Liver replacement with a homograft was first attempted in a human on 1 March 1963 (29). Between then and Thanksgiving Day 1974, 92 more patients were similarly treated at our center. In this review, it will be emphasized that 27 of the 93 recipients achieved survival of at least one year after transplantation with a maximum of six years and that many of these patients have been able to return to a full and useful life. At the same time, attention will be focused upon the causes for the heavy mortality that has retarded the acceptance of this new procedure and upon means by which the record might be improved.
METHODS
Material
Pediatric patients
Fifty-six of the 93 recipients were 18 years of age or younger at the time of operation (Table I). Forty of these 56 pediatric recipients had biliary atresia. In the 36 youngest of the 40 patients with atresia, the mean age at operation was 31.3±15.7 (S.D.) months, range 3 to 67. The four oldest children in the atresia group were seven, 11, 11 and 15 years of age. Because of their long survival, they were thought, on clinical grounds, to have intrahepatic biliary atresia, and this diagnosis was compatible with the histopathologic findings of micronodular biliary cirrhosis in their native livers. Two of these latter four livers contained incidental liver cell carcinomas. Only one such malignant condition was found in the diseased livers of the other 36 patients with extrahepatic biliary atresia in whom transplantations were carried out at a younger age.
TABLE I.
PEDIATRIC PATIENTS
| Diagnosis | No. of examples | Survival >1yr. | Alive* now | Follow-up period present survivors, mos. | OT Nos. of survivors |
|---|---|---|---|---|---|
| Congenital biliary atresia | 40 | 11 (27.5) | 7 | 13, 15, 26, 34, 46, 53, 71 | 91, 89, 73, 64, 53, 46, 33 |
| Chronic aggressive hepatitis | 9 | 3(33) | 2 | 13, 22 | 92, 77 |
| Hepatoma, liver cell carcinoma | 3 | 2 (67) | 0 | – | – |
| Wilson’s disease | 2 | 2 (100) | 1 | 57 | 42 |
| Congenital biliary cirrhosis † | 1 | 1 (100) | 1 | 43 | 56 |
| Alpha-1-andtrypsin deficiency | 1 | 1 (100) | 1 | 25 | 74 |
| Total | 56 | 20 | 12 | 13–71 |
Figures in parentheses indicate percentages.
None of the 56 pediatric patients were HBsAg positive preoperatively.
The eight late deaths were after 12½, 13, 13½,14, 26, 30, 41 and 72 months; causes given in text and Table IV.
Associated with congenital deafness.
The 16 pediatric recipients with diagnoses other than biliary atresia (Table I) had a mean age of 12.9 ± 4.6 (S.D.) years, range 1 to 18. Nine had some variant of chronic aggressive hepatitis without HBsAG antigenemia. Three had hepatomas which could not be removed with conventional partial hepatectomy. Two had Wilson’s disease. There was one example each of congenital biliary cirrhosis and cirrhosis associated with alpha-1-antitrypsin deficiency of the homozygous PiZZ phenotype.
Adult patients
The 37 adult patients treated during the same time averaged 39.0 ±11.1 (S.D.) years, range 21 to 68. Their most frequent diagnoses were primary hepatic malignant tumor, chronic aggressive hepatitis and alcoholic cirrhosis (Table II). All of the patients with non-neoplastic disease were profoundly ill before they were considered for transplantation. Five had a diagnosis of the hepatorenal syndrome; several were unconscious; almost all were wasted from the chronic disease.
TABLE II.
ADULT PATIENTS
| Diagnosis | No. | Survived > 1 yr. | Alive† now | Follow-up period present survivors mos. | OT Nos. of survivors |
|---|---|---|---|---|---|
| Malignant tumor* | 12 | 2 (17) | 2 | 15, 22 | 90, 78 |
| Chronic aggressive hepatitis | 9 | 3 (33) | 0 | – | – |
| Alcoholic cirrhosis | 9 | 1 (11) | 1 | 20 | 82 |
| Primary biliary cirrhosis | 3 | 0 (0) | – | – | – |
| Secondary biliary cirrhosis | 1 | 0 (0) | – | – | – |
| Sclerosing cholangitis with ulcerative colitis | 1 | 0 (0) | – | – | – |
| Massive hepatic necrosis due to hepatitis B virus | 1 | 0(0) | – | – | – |
| Budd-Chiari syndrome | 1 | 1 (100) | 1 | 13 | 93 |
| Total | 37 | 7 (19) | 4 | 13–22 |
Figures in parentheses represent percentages.
The patient with massive hepatic necrosis and two of the patients with chronic aggressive hepatitis had HBsAg positive tests preoperatively.
Included seven liver cell carcinomas–three with cirrhosis–three duct cell carcinomas at the confluence of the right and left hepatic ducts which caused complete bile obstruction, one cholangiocarcinoma and one hemangioendothelial sarcoma. The two patients who are still alive had small obstructing duct cell carcinomas, but in one of these, there have been hepatic recurrences after 21 months.
The four deaths after one year occurred after 12, 13½, 19 and 20½ months. Causes are given in text and in Table VI.
For specific patients of either the adult or pediatric subgroup, orthotopic transplant or OT code numbers have been given, so that the reader may follow given recipients through different publications from our center. This method of patient identification has been used for most of our past publications, beginning with a major review of our experience in 1969 (31).
Management
The operative care and postoperative care of patients with liver transplantation have been described before (25, 31), but some details deserve emphasis. Since 1968 when the concept of brain death became widely accepted in the United States, cadavers usually have not been considered for liver donation unless there is still effective circulation and unless aortography can be performed to delineate the hepatic arterial supply in advance. Complicated preservation devices are no longer used. The livers of infants and children are usually perfused with 1 to 3 liters of a chilled electrolyte solution through the portal vein just before and after organ removal (31). Sometimes, the livers of adults and large adolescents may be more effectively cooled by connecting these cadaveric donors to a cardiopulmonary bypass into which a temperature reducing heat exchanger has been placed (31). Even after such preliminary total body chilling by means of a heart-lung machine, a final intraportal infusion of cold electrolyte solution is used. Incidentally, the technique of hypothermic cardiopulmonary bypass is excellent to rescue cadaveric donors who become unstable in the hours preceding the transplantation or in the course of removing the liver.
The HL-A match was not used as a criterion of donor-recipient selection, but these data were obtained in all instances since 1964. Major incompatibilities were present in all but a handful of patients (Table III). The quality of HL-A matching has not seemed to influence the outcome, although the scarcity of good matches has reduced the meaning of such a correlation.
TABLE III.
HL-A TYPING OF PRIMARY GRAFTS IN 93 PATIENTS
| Match* | No. | Survival, one year | Alive now |
|---|---|---|---|
| A | 1 | 1 † | 0 |
| B | 2 | 1 (50) | 0 |
| C | 14 | 3 (21) | 2 (14) |
| D | 23 | 5 (22) | 3 (13) |
| E | 40 | 15 (38) | 11 (28) |
| F | 6 | 1 (17) | 0 |
| Not done | 7 | 1 (14) | 0 |
| Total | 93 | 27 (29) | 16 (17) |
Figures in parentheses represent percentages.
A-match: HL-A identify between recipient and donor; B-match: compatibility between donor and recipient, but fewer antigens determined in the donor; C-match: one antigen incompatible; D-match: two antigens incompatible; E-match: three or four antigens incompatible, and F-match: ABO violation or positive cross match to cytotoxic antibodies.
Retransplanted after 68 days with C-match graft. Thus, the one year survival was mainly due to the second less well matched organ.
Preformed antired cell isoagglutinins that react against donor tissues and cytotoxins, which can be detected by their lysis of donor lymphocytes, immediately destroy many renal homografts that are transplanted in violation of such positive cross matches. The liver is resistant to this so-called hyperacute rejection (26). In our series, three liver transplantations were carried out in spite of red blood group incompatibility and three more were performed in confrontation of cytotoxic antibodies (Table III). There were no unequivocal hyperacute rejections. An account of this experience is being published separately.
Other modifications of technique or changes in our past policy should be mentioned. Originally, splenectomy was performed at the time of transplantation if this procedure had not already been carried out at an earlier time and if it was considered to be safe. Of the first 80 patients, four had prior and 57 concomitant splenectomy. In the last 13 patients of this report, splenectomy was omitted.
