was deposited on the backside of PDMS membranes by using a PDS 2010 labcoater (Specialty Coating Systems) after covering the well side with a PDMS membrane.
Device Fabrication for microfluidic culture of embryo
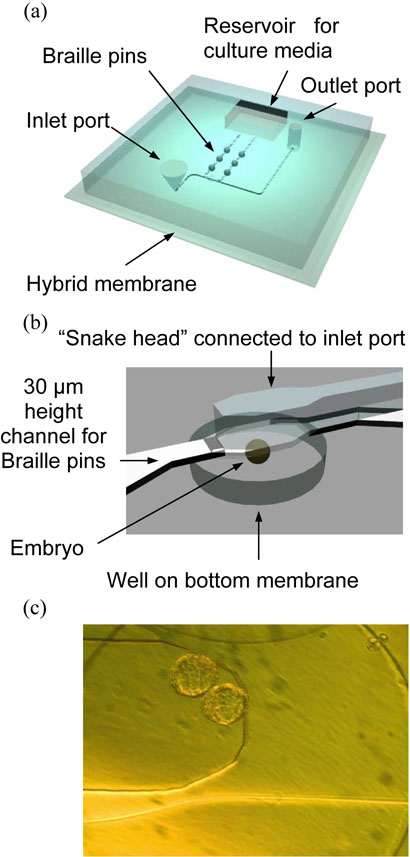
The Microfluidic device (Figure 4(a)) was composed of a thick (~ 8mm) PDMS slab with microfluidic channel features, fabricated by using soft lithography9, attached to a thin PDMS or PDMS-parylene-PDMS hybrid membrane. The thick PDMS slab with channel features was prepared by casting prepolymer (Sylgard 184, Dow-Corning) at a 1:10 curing agent-to-base ratio against positive relief features. The relief features were composed of SU-8 (MicroChem, Newton, MA) and fabricated on a thin glass wafer (200μm thick) by using backside diffused-light photolithography.29 The prepolymer was then cured at 60°C for 60 min, and holes were punched in it by a sharpened 14-gauge blunt needle. The PDMS-parylene-PDMS hybrid membrane was prepared by the following stepwise procedure: spin coating PDMS onto a 4″ silanized silicon wafer to a thickness of 100 μm, curing this layer at 120°C for 30 min, depositing a 2.5 or 5 μm thick parylene layer using a PDS 2010 labcoater, plasma oxidizing the resulting parylene surface for 90 seconds, and spin coating another 100 μm thick layer of PDMS and curing to get a total thickness of ~200 μm.
Figure 4.
(a) Schematic design of PDMS embryo culture device. (b) Enlarged culture area. It consists of three layers; Upper channel (200μm height) for introducing embryo, middle channel for Braille system (30μm height) and PDMS-parylene-PDMS hybrid membrane with 80μm deep well to keep the introduced embryos in the culture chamber i.e. “Snake head” (300μm wide × 200μm depth).
Device Fabrication for Microfluidic Endothelial Cell Culture
The microfluidic device for endothelial cell (EC) culture was fabricated as was described previously.18 Briefly, three layers of cured poly(dimethylsiloxane) (PDMS) at a ratio of 1:10 base to curing agent were sealed together irreversibly using plasma oxidation (SPI supplies, West Chester, PA). Unless stated otherwise, the PDMS layers were cured overnight at 60°C. The top of the three layers (~1 cm thick) contains a rectangular shaped fluid reservoir (Figure 6a). The middle layer (~1 mm thick) consists of bell-shaped channels features29 ~30 μm in height and 300 μm in width formed using soft lithography.9 The channel features of the middle layer face downward and are sealed against a thin membrane bottom layer which is the substrate for cell attachment. PDMS-only thin membranes were fabricated by spin coating freshly mixed 1:10 PDMS onto silanized (75 x 50 mm, 1mm thick) glass slides (Corning Glass Works, Corning, NY) to a uniform thickness of either ~120 and 400 μm and then cured overnight at 120°C. For experiments involving parylene coated membranes, the same PDMS-parylene-PDMS hybrid thin membrane described above with a total uniform thickness of 200 μm was used.
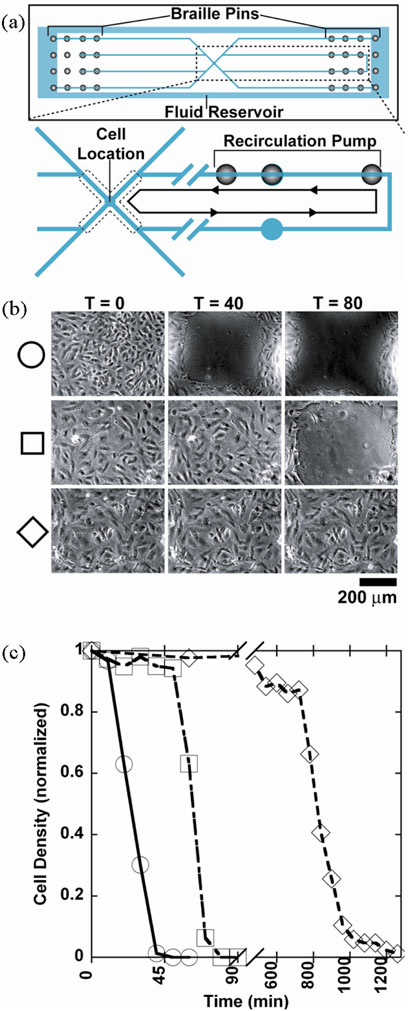
Figure 6.