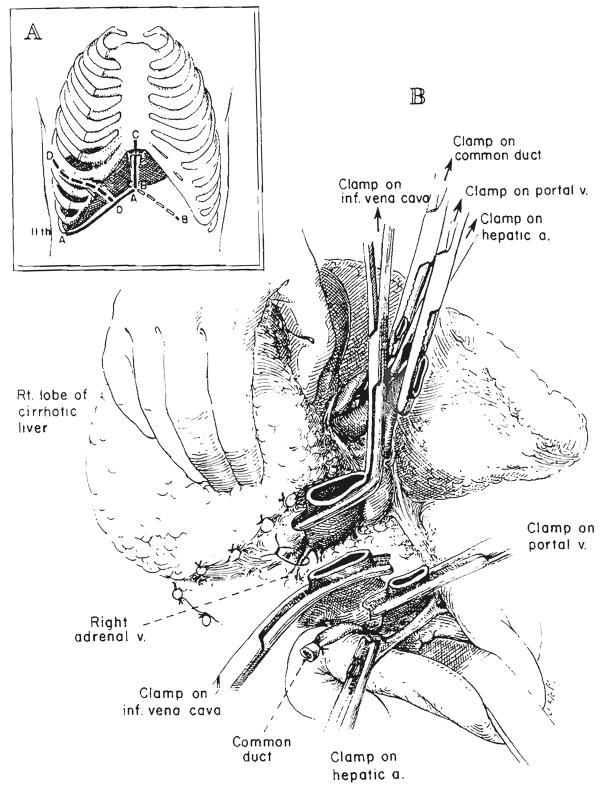
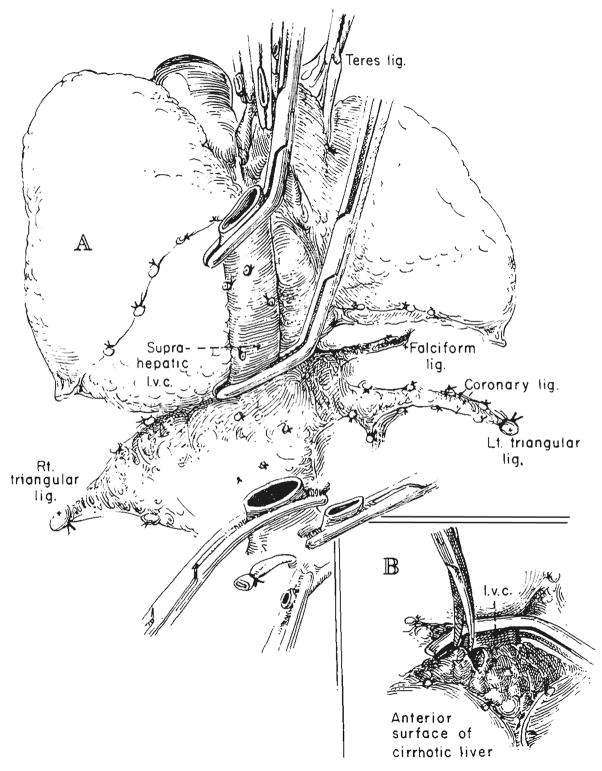
Host hepatectomy is usually still performed by individually dissecting the hilar structures and the vena cava above and below the liver and by then cross clamping and dividing the vessels just as the liver is removed (29, 31). However, in some adults, a safer way has been to transect first the hilar structures and, subsequently, the infrahepatic inferior vena cava when all else is in readiness (Fig. 1). Then, by pulling on clamps which are placed on the hepatic side of these structures, the liver is dissected free from below to above, ligating all cut tissues on the way (Figs. 1 and 2). The suprahepatic inferior vena cava remains intact as the stalk of the specimen until it is clamped just before the liver is removed. This variation in operation has been particularly useful in developing a reasonably long suprahepatic cuff of the inferior vena cava in adults. The vena cava or main hepatic veins may be dissected free from within the cirrhotic liver in a bloodless field (Fig. 2B).
Fig. 1.
Technique of retrograde removal of liver. A, Incisions. AA, Subcostal incision used for all orthotopic liver transplantations. BB, CC and DD, Frequently used extensions from the AA incision. B. Beginning retrograde removal after transection of inferior vena cava and hilar structures. All posterior tissue that is cut should be ligated, although the named vessels encountered, such as the right adrenal vein, are few in number.
Fig. 2.
A, Operative field after retrograde liver mobilization. The last remaining structure, the suprahepatic inferior vena cava, has been clamped above the liver. B, Technique for mobilizing a suitable length of suprahepatic vena cava after placement of clamp. In adults, this usually involves cutting away cirrhotic liver tissue over the frequently distorted and foreshortened right and left hepatic veins.
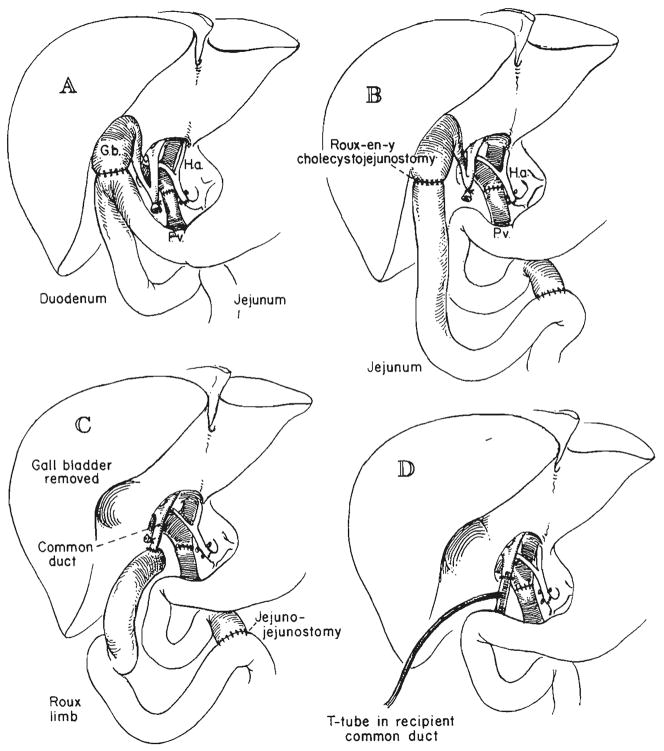
In several of our first recipients who did not have biliary atresia, bile duct reconstruction was with choledochocholedochostomy over a T-tube stent (Fig. 3D). The method lost favor because of a high incidence of bile fistula, and cholecystoduodenostomy after ligation of the common duct (Fig. 3A) became our first choice for a number of years. However, since November 1973, the preferred technique has been cholecystojejunostomy with a Roux-en-Y loop (Fig. 3B), thus removing the homograft from the mainstream of the gastrointestinal tract and draining it through a defunctionalized jejunal limb. Alternatively, Roux-en-Y choledochojejunostomy (Fig. 3C) or choledochocholedochostomy (Fig. 3D) has been used for recently treated patients. In a number of patients, it has been necessary to convert from cholecystojejunostomy to choledochojejunostomy (Fig. 3B and C) because of obstruction at the cystic duct (17).
Fig. 3.
Techniques of biliary duct reconstruction used for most of the transplantation recipients. A, Cholecystoduodenostomy. B, Cholecystojejunostomy. C, Choledochojejunostomy after removal of gallbladder. D, Choledochocholedochostomy. Note that the T-tube is placed, if possible, in recipient common duct.
The first five patients were treated with azathioprine and prednisone. The next 88 recipients were given triple drug immunosuppression, which usually consisted of azathioprine and prednisone, to which a two weeks to four months’ course of horse antilymphocyte globulin was added (31). If hepatotoxicity of azathioprine was suspected, cyclophosphamide was substituted, since it has an immunosuppressive effect comparable with that of azathioprine (32). In a number of later patients, cyclophosphamide was used at the beginning with a switch later to azathioprine.
Histopathologic Studies and Clinical Correlations
During the 12½ years of study, samples were accumulated from the diseased native livers, homografts and selected other tissues and fixed in 10 percent buffered Formalin (aqueous solution of formaldehyde). Frozen sections were stained with Sudan IV for fat, and then, the remainder was processed and the paraffin sections were stained with hematoxylin and eosin, Gordon and Sweet’s silver impregnation method for reticulin fibers, Perls’ Prussian blue method for iron, Weigert’s for elastic counter-stained with hematoxylin and van Gieson, trichrome for collagen and fibrin, periodic acid-Schiff method for glycogen, Pearse’s method for ceroid and lipofuscin, and methyl green pyronin.
Frequently, additional small hepatic samples were initially fixed in glutaraldehyde solution, and then postfixed in osmic acid and embedded in Epon, a synthetic embedding medium. Half micron and ultrathin sections were cut. The former were stained with azure II for examination in the light microscope, while the latter were stained with lead citrate and examined in a Phillips 300 electron microscope.
Many of the findings in the liver grafts have been reported before (21). In preparing this survey, the old specimens were re-examined, and new ones were analyzed. Clinicopathologic correlations were made, so that a final judgment could be made in each about the reasons for failure. The most important element in the final decision was the state of the homograft and the evidence in it of injury from an old or a new rejection. The clinical course and the findings of the autopsy also were taken into consideration.
An effort to assign a single, most important cause of failure inevitably required oversimplification, since the terminal events were always complex. Thus, although infection almost invariably played an important role at the end, as has been emphasized before (17, 25, 31), infections are not mentioned hereinafter unless they seemed to play a triggering and primary role. The all important role of infection in these same patients is to be described in a detailed separate publication.
The effects of hepatic homograft rejection and other postoperative events upon liver function tests have been exhaustively described by us (17, 25, 29, 31) and by Williams and his associates (35). The abnormalities of function with rejection are not diagnostic, since the profile of tests may range from a pattern of obstruction to one of parenchymal necrosis. The single most important test in observing the patients has proved to be serum bilirubin concentration, which frequently will be referred to in the clinicopathologic correlations.