Microfluidic device for HDMEC culture with recirculation. (a) View from the top schematic depicting the location of the Braille pins used for valving and pumping, microfluidic channels, and fluid reservoir. The fluid reservoir has a total volume of ~1 ml and completely surrounds the channel features like a picture frame to provide an unobstructed view of the cells seeded within the channels. Cut-away view shows the location of cells seeded towards the center of the device and recirculation loop with images recorded at the intersection of the “X.” (b) Qualitative data showing effects of evaporation on cell viability. Timelapse images for the three experimental conditions for thin membrane: 120 micron, PDMS-only (“thin PDMS,” ○), 400 micron, PDMS-only (“thick PDMS” □) and 200 micron, PDMSparylene hybrid membrane (“hybrid” ◊). Time listed is in minutes. (c) Quantitative data describing effects of evaporation on cell viability. Cell density was normalized to values at T=0.
Fluid Actuation System
The computer-controlled Braille display fluid actuation scheme is based on a design described previously26 with minor modifications to make the system more compatible with inverted phase contrast microscopy.30 The Braille display used (SC9; KGS, Saitama, Japan) was powered by a universal serial bus (USB) and consisted of 6 actuation cells, each containing 8 piezoelectric Braille pins (6 × 8 = 48 pins).
Indium tin oxide (ITO) Heater
The ITO heater was constructed by an ITO layer deposited on a glass slide with metallic films for electric contact. First, the glass slide was masked using scotch tape to form the pattern for the ITO layer. An ITO with a thickness of ~1500Å was coated on a 75x25x1 mm slide glass using radio frequency sputtering (Enerjet Sputter) as shown in Figure 5(a). This is followed by removing the masking tapes and annealing the device in a 650°C convection oven for 1 hour. The resulting sheet resistance of the annealed ITO layer is ~30 Ω/square. In order to smoothly generate electric current through the ITO layer for uniform joule heating,two aluminum strips (1 mm width and 2000Å thick) were patterned and coated in a similar manner on two edges of the ITO layer. Electrical wires were attached to the aluminum stripes using silver epoxy glue to form connections to external control circuits. A commercially available wire thermocouple (5TC-TT-J; Newport, Santa Ana, CA) was attached onto the heater surface for temperature sensing. All the wires were connected to a microprocessor based temperature control unit (CT16A2088, Minco Products, Inc., Minneapolis, MN) for feedback control of the heater surface temperature. As a result, the heater surface can be maintained constantly at desired temperature. The advantages of this ITO heater are: 1. Excellent optical transparency in visible light wavelength range. 2. Less image distortion than thin film heater (Ref. 26). 3. More uniform heating over large areas.
Figure 5.
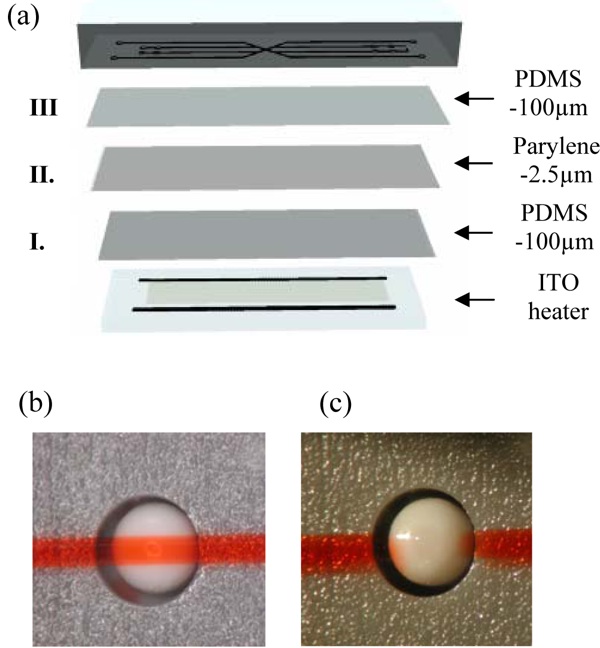
Schematic representation of Braille display-based microfluidics. A typical design for Braille display-based microfluidics is composed of two layers: Upper bulk PDMS with microchannel and bottom membrane. To test the suitability of the parylene coated PDMS with Braille displays, bottom membrane consists of three layers: 100μm-PDMS, 2.5μm-parylene and 100um-PDMS. For cell culture an ITO heater, composed of a glass slide with ITO thin film (thickness about 1500Å) and aluminum electrodes (thickness about 2000Å) patterned on top of it, is placed underneath the membrane. (a). When Braille pin push against the PDMS-Parylene-PDMS hybrid membrane, the channel was fully closed (b). When pin was released, the membrane was restored and channel was opened (c).
(General) Endothelial Cell Culture
Human dermal microvascular endothelial cells (HDMECs, Cambrex, East Rutherford, NJ) were cultured in endothelial growth media-2 MV (EGM-2 MV, Cambrex) in T-25 culture flasks (Corning, Acton, MA) that were placed in a humidified 5% CO2 cell culture incubator. The HDMECs were collected by washing and detaching with 0.25% Trypsin/EDTA (Invitrogen, Carlsbad, CA). The Trypsin solution was neutralized with 10% FBS in DMEM and spun down with a centrifuge (ThermoForma, Marietta, OH) for 5 min, 4° C, 800 RPM. The supernatant was removed and the pellet was resuspended in EGM-2 MV. The spin and resuspension in EGM-2 MV was repeated to ensure removal of Trypsin which inhibits cell adhesion during seeding.
Cell Seeding and Microfluidic Cell Culture
To facilitate cell attachment, the channels were coated for 30-60 min at room temperature with 5-10 μl of human plasma fibronectin (FN) solution (Invitrogen) at a concentration of 100 μg/ml PBS shortly after plasma oxidation (5-10 min). The FN solution was introduced through holes punched with a dermal biopsy puncher (Miltex Inc., York, PA)) though the top and middle layer prior to sealing with plasma oxidation to act as seeding ports by being compatible for use with micropipette tips. After cell seeding (described below), the seeding ports were covered with a sterilized glass slide to avoid contamination when present in non-sterile conditions. After coating, the FN solution was rinsed for 10 minutes with PBS that was pumped through the channels from the fluid reservoir. Afterwards, the device was sterilized by placing under UV light for ~30 minutes. Following UV sterilization, PBS was replaced with endothelial growth media-2 MV (EGM-2 MV, Cambrex, East Rutherford, NJ) which is supplemented as a kit prior to use with 5% fetal bovine serum (FBS) and a host of growth factors/supplements such as vascular endothelial growth factor (VEGF). EGM-2 MV was circulated overnight for the serum proteins to coat the PDMS surface along with FN to facilitate cell attachment. All reagents were added under sterile conditions.
A small amount (3-5 μl) of a dense (~107 cells/ml) HDMEC suspension was pipeted into the cell seeding port and introduced into fluidic regions defined by the Braille pins acting as valves via gentle application of positive pressure. After the HDMECs were seeded, all channels were valved to trap the cells and the PDMS chip and the Braille display were placed in a 37°C/5% CO2 incubator to allow for the cells to attach for 60-90 minutes. After the cells attach, EGM-2 MV culture media was circulated from the fluid reservoir (Supplementary info) for the next 24-72 h until the cells reach confluence.
Recirculating Fluid Actuation
Experiments conducted with recirculation of small amounts of fluid (~500 nl) were conducted on the stage of an inverted microscope (Nikon TS-100F, Japan), imaged with a 10x Ph1 objective (Achromat), and recorded using Coolsnap CF2 Camera with MetaVue software. To account for the lack of controlled temperature and 5% CO2 tension provided by a cell culture incubator, the bottom of the PDMS device was heated to 37°C (ITO heater, PID Temperature Controller, Minco, Minneapolis, MN) and the culture media was specially formulated with a synthetic buffer to maintain stable pH of ~7.3 under ambient conditions.26
Braille pins were reconfigured via computer-control such that flow can only occur in the recirculation loop (Figure 6a) due to complete valving. The initial amount of fluid continuously recirculated was ~500 nl at a pumping frequency of 0.125 Hz. Images were recorded at the intersection of the “X” towards the center of the microfluidic device (Figure 6a). The cell density was recorded for discrete time points and normalized to the value at T=0. Cells were considered still alive if they remained attached and were still moving (visualized with real-time microscopy). For PDMS-only membranes, two experiments were performed in duplicates; for the Parylene membrane device, only one experiment was performed.
RESULTS AND DISCUSSION
Osmolality measurement and diffusion coefficient
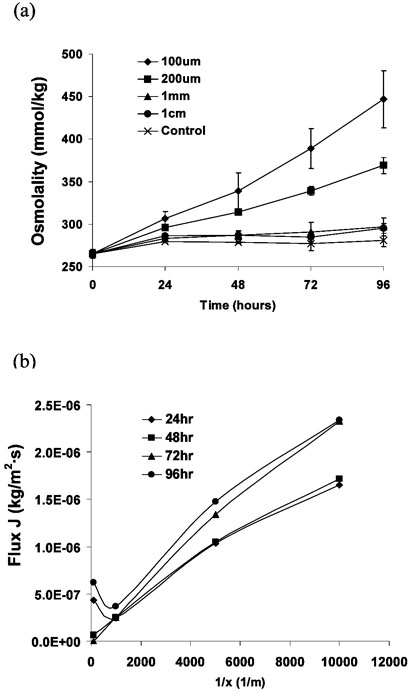
As described above, glass slides were drilled to form 6.5 mm diameter holes and the slide sealed against PDMS membranes with thicknesses of 0.1, 0.2, 1.0 and 10 mm to form wells. Fifty μl of KSOM was added to the wells, overlaid with oil, and the osmolality of the media assessed at 24hr intervals. As shown in Figure 2(a), the osmolality increased significantly over a 96 hour period, with greater shifts observed as the membrane became thinner. The increase in osmolality was particularly significant for thin PDMS membranes of 0.1 and 0.2 mm that are commonly used for pin-actuator based fluid actuation systems.