RESULTS
Pediatric Patients
Other than biliary atresia
Nine of the 16 patients lived for at least one year after transplantation. The seven others who received a total of eight grafts died after one to 188 days. Acute rejection (Fig. 4) was directly responsible for one of these seven deaths (Table IV). Late rejection (Fig. 5) of a primary graft indirectly led to another death, inasmuch as a retransplantation became necessary and was not successful in spite of the fact that the second organ was not rejected (Table IV). The non-immunologic factors of biliary obstruction, tumor recurrence, perforation of a colonic diverticulum and ischemic liver necrosis were responsible for the other fatalities that occurred within the first year (Table IV).
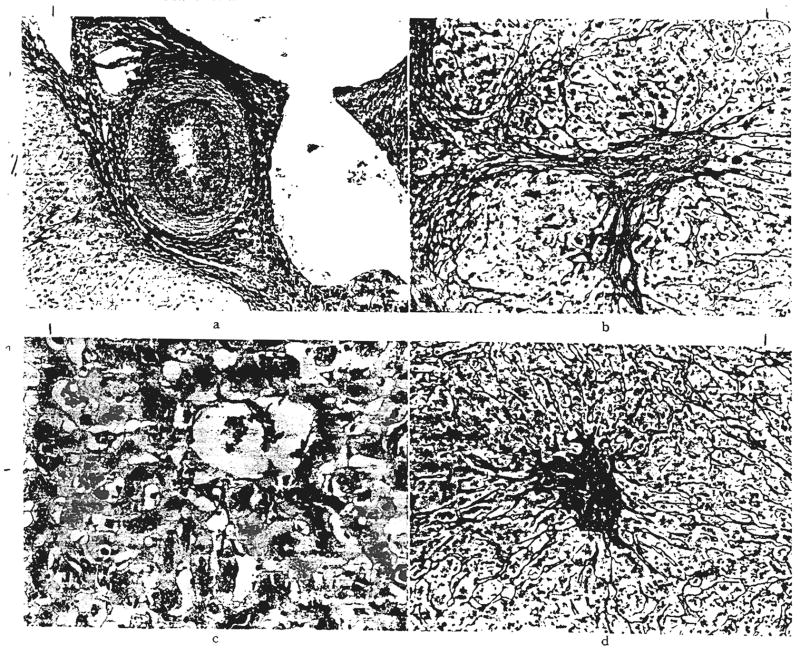
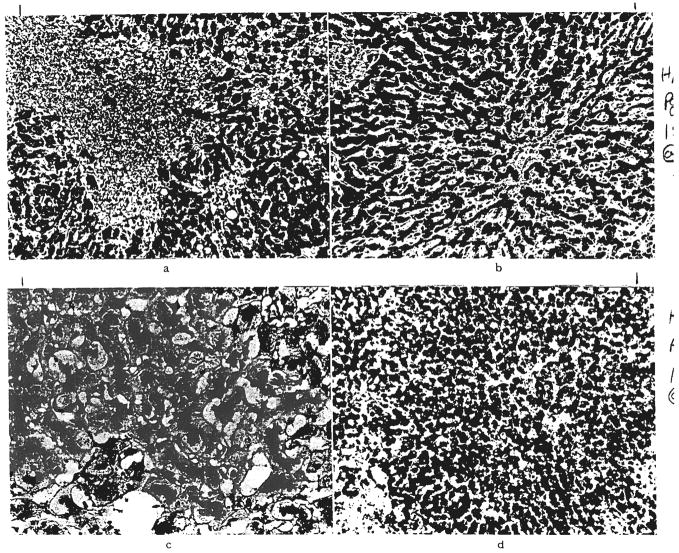
Fig. 4.
Acute rejection. Hepatic homocraft, OT 31, nine days after transplantation. Large numbers of lymphoid cells are present in the portal tracts and between the damaged hepatocytes. Hematoxylin and eosin, a, ×300; b, ×500.
TABLE IV.
CAUSES OF DEATH IN 11 PEDIATRIC PATIENTS WITHOUT BILIARY ATRESIA
| OT No. | Time of death, days | Main cause of death | Rejection | Last bilirubin determination, mgm. per cent |
|---|---|---|---|---|
| 20 | 1 | Ischemic liver necrosis | No | – |
| 31 | 9 | Acute liver failure | Yes | 30 |
| 44 | 34 | Biliary obstruction, Unrecognized | No | 14 |
| 41 | 61 | Biliary obstruction, Corrected | No | 13 |
| 57 | 64 | Perforated diverticulum colon: | No | 3 |
| 23 | 143 | Hepatoma recurrences* | No | 40 |
| 65 | 188 | Infection after second transplantation at 157 days | Yes healing, first | 17 |
| No, second | 1.5 | |||
| 8 | 400 | Hepatoma recurrences;* bile obstruction | No | 15 |
| 14 | 436 | Hepatoma recurrences ;* chronic rejection: complications after second transplantation at 379 days | Yes healing, first | 34 |
| No, second | 5 | |||
| 55 | 780 | Biliary obstruction, unrecognized | No | 30 |
| 27 | 2190 | Chronic liver failure; bile obstruction | Yes healed | 16 |
Hepatoma was original disease.
Fig. 5.
Chronic rejection. a, A small hepatic artery branch in a graft, OT 16b, 339 days after retransplantation is almost occluded by intimal thickening. Elastic stain, ×60. b, Residual scarring after rejection. Same graft, OT 16b. There is increased portal fibrous tissue and connective tissue septums extend into the lobules. Reticulin, ×60. c, Centrilobular cholestasis with bile “thrombi” in dilated canaliculi in graft, OT 14a, one year and 16 days after transplantation. Bile stain, ×200. d, Collapse of reticulin fiber framework around central veins in graft, OT 10, 186 days after transplantation. Reticulin, ×60.
Shortly after one year (Table IV), two more patients died with recurrences of the hepatomas for which the transplantations had been performed. In one of these livers, biliary obstruction developed as the result of a metastasis, and the other one contained evidence of chronic rejection, for which retransplantation was attempted. A third child died 26 months after liver replacement for chronic aggressive hepatitis. At autopsy, a stricture was found at the choledochocholedochostomy site, with dilatation of the duct system which was packed with inspissated bile and debris (Fig. 6). There was no evidence of rejection. The mechanical problem probably could have been corrected if it had been diagnosed. Transhepatic cholangiography had been attempted four times in the six months before death, but the duct system was not entered.
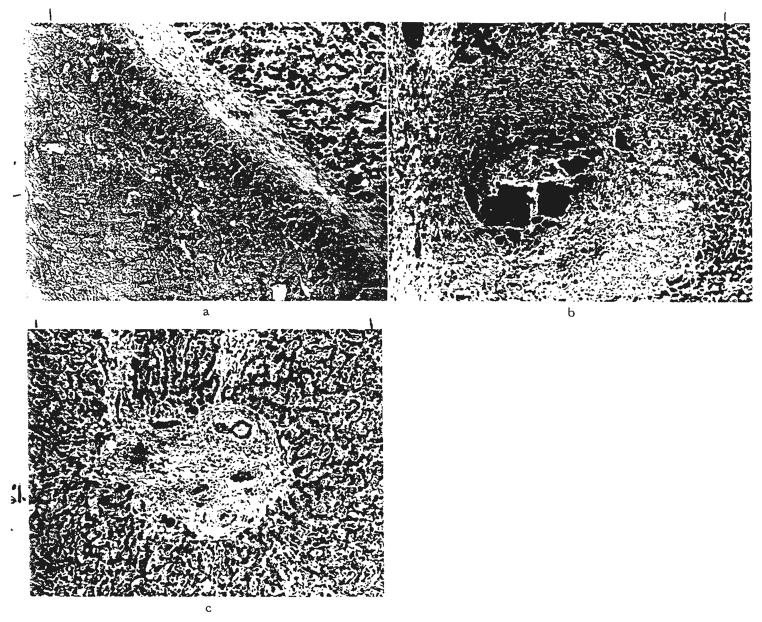
Fig. 6.
Large bile duct obstruction. a, Biliary cast syndrome in graft, OT 55, two years and two months after transplantation. The wall of the distended bile duct is fibrous, and the lumen is blocked by inspissated bile and debris. Hematoxylin and eosin, ×60. b, Biliary cast syndrome in graft, OT 43, 47 days after transplantation. A portal tract is greatly expanded and an intrahepatic bile duct is blocked by inspissated bile. Hematoxylin and eosin, ×50. c, Chronic large duct biliary obstruction. Enlarged fibrous portal tract with proliferated bile ductules containing bile casts. Hematoxylin and eosin, ×70.