Figure 2.
(a) Osmolality of media (KSOM + SSS) over time when cultured in control (organ culture dish) and glass-slide wells with PDMS membrane bottoms of varying thickness. Osmolality changes over time were significantly different for PDMS 0.1mm compared to remaining treatment groups P<0.01. (b) Based on equation 3, Flux J was plotted with respect to 1/x using the measured osmolalities with varying PDMS thicknesses.
Closer analysis of the data provides useful quantitative insights into the characteristics of PDMS as well as how careful one needs to be when handling small amount of media. Silicon elastomer membranes are known for their selective permeation properties with gases and liquids. The diffusion of water through silicone has been measured experimentally,31-32 and interpreted theoretically.33 These reports suggest that the classical Fick's model of diffusion, although not perfect, describes diffusion of water through PDMS reasonably well. In our experiments, we calculate the flux of water, and the diffusion coefficient of water through PDMS using measured osmolality values and Fick's laws of diffusion.
Osmolality is defined as a measure of the number of dissolved particles (ions and undissociated molecules, such as glucose or proteins) per kg of water.34 O* represents the total number of dissolved particles, assuming constant over the time, O is the measured osmolality (mmol/kg), m is the total mass (kg) of the media. The change in mass and change in osmolality is inversely proportional. Thus,
| (1) |
| (2) |
When J is defined as the mass flux through a unit area per unit time [kg/m2·s]. Δm is mass change with given time. A is area [m2], which is area of 6.5 mm well in this case. t is time [s], which is 24hour in this case.
| (3) |
By Fick's 1st law, J can be also described as:
| (4) |
Equation 3 provides a plot of J with respect to 1/x as shown in Figure 2 (b) where the slopes, k, are between 10−10 ~ 2·10−10[kg/m·s]. Based on equation 3 and Fick's 1st law (eq 4) we find that these slopes give diffusion coefficients, D, of 3×10−9 ~ 6×10−9[m2/s], which is consistent with previously reported value31-33. Here, the humidity at the media surface is considered to be 1 (100% humidity), the humidity of our incubator was measured to be approximately .85 (85% humidity), the saturated vapor pressure (Ps) at 37°C is 6.33×103 Pa, P is the vapor pressure outside of the chip's environment, P/Ps is 0.85, the water solubility coefficient (S) in PDMS is approximately 1.04 [(cm3)water/cmHg·(cm3)PDMS]35 and the saturated vapor specific volume (Vg) at 37°C is Vg ≈ 22.94(m3 / kg), ρg = 0.0436(kg /m3).36
An observation not explained by evaporation of water through PDMS is the osmolility shift from 265 to 280 (mmol/kg) over the first 24 hours, even in control experiments that did not use PDMS. Absorption of water by the oil used to cover the media only account for a very small portion (2% or 0.0540mg) of the lost water.37 Thus, the majority of the loss is due to evaporation occurring during the few minutes of handling between transfer of droplets from a macroscopic bottle of media to a well and covering it with oil. This underscores not only the importance of preventing evaporation from media in the chip, but also how crucial it is to prevent evaporation during transfer of fluids onto the chip.
Embryo culture and Osmolality
As described above for measurement of osmolality shifts, the collected zygotes were placed into 50μl of KSOM + 0.1% SSS overlaid with mineral oil in either organ culture dishes (control), or in coverglass wells with PDMS floors of 10 (PDMS-10), 1.0 (PDMS-1), or 0.1 (PDMS-0.1) mm thickness. As shown in Figure 3 (a), there was no significant difference in the ability of zygotes to develop to/beyond the 2-cell stage at 24hrs of culture in control (73%), PDMS-10 (81%), PDMS-1 (88%), or PDMS-0.1 (80%). However, at 48hrs, significantly fewer zygotes (P<0.01) had developed to/beyond the 4-cell stage in PDMS-0.1 (22%) compared to control (67%), PDMS-10 (67%) and PDMS-1 (72%). The compromised development of embryos in PDMS-0.1 (Figure 3a), where the measured osmolality at 24-48 hrs is well above 300 mmol/kg (Figure 2a), is consistent with the known tendency of embryo development to be arrested at high osmolalities.17 Thus, one cannot perform embryo culture in microfluidic devices with thin membranes composed solely of PDMS or the type of membrane commonly used for deformation-based microfluidic actuation systems. Although embryos have been previously cultured in PDMS/borosilicate hybrid microfluidic devices under static conditions,38 little consideration was given to the evaporation through PDMS due to its thickness. In both the previous system and our current system, most of the evaporation occurs through the bottom PDMS layer but the amount of evaporation in our system is much greater because the PDMS membrane is much thinner. We also investigated the effect of pre-soaking PDMS in culture media or mineral oil for 24 hours, but saw little or no improvement (data not shown).
Figure 3.
(a) Mouse pronuclei (PN) zygote development in control (organ culture dish) and glass-slide wells with different thickness PDMS membranes. (b) Parylene was coated on the mounted PDMS outside surface with 2.5um thickness and then osmolality change was measured. Osmolality of KSOM drifted from 265 to 285 mmol/kg during 96hrs of incubation in parylene coated PDMS-0.1. (c) Percent blastocyst development was significantly improved with parylene coating (87±14 blastocyst development) compared to no parylene (2±4; P<0.01). (d) blastocyst images taken from parylene coated PDMS-0.1 well.
Next, we coated 2.5 um parylene on the outer side of a 0.1mm-PDMS membrane or simply applied plastic tape. Parylene is deposited by a unique vapor deposition polymerization process, and the resulting coatings are pinhole free and can easily conform to surface shapes while maintaining a uniform thickness. Both parylene coated PDMS and tape made evaporation through the membrane negligible, as shown in Figure 3 (b). (The slight shift from 265±5.1 to 285±1.2 mmol/kg is attributed to evaporation during transfer of fluid not evaporation through the membrane.) Importantly, the parylene coating significantly improved blastocyst development (87±14 blastocyst development) compared to the identical system with no parylene (2±4; P<0.01; Figure 3 (c)). Tape was not tested for embryo development, however, because it is thick and unfavorable for deformation-based microfluidic actuation systems and the adhesive on the tape may be toxic to cells.
To perform microfluidic embryo culture, we fabricated a two-level fluidic system using a two-step photolithography procedure. First, a 30μm high bell-shaped channel for Braille actuation was formed by backside diffused-light photolithography29 then a 200μm high rectangular channel for introducing embryos was aligned and formed by conventional photolithography. Next the PDMS-Parylene-PDMS “hybrid” membrane with a bottom well (80μm depth) for trapping39 was used to seal the channels. Embryos were introduced into the “Snake head-like” culture chamber with a well, as shown in Figure 4 (b) using fluid pumping action generated by manual deformation and release of the culture media reservoir with fingers. As shown in Figure 4 (c), one cell embryos were successfully cultured into blastocysts using the PDMS micro device with the “hybrid” membrane, under static conditions, without changing the culture medium over the 96 hour culture period. When one cell embryos were cultured using a PDMS micro device with the same thickness, but without the “hybrid membrane”, the cells did not develop into blastocysts. The insights and methods described should be broadly useful for advancement of microfluidic cell cultures and assays in general and for increasing application, acceptance, and subsequent efficiencies in mammalian gamete/embryo culture and success of assisted reproductive technologies in biomedical research (transgenic mice), genetic gain and domestic animal production, and treating human infertility.
Use of Parylene Coated PDMS Membranes in Deformation-Based Fluid Actuation
We further tested the applicability of the parylene coated PDMS devices to actively actuated Braille microfluidic systems and to other cell types. When parylene was simply coated on the outside and the PDMS microfluidic chips which are subjected to repeated deformation-based fluid actuation using piezoelectric pin actuators of a refreshable Braille display, the parylene membrane became scratched, scarred, and cracked. To address this problem of cracking, a “hybrid” membrane was developed that sandwiched parylene between two layers of thin PDMS as seen in Figure 5. First, a 100μm PDMS membrane (Figure 5 (a) I) was formed on a silanized 4″ silicon wafer followed by deposition of a 2.5μm film of parylene (surface II). The resulting parylene surface was plasma oxidized and covered with a spin-coated layer of PDMS (100μm) to form surface III. This “hybrid” membrane (surface I, II, III) was irreversibly bonded to a PDMS mold containing channel features to form the microfluidic chip. As shown in Figure 5 (b) and (c), the channels are fully compatible with deformation-based fluid actuation using pin actuators.
Endothelial Cell Survival Under Recirculating Fluid Actuation
Using the deformation-based fluid actuation described above that incorporates a PDMS-parylene-PDMS hybrid membrane, we also tested sub-microliter recirculating culture of human dermal microvascular endothelial cells (HDMEC) in a non-humidified environment with on device heating. This type of capability is expected to be important for future studies of the effect of autocrine and paracrine effects on endothelial cells under fluid perfusion conditions.
Confluent monolayers of HDMECs were seeded and cultured within the microfluidic device and imaged at the intersection of the “X” region (Figure 6a). Figure 6b is a timelapse comparison of HDMEC survival under continuous recirculation of ~500 nl of media with deformation-based Braille fluid actuation. The experimental conditions were 120 μm thick, PDMS-only membrane (“thin PDMS”); 400 μm thick, PDMS-only membrane (“thick PDMS”); and 200 μm thick, PDMS-parylene hybrid membrane (“hybrid”). At T = 40 (time given in min), virtually all the cells in the visualized region for the “thin PDMS” membrane are dead and detached whereas the cells for the “thick PDMS” and “hybrid” membranes remain confluent. At T = 80, virtually all the cells for the “thin PDMS” and “thick PDMS” membranes are dead and detached whereas the “hybrid membrane” still remains confluent.