The last late death occurred six years after transplantation. The original diagnosis in this patient was Wilson’s disease. After liver replacement, the homograft had no tendency to accumulate copper (30). About four years postoperatively, the patient was thought to have discontinued the immuno-suppressive therapy for several months. Liver function, which had been perfect, deteriorated, and he became jaundiced. Resumption of treatment did not reverse the process in spite of the fact that the liver biopsy now had relatively minor abnormalities, Because a transhepatic cholangiogram showed a partial biliary obstruction, bile duct reconstruction was revised with conversion of cholecystoduodenostomy to choledochojejunostomy; jaundice persisted. At autopsy, almost two years later, the main finding in the liver homograft was cholestasis, similar to that seen in Figure 6, suggestive of chronic bile duct obstruction.
Five of the 16 recipients in this nonatresia pediatric subgroup are still alive after 13 months to almost five years (Table I). Each attends junior high school, high school or a university. They all have normal, or nearly normal, liver function, with serum bilirubin concentrations ranging from 0.5 to 1.5 milligrams percent.
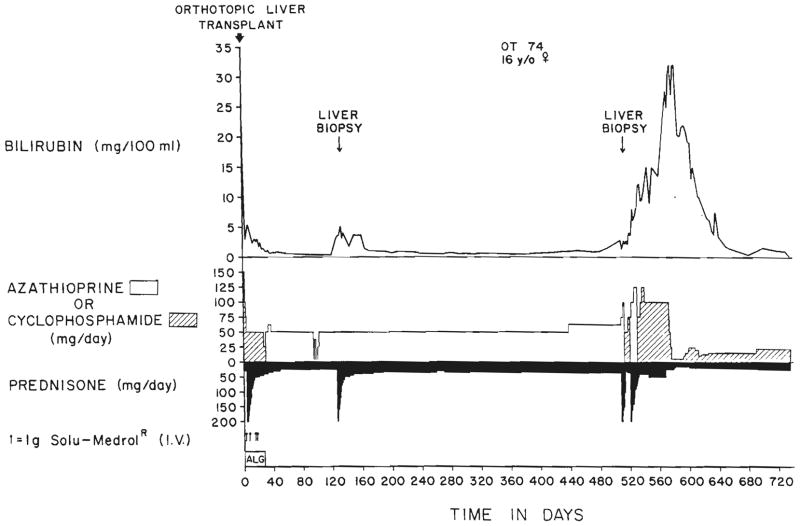
One of these patients who is now well became deeply jaundiced and profoundly ill almost a year and a half after transplantation. A liver biopsy was interpreted as showing drug toxicity (Fig. 7). Cyclophosphamide was substituted for azathioprine with subsequent slow improvement of liver function during the next few months (Fig. 8).
Fig. 7.
Presumed drug toxicity. Biopsy of a graft in a patient, OT 74, who was deeply jaundiced at one year and five months after transplantation. The liver functions returned to normal after azathioprine was replaced with cyclophosphamide and after the prednisone dosage was lowered to less than half of its previous level. a, Centrilobular hepatocytes are swollen and show abnormal variation in nuclear size. Hematoxylin and eosin, ×200. b, Centrilobular cholestasis with bile thrombi in dilated canaliculi. Bile stain, ×200. c, Normal portal tract. Hematoxylin and eosin, ×200.
Fig. 8.
The first two post-transplantation years of patient, OT 74. The second liver biopsy, which is shown in Figure 7, suggested drug toxicity. Substitution of cyclophosphamide for azathioprine and reductions in steroid dosage were followed by gradual resolution of the profound hyperbilirubinemia.
Biliary atresia
The 40 recipients were given a total of 45 livers. One of the second grafts was from a chimpanzee (26, 30). The other four organs for retransplantation were from cadavers as were all the 40 primary grafts. The one year survival figure was 11 of 40 patients or 28 per cent.
In a recent publication, clinical or pathologic analyses were given for each of the 40 individual patients (30). Our interpretations from that raw data of the main reasons for graft losses and deaths are summarized in Table V. The complications came in progressive waves, to which specific etiologic factors selectively contributed at successive times.
TABLE V.
REASONS FOR 37 ORTHOTOPIC HOMOGRAFTS AND ONE CHIMPANZEE HETEROGRAFT BEING LOST AFTER TRANSPLANTATION INTO 33 CHILDREN WITH BILIARY ATRESIA WHO EVENTUALLY DIED
| Category | No. of examples | Time to death or graft loss, days | OT No. |
|---|---|---|---|
| Acute technical or management | 11 | ||
| Hepatic artery clot (3) | 4, 20, 1 | 18, 38, 52b | |
| Hemorrhage (2) | 0,2 | l,76b | |
| Graft necrotic (2) | 11,2 | 24, 48 | |
| Portal vein clot (1) | 1 | 21 | |
| Outflow venous obstruction (1) | 7 | 34 | |
| Uncorrected hypovolemia (1) | 1 | 80 | |
| Pulmonary insufficiency (1)§ | 14 | 71b | |
| Delayed technical | 7 | ||
| Biliary obstruction (4)* | 37, 47, 73, 84 | 30, 43, 49, 84 | |
| Biliary fistula (2)* | 81,28 | 47, 68 | |
| Fungus peritonitis and traumatic pancreatitis (1) | 76 | 26 | |
| Acute rejection | 4 | 7, 31, 10,21 | 7, 50, 71a, 86 |
| Chronic rejection | 3 | 881; 65, 339 | 13a, 16a, 16b |
| Septic hepatic infarction | 4† | 133, 186, 61, 105 | 9, 10, 11, 12 |
| Infection of graft | 3 | ||
| Hepatitis (2) | 377, 33 | 29, 76a | |
| Aspcrgillus (1) | 20 | 13b | |
| Systemic infection | 5‡ | ||
| ? gram-negative bacillus (2) | 36, 175 | 35, 59 | |
| Hemophilus (1) | 1238 | 19 | |
| Nocardia and Candida (1) | 51 | 37 | |
| Herpes (1) | 59 | 67 | |
| Transplantation of liver with atresia | 1 | 85 | 52a |
a Primary graft in patient who underwent retransplantation; b second graft.
Bile duct reconstruction was attempted in three of these six patients but unsuccessfully.
Three of these four livers had vascular or other findings of chronic rejection.
Two of these five livers had vascular findings of chronic rejection.
This child received a. chimpanzee heterograft at retransplantation.
Acute problems of surgical technique or management were the most common causes of failure and resulted in the deaths of 11 children in 5.7 ± 6.6 (S.D.) days, range zero to 20. Graft vascular thrombosis, bleeding and the use of necrotic livers headed the list (Table V). The deaths occurred too soon to permit an effect upon the pre-existing jaundice, except in a child in whom the hepatic artery clotted but who had the longest survival time of 20 days. The serum bilirubin level fell promptly and was 1.5 milligrams per cent just before death, which resulted from infection in multiple areas of liver necrosis. Birtch and Moore (4) have reported a similar experience of survival of 45 days by a patient who had a dearterialized and partly necrotic but functioning homograft. The recipient of the chimpanzee heterograft had a serum bilirubin concentration of 14 milligrams per cent after 14 days when he died from the pulmonary insufficiency that had been present since the time of retransplantation.
Another seven children died after 60.9 ± 22.9 (S.D.) days from additional mechanical complications which usually involved the reconstructed biliary duct system in one of the ways shown in Figure 9 and which were not immediately lethal (Table V). Infection was the usual final event in the downhill course. The last serum bilirubin concentrations in these patients were 9.9 ± 6.1 (S.D.) milligrams per cent, range 3 to 22. There was no histopathologic evidence of rejection in any of the grafts, nor was cholangitis a prominent feature. Virus infestation of the cells lining the bile ductules or of the hepatocytes was diagnosed in three of these seven livers (Fig. 10). It has been suggested by Martineau and his associates (17) that such virus infestation of the biliary tree can cause swelling, necrosis and shedding of the infected cells to form obstructing casts.
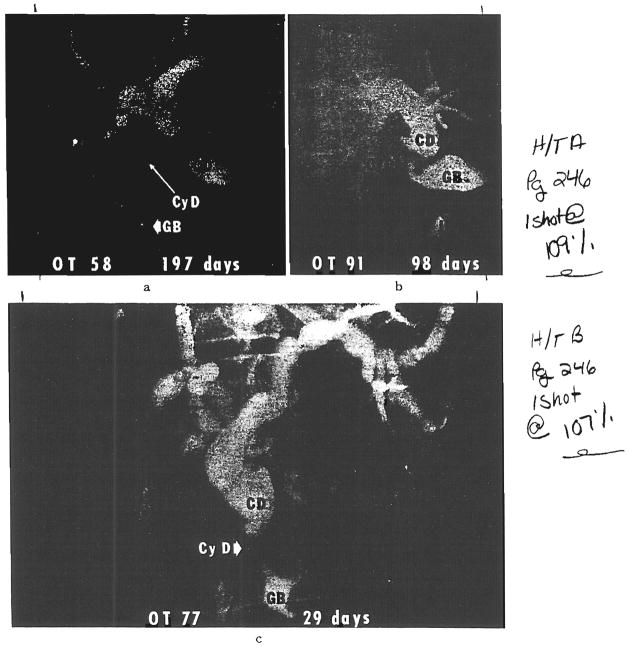
Fig. 9.