The results were quantified by counting the changes in cell density with time due to continuous recirculation (Figure 6c). For the “thin PDMS” membrane, about 50 percent of the cells were dead and detached by about T = 25 (extrapolating from data in Figure 6c) and all of the cells were gone by T=50. For the “thick PDMS” membrane, about 50 percent of the cells were dead and detached by T = 65 and all of the cells were gone by T = 90. With the “hybrid” membrane, cells survive much longer under continuous recirculation than the PDMS-only membranes. Cells remain roughly confluent (>85 percent of the original cell density) up to T = 720 (or 12h). Afterwards, cells begin to die more rapidly with 50 percent of the cells remaining alive and attached at about T=800 (13.3h) and less than 5 percent at T = 1080 (18h) (Figure 6c). By comparing the times it takes for 50 percent of the cells to die, we conclude that cells are able to survive about 2.6 times longer with the “thick PDMS” membrane compared to the “thin PDMS” membrane. In addition, cells are able to survive about 29 times as long with the “hybrid” membrane when compared with the “thin PDMS” membrane and 11 times as long when compared with the “thick PDMS” membrane. Thus, the integration of parylene in the “hybrid” membrane substantially increases the time HDMECs survive under continuous recirculation of ~500 nl of fluid even though at 200 μm, the “hybrid” membrane is slightly thicker than the “thin PDMS” membrane (120 μm) and half as thick as the “thick PDMS” membrane (400 μm).
Further analysis of evaporation rates, using the simple diffusion equation suggested above, is useful in understanding the different cell survival ratios under continuous recirculation with diverse membranes. Simply comparing 100μm-PDMS with 8mm-PDMS, the flux J through the 8mm-PDMS is approximately 1.25 % of the flux through the 100μm-PDMS. So the majority of evaporation on the PDMS chip with a thin membrane occurs from the bottom thin PDMS membrane. In addition, the amount of evaporated water escaping through the thicker, top PDMS (~8 mm thickness) can be compared with the amount of water evaporated through the thin bottom parylene (~2.5 μm thickness)using the simple diffusion equation: . The diffusion coefficient of Parylene C for water at 30°C is 2.60 ×10−13(m2/s),40 which is 4 orders lower than that of PDMS. The thickness of 2.5μm-parylene is 3200 times smaller than that of 8mm-PDMS. The solubility coefficient of Parylene C for water at 40°C is 0.62[(cm3)water/cmHg·(cm3)Parylene],40 which is 0.6 times smaller than that of PDMS. Finally, the flux J through the thin 2.5-parylene is only 24.8% of the flux through the much thicker top PDMS (~8mm). This does suggest that evaporation through the thicker top PDMS as well as partly the bottom 2.5-parylene membrane will be an eventual cause of osmolality shifts even on the “hybrid” membrane chips if the experiment goes on for a long period. In most experiments, partial refreshment of the sub-microliter culture media from a larger on-chip reservoir at least every several hours is envisioned and the current system would function just fine. When longer experiments (days and weeks) are desired in non-humidified environments without refreshing the submicroliter volumes of media, then additional considerations would be needed, such as moisture barriers for the top side of the chip and increasing the bottom parylene thickness, to further reduce loss of water.
Analysis of osmolality shifts using the simple diffusion equation also gives us a better explanation of why the HDMECs died within 25 min with a 120μm–PDMS bottom membrane. Combining equation (3) and (4),(5). Based on the channel design in figure 6 (a), approximate values of variables are: channel area, A = 1.473×10−5 m2, Δm = 2.21×10−7kg [1000 kg/m3 × 0.5x channel volume (4.42×10−10 m3)], D = (3~6)·10−9 m2/s, S = 1.04 [(cm3)water/cmHg·(cm3)PDMS],35 Ps at 37°C = 6.33×103, P/Ps = 0.25, dx = 120×10−6 (m). Assuming the initial osmolality of media for HDMECs as ~260 mmol/kg, the osmolality inside the microfluidic channels after 30~60 minutes is expected to be at least 520 mmol/kg and explains why the majority of HDMECs died. Here, the humidity at the media surface is considered to be 100% humidity at 37°C, the humidity of the room was measured to be approximately 25% humidity at 20~C, and the saturated vapor specific volume (Vg) at 37~C is V ≈g 22.94(m3 / kg), pg =0.0436(kg / m3).36
In addition to osmolality, the oxygen in the media present in chips is most likely the limiting nutrient source and its depletion may affect cell survival over time. Oxygen is an important substrate for cell growth and severe depletion would affect cell growth. However, thinner membranes have faster oxygen permeation and less depletion. Therefore, we do not believe that the difference in cell survival described is due to oxygen depletion. This conclusion is supported further by Ref. (30) which details oxygen measurement and consumption in microfluidic PDMS cell culture devices.