Examples revealed by transhepatic cholangiography of homograft cystic duct obstruction after biliary reconstruction with cholecystoenterostomy. a, Original procedure was cholecystoduodenostomy. After this transhepatic cholangiogram, conversion was made to choledochoduodenostomy. At operation, the filling defect near the exit of the cystic duct was found to consist of a chalk-like sludge. There was not complete relief of jaundice. When the patient died 13 months after transplantation, the homograft still had intrahepatic evidence of large duct obstruction. b, The original reconstruction was with cholecysto-Roux-en-Y-jejunostomy. This was converted to a choledochojejunostomy. The patient is well a year later. c, The original reconstruction was with cholecysto-Roux-en-Y-jejunostojrny. This was converted to a choledochojejunostomy. The patient is well about two years later. CD, Common bile duct; CyD, cystic duct; GB, gallbladder, and J, Roux-en-Y limb of jejunum.
Fig. 10.
Virus infestation of cells lining bile ducts. a, Several bile ducts obstructed by swollen virus infected cells in an expanded portal tract in a liver graft, OT 30,37 days after transplantation. Hematoxylin and eosin. ×50. b, Detail of similar obstructed duct in another infected graft, OT 44, shows enlarged lining eoithelial cells containing nuclear inclusions. Cytomegalovirus was identified in this liver. Hematoxylin and eosin, ×700.
The foregoing pooled 18 examples of predominantly technical or mechanical difficulties accounted for 55 per cent of all the deaths in the atresia experience. The staggering total actually may have understated the true contribution of surgical technical complications to the failures, since it excluded the four examples of septic hepatic infarction (Table V) in which portions of the liver became dearterialized and necrotic with invasion by bacteria from the intestinal tract. The latter complication has been suggested to be due to a combination of insufficient immunosuppression plus technical or mechanical factors which twist or otherwise jeopardize the hepatic arterial patency (25, 31).
Rejection played a surprisingly minor role in causing graft loss or death, at least insofar as this could be detected by histopathologic examination. Four patients acutely rejected the primary graft (Fig. 4) after 17.3 ± 11.0 (S.D.) days. The last serum bilirubin concentrations were 17.8 ± 7.5 (S.D.) milligrams per cent, range 13 to 29.
Three grafts were designated as being lost primarily from chronic rejection because of the severe arterial narrowing, lymphoid cell infiltration and other findings that are characteristic of this process (Fig. 5). These transplants had been in place for two months to two and one-half years and were supporting serum bilirubin concentrations of 19, 10 and 20 milligrams per cent. Three of the four livers that were lost primarily because of septic hepatic infarction had chronic rejection as well.
Systemic infections or else infestations of the homografts were thought to be the main causes of failure in eight patients, generally at an intermediate time. Concomitant chronic rejection had damaged two of the homografts of the five patients who had lethal systemic infections (Table V). Serum bilirubin concentrations were variable in these eight instances, ranging from 1 to 52, with a mean of 16.0 ± 16.4 (S.D.) milligrams per cent. The three patients with infected livers had bilirubin levels of 52, 15 and 10 milligrams per cent.
One liver which was obtained from an anencephalic monster never cleared bilirubin. Ironically, it had the histopathologic findings of intrahepatic biliary atresia when it was removed 85 days later. An attempt at retransplantation failed when the hepatic artery of the second transplant clotted.
Eleven or 28 per cent of the 40 patients survived for at least one year after operation, and seven are still alive with good or perfect function from their original hepatic homografts after 13, 15, 26, 34, 46, 53 and 71 months (Table I). All are living essentially normal lives. Their return visits to Colorado range from every three to 12 months. None are jaundiced. In two of these patients, serial biopsies were obtained. Some of the early specimens showed classical cell mediated rejection (30) which was readily controlled as judged by the final clinical outcome. As already recounted, the late deaths occurred 12½, 13½, 30 and 41 months after transplantation.
Adult Patients
Only seven or 19 per cent of the 37 adult recipients lived for as long as one year postoperatively. The results were not satisfactory after transplantation for any of the main indications for liver transplantation (Table II). Only one of nine alcoholics survived for as long as a year. Three of nine patients with chronic aggressive hepatitis lived for this long, as well as two of 12 patients with primary tumors of the liver. Two patients in the malignant category are still alive 15 and 22 months after liver replacement for the small intrahepatic duct cell carcinomas described by Klatskin (15) that obstruct the confluence of the right and left hepatic ducts and cause early jaundice. The recipient with the longer follow-up period has multiple recurrences in the transplanted liver. The patient (Table II) who had the Budd-Chiari syndrome is the only one with this diagnosis known to have been treated with liver transplantation.
In Table VI, a single principal reason was identified for the loss of each of the 35 homografts that were transplanted to the 33 adult recipients who eventually died. Seventeen of the recipients or 59 per cent of the 29 who died in the first postoperative year had lethal complications which had their genesis intraoperatively or just afterward (Table VI).
TABLE VI.
PRINCIPAL REASONS FOR 35 ORTHOTOPIC HOMOGRAFTS BEING LOST AFTER TRANSPLANTATION INTO 33 ADULTS WHO EVENTUALLY DIED
| Category | No. of examples | Time to death or graft loss, days | OT no. |
|---|---|---|---|
| Acute technical | 11 | ||
| Graft necrotic(3) | 7, 8, 8 | 6,75,85 | |
| latrogenic duct obstruction (3)* | 10, 39, 20 | 22, 25, 79 | |
| Biliary fistula (5)* | 23, 13, 27, 29, 34 | 5, 28, 63, 69, 70 | |
| Acute neurologic | 6 | 6, 3, 26, 32, 41, 22 | 4, 32, 39, 40, 61, 72 |
| Systemic infection§ | 7 | 22, 7, 35, 22, 11, 15, 9 | 2, 3, 17, 54b, 62b,81, 83 |
| Delayed biliary obstruction | 6† | 564, 408, 62, 111, 62, 47 | 54a, 58, 60, 62a, 87, 88 |
| Acute rejection | 1‡ | 9 | 51 |
| Chronic Rejection | 0 | – | – |
| Recurrent tumor | 2 | 349, 87 | 15, 45 |
| Recurrent hepatitis | 1 | 623 | 36 |
| No satisfactory explanation | 1 | 161 | 66 |
a Primary graft in patient who underwent retransplantation; b second graft.
Reoperation was attempted in three of these patients, one with obstruction and two with fistula.
Attempts were made to operate secondarily upon four of these patients.
Four other grafts contained residual changes or repair following acute rejection, but this was not considered to be the main cause of failure.
Bacterial or fungal or both.
Eleven of these 17 early accidents were classed as technical and led to death in 19.8 ± 11.4 (S.D.) days. Only one of these 11 patients was not jaundiced before death. The other ten had final serum bilirubin concentrations that ranged from 7 to 46 milligrams per cent, with a mean of 26.8 ± 12.7 (S.D.). Three of the patients were given livers that were irreparably damaged by ischemia in operations that were long and bloody. The grafts of three more recipients were ruined by tying off an anomalous double barreled channel of cystic and common duct preparatory to performance of cholecystoenterostomy (31). The complication was not recognized or treated in two patients. An attempt to relieve the consequent total biliary obstruction in the third patient resulted in a bile fistula and lethal peritonitis. In five more patients, bile fistulas developed with peritonitis, and they died after 13 to 34 days. The leaks were at the site of choledochocholedochostomy or at the T-tube insertion site in four instances. The fifth fistula was caused by necrosis of the gallbladder after cholecystoenterostomy.
Cerebrovascular accidents accounted for six other puzzling intraoperative or early postoperative complications. Two of the patients were in Stage IV hepatic coma at the time of operation, but the other four were more or less alert. After operation, five of the six recipients, including one in previous coma, had a lucid interval that lasted from a few hours to three days before they were crippled by neurologic disabilities that were suggestive of major brain stem damage. The transplant operations in each instance had been long and difficult, with many blood transfusions which further reduced thrombocyte counts that had already been low. The neurologic damage was so profound in each instance that death followed within a few days, or else a decision was later made to stop active treatment. The six patients died after 21.7 ± 14.8 (S.D.) days, range 3 to 41. By then, two patients had serum bilirubin concentrations of 28 and 7 milligrams per cent, respectively, but the other four had bilirubin values which ranged from 0.7 to 2.7 milligrams per cent. All the liver grafts were free of rejection, and two were completely normal. The brains had areas of myelinolysis in the brain stem. In addition, there were other cortical and subcortical abnormalities in some of the brains. These latter included small hemorrhages, infarcts and edema.
There were seven deaths 17.3 ± 9.8 (S.D.) days after transplantation from systemic infections which usually reflected the poor preoperative state of the patients. Two were receiving second grafts, one patient had candidemia at the time of transplantation, one was a fragile end-stage alcoholic and three were patients who were treated early in our experience before infectious disease care under conditions of immunosuppression had been standardized. Two of these last three patients had also had pulmonary embolization from clots which had formed in a venovenous bypass tube which is no longer used (29). The final serum bilirubin concentrations were variable, ranging from 1.5 to 22 milligrams per cent, with a mean of 9.9 ± 7.6 (S.D.).