CONCLUSION
Compared to conventional cell cultures performed in Petri dishes with low cell volume to extracellular fluid volume (CV/EV) ratios, microfluidic environments with large CV/EV ratios have many advantages in terms of cellular self-conditioning of their surrounding medium3. Systems with large CV/EV ratios, however, typically also possess large surface to volume (SAV) ratios which increases the rate of evaporation and presents a challenge, particularly when using microfluidic devices made of water vapor permeable materials such as PDMS. Although understanding and preventing evaporation is important for microfluidic applications generally, it is particularly crucial for sensitive mammalian cell culture applications where even relatively small shifts in osmolality can drastically alter cell behavior. Our study provides five main conclusions: i) Osmolality shifts of media in PDMS devices are well approximated by simple diffusive flux models of water vapor through PDMS. Thus, evaporation-mediated osmolality shifts are particularly rapid when using devices with thin PDMS components; ii) Preimplantation mouse embryos are highly sensitive cells and their development is affected greatly by osmolality shifts such as will occur in devices with thin PDMS membranes even in typical humidified cell culture incubators; iii) Under non-humidified conditions with heating, both the 120μm- and 400μm–PDMS bottom membranes limit HDMEC survival to 25-65 minutes. iv) PDMS-parylene-PDMS “hybrid” membranes can prevent such evaporation while also providing the mechanical flexibility needed to be compatible with deformation-based microfluidic actuation systems and the optical clarity needed for cell imaging; and v) Microfluidic systems that incorporate the thin hybrid membrane can successfully develop single cell embryos to the blastocyst stage and culture human endothelial cells for prolonged periods under a non-humidified environment, whereas devices that use PDMS-only membranes cannot. The better quantitative understanding of how the osmolality of cell culture media changes in PDMS devices may be useful in accounting for and remedying such shifts in culture or for application where changes in the osmolality of media in a controlled manner during culture is necessary.41 The ability to stabilize evaporation in PDMS chips compatible with pin actuatorbased computer controlled pumps and valves using the PDMS-parylene-PDMS hybrid membrane expands the ability to perform convenient and versatile microfluidic cell and embryo culture experiments where fluid circulation and exchange can be regulated to mimic the dynamic culture environments in vivo or to manipulate reagents for long-term on-chip assays as well as to better control passive pumping using permeation-driven flow.42-43
ACKNOWLEDGMENT
This research is supported by National Institutes of Health (NIH) (HD 049607-01), United States Department of Agriculture (USDA;2005-35203-16148), the Michigan Economic Development Corporation (MEDC; GR 696), the U.S. Army Research Laboratory and the U.S. Army Research Office under Contract/Grant DAAD19-03-1-0168. We thank Professor Mark Burns (University of Michigan, Ann Arbor) and his group for their assistance with parylene coating and use of clean-room facilities and Wei Gu for Braille displays. JWS thanks funding from NIH T32 DE07057 training grant.
REFERENCES
- 1.Dertinger SKW, Chiu DT, Jeon NL, Whitesides GM. Anal. Chem. 2001;73:1240–1246. [Google Scholar]
- 2.Figeys D, Pinto D. Anal. Chem. 2000;72:330A–335A. doi: 10.1021/ac002800y. [DOI] [PubMed] [Google Scholar]
- 3.Walker GM, Zeringue HC, Beebe DJ. Lab Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 4.Leclerc E, Sakai Y, Fujii T. Biotechnol Prog. 2004;20:750–755. doi: 10.1021/bp0300568. [DOI] [PubMed] [Google Scholar]
- 5.Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Chu LY, Chueh B-H, Shen M, Hazarika B, Phadke N, Takayama S. Analyst. 2004;129:1026–1031. doi: 10.1039/b407623k. [DOI] [PubMed] [Google Scholar]
- 7.Quake SR, Scherer A. Science. 2000;290:1536–40. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TJ, Ross D, Gaitan M, Locascio LE. Anal. Chem. 2001;73:3656–3661. doi: 10.1021/ac010269g. [DOI] [PubMed] [Google Scholar]