At an intermediate time of 209 ± 221 (S.D.) days, ranging from 47 to 564 days, six grafts were retrieved by death or retransplantation that had histopathologic findings of extrahepatic biliary obstruction. The original biliary duct reconstruction was with cholecystoduodenostomy or Roux-en-Y cholecystojejunostomy. There was clinical evidence of adequate bile excretion initially. However, it was either proved later in four patients or assumed in retrospect that the site of obstruction was at the cystic duct, as shown in Figure 9. Reoperation was performed in four patients, and a secondary choledochoenterostomy was attempted with what was thought to be success. However, histopathologic findings persisted within the liver of large bile duct obstruction weeks or months later. Furthermore, jaundice was not permanently relieved. The final mean serum bilirubin concentration reflecting the function of these grafts was 18.4 ± 8.4 (S.D.) milligrams per cent, range 5 to 28. Two of these apparently obstructed hepatic homografts were removed after 564 and 111 days and replaced under the probably erroneous assumption that they had been rejected. The patients died 11 and 22 days later.
The most surprising observation was that only one of the 35 livers transplanted to adult recipients contained the classical findings of acute cell mediated rejection. Four other grafts contained residual changes of acute rejection or evidence of repair following this process, but rejection was not considered to be the main cause of failure. Furthermore, there were no examples of chronic rejection.
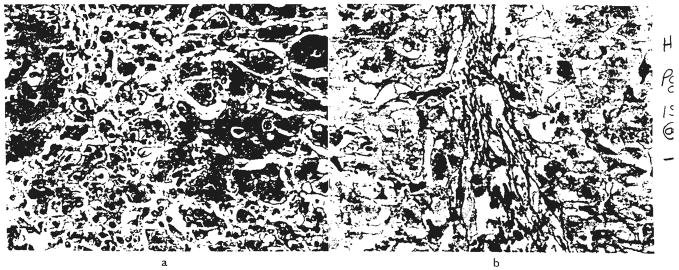
However, only three of the remaining 30 livers were structurally normal. The majority of the others contained such diverse and nonspecific findings (Fig. 11) as fatty infiltration, venous congestion, centrilobular cholestasis, atrophy or necrosis of the centrilobular hepatocytes and focal necrosis or infarction that were thought to be secondary to ischemia or sepsis. One liver had severe centrilobular atrophy and reticulin collapse at the time of death from hepatic failure after 161 days. A convincing final diagnosis was not made. Several livers were infested with cytomegalovirus particles (Fig. 10) which were in hepatocytes and in the cells lining ducts.
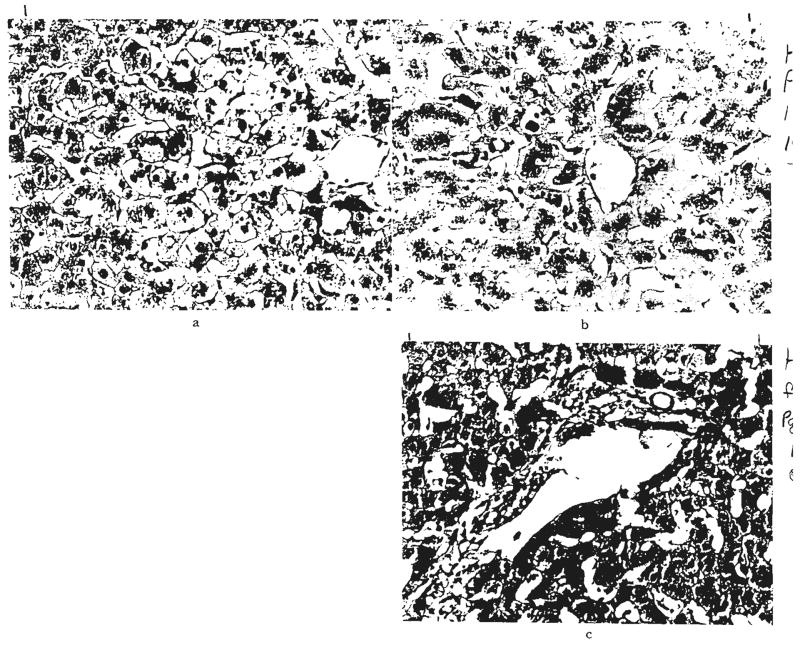
Fig. 11.
Patterns of liver injury not thought to be rejection or obstruction. a, Focal areas of necrosis, OT 69. Hematoxylin and eosin, ×60. b, Acute venous congestion, OT 17. Hematoxylin and eosin, ×60. c, Centrilobular cholestasis with bile thrombi, OT 97. There was no evidence of large duct obstruction, drug toxicity or rejection in this patient. Hematoxylin and eosin, ×200. d, Diffuse fatty infiltration of hepatocytes, OT 94. Frozen section stained with Sudan IV, ×60.
Two hepatic grafts were invaded with recurrent malignant tumors of the same kind that had destroyed the native liver, leading to death after 349 and 87 days. One was a hepatoma and the other was a hemangioendothelial sarcoma.
Four of the seven patients who survived for one year are still alive with follow-up periods of 13 to 22 months. They have perfect liver function. All four live at home and work full time or attend school. The patient with the longest follow-up period has recurrences of intrahepatic duct cell carcinoma.
The three deaths after one year were at 13½, 19½, and 20½ months. All were patients whose original diagnosis was chronic aggressive hepatitis. However, recurrence of the aggressive hepatitis was observed only in the recipient who was an HBsAg virus carrier (Fig. 12). Candidiasis and nocardiosis contributed to her death as Corman and his associates have reported (7). In the other two recipients, the grafts were destroyed by the biliary obstruction already cited, which was not recognized in one instance leading to an ill-advised retransplantation and which was apparently not effectively treated in the other.
Fig. 12.
Recurrence of viral hepatitis. a, Hepatitis affecting graft, OT 36. Hematoxylin and eosin, ×250. b, Same graft stained with orcein. Granular material is present in the hepatocyte cytoplasm. This usually indicates the presence of hepatitis B viral antigen. Shikata stain, ×300.
DISCUSSION
In a positive sense, the most important conclusion that has emerged from this experience with liver replacement was that prolonged survival repeatedly was possible. A total of 27 patients lived for at least a year following operation, and 16 of this group are still alive after more than one to almost six years. The outlook has slowly improved, although not to a satisfactory state. The first 25 recipients who formed the basis of a monograph on liver transplantation (31) included only five one year survivors. The next group of 25 contained six, and the group after that had eight. There have already been eight one year survivors among the 18 patients beginning with No. 76.
The chronic survivors, particularly those in recent times, have had remarkably stable liver function, and usually, they have achieved complete social rehabilitation. Survival of more than a year after orthotopic liver transplantation has been recorded from other centers by Williams (35), Waldram and Calne and their associates (34) in England, by Daloze and his colleagues (8)1 in Canada and by Hume and his associates (12) in the United States.
One reason the results were not better was the great technical difficulty of performing orthotopic liver transplantation under the trying conditions of end-stage hepatic disease with consequent portal hypertension and multiple coagulation and metabolic defects. Even excluding the biliary duct complications, about a fourth of the patients who died left the operating room with situations that were incompatible with survival, such as ischemic liver necrosis, graft vascular thrombosis and uncontrolled hemorrhage. The mortality from technical accidents involving vessels was particularly high in infants with biliary atresia, partly because of the small size of the structures for anastomosis. The recent use of microsurgical techniques has almost completely eliminated such losses. In future trials, children with a wide range of diseases, including biliary atresia, undoubtedly will be the most favorable group of recipients.
More critical questions must be asked about the six crippling cerebrovascular accidents which constituted the single most common lethal complication intraoperatively and early postoperatively among the 37 adults. The most consistent, although not the only, damage was found in the brain stem and consisted of the kind of myelinolysis that is associated with end-stage liver disease, Williams and his associates (35) also may have encountered cerebrovascular accidents, inasmuch as two of their 26 recipients failed to awaken from anesthesia. Lampe and his co-workers (16) have reported a serious, but partly reversible, diffuse brain injury in an 11 year old recipient of an orthotopic liver.
They ascribed the complication to intraoperative hyperosmolarity that had been caused by excessive glucose administration. Hyperglycemia was not a regular feature of our patients, although it was present in some of them. Intraoperative thrombocytopenia and hypocoagulability may have led to small hemorrhages into areas of pre-existing brain disease. Air embolus as the causation has not been ruled out. The neuropathologic findings in these autopsies are being restudied, so that preventive measures may be developed for future patients. In the meanwhile, the high risk of brain damage in adult patients who had a wide variety of diagnoses suggests that as a general policy candidacy for transplantation was being considered too late in the terminal course of the disease.