- 9.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 10.Moor AN, Murtazina R, Fliegel L. J. Mol. Cell. Cardiol. 2000;32:925–936. doi: 10.1006/jmcc.2000.1133. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Chen CC, Buckland B, Aunins Biotechnol. Bioeng. 1997;55:783–792. doi: 10.1002/(SICI)1097-0290(19970905)55:5<783::AID-BIT8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Ozturk SS, Palsson O. Biotechnol. Bioeng. 1991;37:989–993. doi: 10.1002/bit.260371015. [DOI] [PubMed] [Google Scholar]
- 13.Takagi M, Hayashi H, Yoshida T. Cytotechnology. 2000;32:171–179. doi: 10.1023/A:1008171921282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Takagi M, Qu Y, Gao P, Yoshida T. Cytotechnology. 1999;29:27–33. doi: 10.1023/A:1008016806599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lezama R, Diaz-Tellez A, Ramos-Mandujano G, Oropeza L, Pasantes-Morales H. Neurochem. Res. 2005;30:1589–1597. doi: 10.1007/s11064-005-8837-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu MH, Dimopoulos G, Mantalaris A, Varley J. Biotechnol. Appl. Biochem. 2004;40:41–46. doi: 10.1042/BA20030170. [DOI] [PubMed] [Google Scholar]
- 17.Brinster RL. J. Exp. Zool. 1965;158:49–58. doi: 10.1002/jez.1401580105. [DOI] [PubMed] [Google Scholar]
- 18.Song JW, Gu W, Futai N, Warner KA, Nor JE, Takayama S. Anal. Chem. 2005;77:3993–3999. doi: 10.1021/ac050131o. [DOI] [PubMed] [Google Scholar]
- 19.Urbanski JP, Thies W, Rhodes C, Amarasinghe S, Thorsen T. Lab Chip. 2006;6:96–104. doi: 10.1039/b510127a. [DOI] [PubMed] [Google Scholar]
- 20.Zheng B, Roach LS, Ismagilov RF. J. Am. Chem. Soc. 2003;125:11170–11171. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
- 21.Koh CG, Tan W, Zhao MQ, Ricco AJ, Fan ZH. Anal. Chem. 2003;75:4591–4598. doi: 10.1021/ac0343836. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z, Cui Z, Cui D, Xia S. Sensor Actuat A-phys. 2003;108:162–167. [Google Scholar]
- 23.Khandurina J, McKnight TE, Jacobson SC, Waters LC, Foote RS, Ramsey JM. Anal. Chem. 2000;72:2995–3000. doi: 10.1021/ac991471a. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, Park SH, Yang H, Chung KH, Yoon TH, Kim SJ, Kim K, Kim YT. Lab Chip. 2004;4:401–407. doi: 10.1039/b313547k. [DOI] [PubMed] [Google Scholar]
- 25.Chang WJ, Akin D, Sedlak M, Ladisch MR, Bashir R. Biomedical Microdevices. 2003;5:281–290. [Google Scholar]
- 26.Futai N, Gu W, Song JW, Takayama S. Lab Chip. 2006;6:149–154. doi: 10.1039/b510901a. [DOI] [PubMed] [Google Scholar]
- 27.Shin YS, Cho K, Lim SH, Chung S, Park SJ, Chung C, Han DC, Chang JK. J. Micromech. Microeng. 2003;13:768–774. [Google Scholar]
- 28.Gu W, Zhu X, Futai N, B. Cho S, Takayama S. Proc. Natl. Acad. Sci. USA. 2004;101:15861–15866. doi: 10.1073/pnas.0404353101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Futai N, Gu W, Takayama S. Adv. Mater. 2004;16:1320–1323. [Google Scholar]
- 30.Mehta G, Mehta K, Sud D, Song JW, Bersano-Begey T, Futai N, Heo YS, Mycek MA, Linderman JJ, Takayama S. Biomedical microdevices. 2006. accepted. [DOI] [PubMed]
- 31.Favre E, Schaetzel P, Nguyen QT, Clement R, Neel J. J. Membrane Sci. 1994;92:169–184. [Google Scholar]
- 32.Watson JM, Baron MG. J. membrane Sci. 1996;110:47–57. [Google Scholar]
- 33.Tamai Y, Tanaka H, Nakanishi K, Macromolecules 1995. p. 2544.
- 34.Kaplan A, Jack R, Opheim KE, Toivola B, Lyon AW. 4th. Williams&Wilkins; Malvern, PA: 1995. Clinical Chemistry: Interpretation and Techniques; p. 151. [Google Scholar]
- 35.Barrie JA, Machin D. J. Macromol. Sci.-Phys. 1969;3(4):645–672. B. [Google Scholar]
- 36.Annamalai K, Puri KI. CRC press; Washington, D.C.: 2002. Advanced Thermodynamics engineering; p. 673. [Google Scholar]
- 37.Du Y, Mamishev AV, Lesieutre BC, Zahn M, Kang SH. IEEE Transactions on Dielectrics and Electrical Insulation. 2001;8:805–811. [Google Scholar]
- 38.Raty S, Walters EM, Davis J, Zeringue H, Beebe DJ, Rodriguez-Zas SL, Wheeler MB. Lab Chip. 2004;4:186–190. doi: 10.1039/b316437c. [DOI] [PubMed] [Google Scholar]
- 39.Carlo DD, Aghdam N, Lee LP. Anal. Chem. 2006;78:4925–4930. doi: 10.1021/ac060541s. [DOI] [PubMed] [Google Scholar]
- 40.Hubbell WH, Jr, Munir ZA. Journal of polymer science. 1975;13:493–507. [Google Scholar]
- 41.Hay-Schmidt A. J. Assist. Reprod .Genet. 1993;10:95–98. doi: 10.1007/BF01204448. [DOI] [PubMed] [Google Scholar]
- 42.Verneuil E, Buguin A, Silberzan P. Europhys. Lett. 2004;68:412–418. [Google Scholar]
- 43.Randall GC, Doyle PS. Proc. Natl. Acad. Sci. USA. 2005;102:10813–10818. doi: 10.1073/pnas.0503287102. [DOI] [PMC free article] [PubMed] [Google Scholar]