In the livers that were eventually retrieved for study by death of the recipient or removal at retransplantation, a potentially encouraging but perplexing observation was the paucity of unequivocal findings of either acute or chronic rejection. Early biopsies from a few of these grafts contained the unmistakable lymphoid infiltrations of acute cell mediated rejection (30, 31), but this process usually had been controlled at least by histopathologic criteria by the time the whole organ was examined. Moreover, the kind of chronic rejection that has been so well described in dogs (21, 28) and which is characterized by obliterative vascular lesions with or without lymphoid infiltration and fibrosis was not common either.
About one in four of all the grafts studied had strong histopathologic evidence of large bile duct obstruction, and in most of these instances, there was confirmation that such mechanical problems had been present from premortem clinical studies or from postmortem gross examination. Some of the livers had demonstrable infestation and injury by bacteria, fungi or viruses. However, the majority of the livers contained changes that might be considered nonspecific under other circumstances. Examples were fatty infiltration, venous congestion, centrilobular cholestasis, centrilobular atrophy or necrosis and focal necrosis or infarction.
The interpretation from these findings may prove to be that liver recipients are being systematically overimmunosuppressed, particularly since, as a final event, almost all liver recipients who die have significant extrahepatic infections (31). If over-treatment is the problem, a change in management policy will be called for, whereby lightening instead of intensification of immunosuppression would usually be an appropriate response to deterioration of postoperative liver function.
This kind of radical approach can hardly be justified yet since many patients with a graft that is failing early after transplantation respond to increased dosages of prednisone. It may be that some of the structural abnormalities that are presently thought to be nonspecific are actually subtle manifestations of hepatic rejection or recovery from this process. Such an interpretation would fit with observations in dogs that start to recover from rejection, either spontaneously or as a result of treatment with immunosuppressive drugs (11, 21, 28, 31). Characteristically, the homografts of such animals lose the lymphoid cell infiltration and develop prominent centrilobular bile stasis, atrophy of the centrilobular hepatocytes, collapse and condensation of the centrilobular reticulin and increased amounts of reticulin and collagen in the portal tracts. Besides any, or all, of these findings, fat deposition is not uncommon.
Similar observations, including centrilobular bile stasis, have been made in the homografts of untreated, but long surviving, pigs by Hunt (13) and Battersby and his colleagues (2) and in treated baboons by Myburgh and his associates (19). Autografts of dog livers reported by us (31), Alican and Hardy (1) and McBride and his associates (18) did not have the cholestatic or other findings. The same absence of these findings in pig and baboon hepatic autografts has been described by Battersby (2) and Myburgh (19) and their colleagues.
The critical and, as yet, unanswered questions are whether or not the foregoing changes in homografts and particularly the finding of intrahepatic cholestasis could represent ongoing rejection and, if so, how? Myburgh and his associates (19) have suggested the possible dynamic and immunologic nature of the cholestatic lesion on the basis of their own observations as well as a more general hypothesis of Schaffner and Popper (22). In essence, their argument holds that a continuous and often sublethal injury to the hepatocytes is mediated by humoral antibody or by small numbers of leftover lymphoid cells. The hypothesis continues that hepatocyte organelles, including the smooth endoplasmic reticulum, are damaged, causing perturbations in the secretion and relative concentrations of bile salts, cholesterol, phospholipids and bilirubin with the consequent formation of intrahepatic sludge and the establishment then of a reinforcing vicious cycle of sludging and obstruction. Studies will need to be designed to test this potentially important theory, an extension of which Waldram and his colleagues (34) have applied to partially explain sludge formation in the large bile ducts.
Unfortunately, similar histopathologic abnormalities can be caused by many agents. Fahrländer and his co-workers (9) reported that, in eight patients, severe bacterial infection of organs other than the liver caused intrahepatic cholestasis with minimum hepatocellular injury or inflammation. Electron micrographs revealed dilatation of the canaliculi and rough endoplasmic reticulum. A clinical syndrome of liver failure associated with infection recently reported by Norton and his colleagues (20) may well represent a more severe version of the same process.
It is self evident that intrahepatic cholestasis or the other manifestations of liver injury could also be caused by drugs, particularly those used for immunosuppression. The possibility that either azathioprine or prednisone can harm animal or human livers was already well known a decade ago (27, 31) from experience in kidney transplantation, although there was, at that time, no good way of separating out the instances of serum hepatitis. With the development of the HBsAg screening tests by Blumberg and his co-authors (5), Torisu and his associates (33) were able to survey the serums of 83 of our renal recipients. Because serial serum samples had been systematically stored, it was possible to develop a longitudinal profile of the HBsAg state going back as long as five or six years. The serum of nearly 20 per cent of the patients had become HBsAg positive, but the ultimate incidence of severe liver disease in this group was not substantially different from that in the kidney recipients who had been consistently HBsAg negative. Because of these observations incriminating factors other than serum hepatitis in the cause of post-transplantation hepatic dysfunction, a number of our renal recipients with serious postoperative liver impairment have been switched to cyclophosphamide (32). The improvement that may follow in the hepatic state without deterioration of renal hemograft function has been confirmed by Berne and his associates (3) and in at least one of the liver recipients herein reported. A similar program of drug change in liver recipients with hepatic malfunction is likely to be important in future patients, notwithstanding the belief of Briggs (6) and Ireland (14) and their associates that azathioprine is totally devoid of hepatotoxicity.
Because the definition of the cause for postoperative hepatic malfunction is emerging as the most critical element in the care of liver recipients, other active diagnostic steps will need to be taken, of which repeated needle biopsies and cholangiography are probably the most important. Even if unequivocal diagnosis of conditions like hepatitis, drug hepatotoxicity, rejection and intrahepatic infection is not possible, repeated tissue study findings could give a better insight into the evolution of recovery or failure of the transplants which will be applicable in future patients. Clearly, radical decisions about therapy, such as one for retransplantation, should not be made without prior biopsies and without multiple efforts at transhepatic cholangiography to rule out large duct obstruction.
The greatest dividend from improved diagnosis may be the better management of the biliary duct complications that have so beset liver transplantation in our hands (17, 26, 31) and as reported by Williams and his associates (35) in the King’s College-Cambridge series in England. Bile fistulas usually require wide drainage. With duct obstruction, reoperation is mandatory before there is incurable infection of the homograft.
In our series of obstructions, the most common reoperation has been conversion from cholecystojejunostomy to choledochojejunostomy (Fig. 3B and C). In the series herein reported, there were about 20 livers in which the ducts were certainly, or probably, obstructed. Only a few were successfully corrected, virtually all within our recent experience, including five of the 16 patients who are still alive.
With such a high incidence of biliary tract complications, continuous reassessment of the initial choice of reconstructive procedure will be necessary. Although our usual first choice is a Roux-en-Y cholecystojejunostomy, choledochocholedochostomy with a T-tube stent has been used frequently, taking pains to provide broad wound drainage. The former operation has had a high incidence of obstruction, and the latter has carried a high risk of fistula (26). With either complication, we (17) and Waldram and his group (34) have noted a remarkable tendency for the ducts to become filled with a chalk-like debris, leading to what has been called the inspissated bile syndrome.
Periodic reassessment also will be necessary of the influence of the original host disease upon the outcome insofar as this factor influences future patient selection. None of the diseases for which liver transplantation has been used so far can be categorically precluded as an indication for further trials, especially in children. The brightest chapter in liver transplantation has been in the treatment of inborn errors of metabolism in children, including our two patients with Wilson’s disease, our patient with alpha-1-antitrypsin deficiency and the child of Daloze (8) with Niemann-Pick disease.
Although the experience with adults with cirrhosis in general and with alcoholic cirrhotics in particular has been dismal, this has often been because of their appalling condition at the time of treatment. Earlier decisions need to be made about treatment with transplantation. Even continued efforts to treat recipients with chronic HBsAg antigenemia are probably warranted, especially if hyperimmune specific gamrna globulin therapy can be offered.
The treatment of hepatic malignant tumors with liver replacement remains a controversial issue. One of our patients is cured of a hepatoma after almost six years, but that neoplasm was small and was an incidental finding in a liver that had biliary arresia. Five other patients with hepatornas who lived long enough for observations to be made had recurrences as did a sixth patient whose native liver and, later, whose liver graft were destroyed by a hemangioendothelial sarcoma. Two of our three patients with intrahepatic duct cell carcinomas are alive one and one-quarter and one and three-quarter years postoperatively. Unfortunately, the patient with the long survival period has massive recurrences which are predominantly in the homograft.
Although details have varied, the over-all message from Williams and his associates (35) has been the same, although somewhat more optimistically expressed. In their series of transplantations for four hepatomas, five duct cell carcinomas, two meta-static malignant lesions and one cholangiocarcinoma, eight of the 12 patients were afflicted with recurrent neoplasm. In four of their five patients with duct cell carcinoma, obvious metastases developed, the exception being a recipient who died after three weeks. On the other hand, hepatoma recurred once in four instances, but only one of these recipients lived for as long as a year. That exceptional patient who died of biliary obstruction after more than five years was free of metastases at autopsy, However, the original tumor was apparently slow growing, since she had been aware of an abdominal mass for more than six years preceding transplantation.
Recurrences after liver transplantation for primary hepatic malignant tumors also have been recorded by Hume (12) and Fortner (10) and their associates. It is axiomatic that the outcome in any given patient will depend on the extent of the neoplasm at the time of transplantation. In this connection, a precautionary note about what constitutes genuine candidacy for liver replacement may be introduced on the basis of our unusually large experience with hepatic trisegmentectomy, a partial liver resection in which approximately 85 per cent of the liver is removed (24). A number of the patients in that series who, as it turned out, could be treated by conventional means had been referred to us for consideration of liver transplantation after an erroneous decision of nonresectability had been made at earlier operations. The same point has been made by Smith (23).
summary
During the 11½ year period ending 13 months ago, 93 consecutive patients were treated with orthotopic liver transplantation. Fifty-six of the recipients were 18 years old or younger, and the other 37 were adults. The most common indications for operation were biliary atresia, primary hepatic malignant tumor, chronic aggressive hepatitis and alcoholic cirrhosis.
There has been a gradual improvement in results throughout the period of study, although not to a satisfactory level. Twenty-seven of the 93 patients survived for at least one year after liver replacement with a maximum of six years, and 16 are still alive after 13 to 71 months. The 11 late deaths after one to six years were caused by chronic rejection, biliary obstruction, recurrence of hepatoma, systemic infection or hepatitis of the homograft.
Rejection of the liver as judged by classical histopathologic criteria played a surprisingly small role in the heavy over-all mortality, accounting for less than 10 per cent of the deaths. Technical or mechanical problems, especially those of biliary duct reconstruction, were a far greater cause of failure, as were systemic infections. Six of the 37 adult recipients had lethal cerebrovascular accidents during, or just after, operation. When abnormalities of liver function developed in the post-operative period, the nearly automatic diagnosis of homograft rejection, in retrospect, proved to have been wrong in most instances.
Further development of liver transplantation depends upon two kinds of progress. There must be reduction of operative and early postoperative accidents and complications by more discriminating patient selection, purely technical improvement and better standardization of biliary duct reconstruction. The second area will be in sharpening the criteria for the differential diagnosis of postoperative hepatic malfunction, including the liberal use of transhepatic cholangiography and needle biopsy. Only then can better decisions be made about changes in medication or about the need for secondary corrective surgical procedures.
Acknowledgments
The work was supported by research grant Nos. MRIS 8118-01 and 7227-01 from the Veterans Administration; by Grant Nos. AM-17260 and AM-07772 from the National Institutes of Health, and by Grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health, Bethesda, Maryland.
Footnotes
The patient described in this report is alive one and one-half years after transplantation. The other two one year survivors in the Montreal series of six patients died after two and three-quarters and three and three-quarters years as learned from personal communication on 3 December, 1975.
References
- 1.Alican F, Hardy J. Replantation of the liver in dogs. J Surg Res. 1967;7:368. [Google Scholar]
- 2.Battersby C, Egerton WS, Balderson G, et al. Another look at rejection in pig liver homografts. Surgery. 1974;76:617. [PubMed] [Google Scholar]
- 3.Berne TV, Chatterjee SN, Craig JR, et al. Hepatic dysfunction in recipients of renal allografts. Surg Gynecol Obstet. 1975;141:171. [PubMed] [Google Scholar]
- 4.Birtch AG, Moore FD. Experience in liver transplantation. Transplant Rev. 1969;2:90. doi: 10.1111/j.1600-065x.1969.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg BS, Gerstley BJS, Hungerford DA, et al. A serum antigen (Australia antigen) in Down’s syndrome, leukemia, and hepatitis. Ann Intern Med. 1967;66:924. doi: 10.7326/0003-4819-66-5-924. [DOI] [PubMed] [Google Scholar]
- 6.Briggs WA, Lazarus JM, Birtch AG, et al. Hepatitis affecting hemodialysis and transplant patients. Arch Intern Med. 1973;132:21. [PubMed] [Google Scholar]
- 7.Corman JL, Putnam CW, Iwatsuki S, et al. Liver homotransplantation for chronic active hepatitis with macronodular cirrhosis, HBsAg positive. Gastroenterology. in press. [Google Scholar]
- 8.Daloze P, Corman J, Block P, et al. Enzyme replacement in Niemann-Pick disease by liver homotransplantation. Transplant Proc. 1975;7(Suppl 1):607. [Google Scholar]
- 9.Fahrlander H, Huber F, Gloor F. Intra-hepatic retention of bile in severe bacterial infections. Gastroenterology. 1964;47:590. [PubMed] [Google Scholar]
- 10.Fortner JG, Beattie EJ, Jr, Shiu MH, et al. Orthotopic and heterotopic liver homografts in man. Ann Surg. 1970;172:23. [PMC free article] [PubMed] [Google Scholar]
- 11.Groth CG, Porter KA, Otte JB, et al. Studies of blood flow and ultrastructural changes in rejecting and non-rejecting canine orthotopic liver homografts. Surgery. 1968;63:658. [PMC free article] [PubMed] [Google Scholar]
- 12.Hume DM, Wolf JS, Lee HM, Abouna G. Liver transplantation. Transplant Proc. 1972;4:781. [PubMed] [Google Scholar]
- 13.Hunt AC. Pathology of liver transplantation in the pig. In: Read AE, editor. The Liver. Vol. 337. London: Butterworth & Co., Ltd.; 1967. [Google Scholar]
- 14.Ireland P, Rashid A, Lichtenberg FV, et al. Liver disease in kidney transplant patients receiving azathioprine. Arch Intern Med. 1973;132:29. [PubMed] [Google Scholar]
- 15.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis; an unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 16.Lampe EW, II, Simmons RL, Najarian JS. Hyperglycemic nonketotic coma after liver transplantation. Arch Surg. 1972;105:774. doi: 10.1001/archsurg.1972.04180110091023. [DOI] [PubMed] [Google Scholar]
- 17.Martineau G, Porter KA, Corman J, et al. Delayed biliary duct obstruction after orthotopic liver transplantation. Surgery. 1972;72:604. [PMC free article] [PubMed] [Google Scholar]
- 18.McBride RA, Wheeler HB, Smith LL, et al. Homotransplantation of the canine liver as an orthotopic vascularized graft; histologic and functional correlations during residence in the new host. Am J Pathol. 1962;41:501. [PMC free article] [PubMed] [Google Scholar]
- 19.Myburgh JA, Abrahams C, Mendelsohn D, et al. Cholestatic phenomenon in hepatic allograft rejection in the primate. Transplant Proc. 1971;3:501. [PubMed] [Google Scholar]
- 20.Norton L, Moore G, Eiseman B. Liver failure in the postoperative patient; the role of sepsis and immunologic deficiency. Surgery. 1975;78:6. [PubMed] [Google Scholar]
- 21.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, Putnam CW, editors. Experience in Hepatic Transplantation. Vol. 422. Philadelphia: W. B. Saunders Co.; 1969. [Google Scholar]
- 22.Schaffner F, Popper H. Cholestasis is the result of hypoactive hypertrophic smooth endoplasmic reticulum in the hepatocyte. Lancet. 1969;2:355. doi: 10.1016/s0140-6736(69)92704-4. [DOI] [PubMed] [Google Scholar]
- 23.Smith R. Liver resection. J R Coll Surg Edinb. 1972;17:154. [PubMed] [Google Scholar]
- 24.Starzl TE, Bell RH, Beart RW, Putnam CW. Hepatic trisegmentectomy or extended right lobectomy; relation to other liver resections. Surg Gynecol Obstet. 1975;141:429. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Ishikawa M, Putnam CW, et al. Progress in and deterrents to orthotopic liver transplantation; with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Marchioro TL, Porter KA. Progress in. homotranspiantation of the liver. In: Welch C, editor. Advances in Surgery. Vol. 295. Chicago: Year Book Medical Publishers, Inc.; 1966. [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Marchioro TL, von Kaulla K, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Porter KA, Putnam CW, et al. Liver replacement in children. Proceedings of the Josiah Macy, Jr., Foundation International Conference on Liver Disease in Infancy and Childhood; International Children’s Centre, Paris. 26–28 June 1975; In press. [Google Scholar]
- 31.Starzl TE, Putnam CW. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Co.; 1969. [Google Scholar]
- 32.Starzl TE, Putnam CW, Halgrimson CG, et al. Cyclophosphamide and whole organ transplantation in humans. Surg Gynecol Obstet. 1971;133:981. [PMC free article] [PubMed] [Google Scholar]
- 33.Torisu M, Yokoyama T, Amemiya H, et al. Immunosuppression, liver injury, and hepatitis in renal, hepatic, and cardiac homograft recipients; with particular reference to the Australia antigen. Ann Surg. 1971;1974:620. doi: 10.1097/00000658-197110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldram R, Williams R, Calne RY. Bile composition and bile cast formation after transplantation of the liver in man. Transplantation. 1975;19:382. doi: 10.1097/00007890-197505000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Williams R, Smith M, Shilkin KB, et al. Liver transplantation in man; the frequency of rejection, biliary tract complications, and recurrence of malignancy based on an analysis of 26 cases. Gastroenterology. 1973;64:1026. [PubMed] [Google Scholar]